Abstract
Many proteins can be split into fragments that exhibit enhanced function upon fusion to interacting proteins. While this strategy has been widely used to create protein-fragment complementation assays (PCAs) for discovering protein–protein interactions within mesophilic organisms, similar assays have not yet been developed for studying natural and engineered protein complexes at the temperatures where thermophilic microbes grow. We describe the development of a selection for protein–protein interactions within Thermus thermophilus that is based upon growth complementation by fragments of Thermotoga neapolitana adenylate kinase (AKTn). Complementation studies with an engineered thermophile (PQN1) that is not viable above 75°C because its adk gene has been replaced by a Geobacillus stearothermophilus ortholog revealed that growth could be restored at 78°C by a vector that coexpresses polypeptides corresponding to residues 1–79 and 80–220 of AKTn. In contrast, PQN1 growth was not complemented by AKTn fragments harboring a C156A mutation within the zinc-binding tetracysteine motif unless these fragments were fused to Thermotoga maritima chemotaxis proteins that heterodimerize (CheA and CheY) or homodimerize (CheX). This enhanced complementation is interpreted as arising from chemotaxis protein–protein interactions, since AKTn-C156A fragments having only one polypeptide fused to a chemotaxis protein did not complement PQN1 to the same extent. This selection increases the maximum temperature where a PCA can be used to engineer thermostable protein complexes and to map protein–protein interactions.
Keywords: adenylate kinase, hyperthermophile, protein-fragment complementation, protein–protein interaction, thermophile
Introduction
Microbes that grow optimally above 60°C (thermophiles) and 80°C (hyperthermophiles) populate ecosystems where many biomolecules exhibit reduced stability (Rothschild and Mancinelli, 2001). To support cellular growth, their proteins have evolved distinct amino acid compositions (Zeldovich et al., 2007) and higher thermostability (Vieille and Zeikus, 2001) compared to orthologs found in mesophilic organisms that grow at lower temperatures. This latter feature has been exploited for a variety of biotechnological applications. Thermostable DNA polymerases have revolutionized molecular biology (Niehaus et al., 1999), xylanases have made paper processing greener (Bajpai et al., 2006) and oligosaccharide-modifying enzymes have been harnessed for corn syrup production (Crabb and Mitchinson, 1997). There is interest in further harnessing thermotolerant microbes and their proteins for other industrial processes such as biomass conversion to bioethanol (Shaw et al., 2008) or biohydrogen (Kongjan et al., 2009). However, no in vivo screens have been developed for creating protein complexes that help achieve these high-temperature metabolic engineering and synthetic biology goals (Blumer-Schuette et al., 2008; Chou et al., 2008).
A comparison of the findings from mesophile and hyperthermophile proteomic studies suggests that the lack of high-temperature protein–protein interaction screens may limit the discovery of some protein complexes. A high-throughput screen for pairwise protein–protein interactions among almost 1000 Pyrococcus horikoshii proteins found only 56 hetero-interactions using a two-hybrid assay implemented at a temperature (37°C) far below that of its optimal growth of 98°C (Usui et al., 2005). This finding can be contrasted with similar screens for protein complexes in bacteria and yeast under near physiological conditions, which invariably find protein–protein interactions at a frequency that is more than an order of magnitude higher (Rain et al., 2001; Butland et al., 2005; Tarassov et al., 2008). The creation of an assay that can be used to assess protein complex formation at thermophile growth temperatures would have multiple advantages over available assays in studying natural and engineered proteins (Usui et al., 2005; Tarassov et al., 2008). High temperature assays are predicted to be superior at discovering interactions among proteins that require extreme temperatures to adopt their native conformation (Abd Rahman et al., 1997; Siddiqui et al., 1998; Goda et al., 2005) and among proteins whose interactions weaken as temperature is decreased from the levels where hyperthermophiles grow (Ogasahara et al., 2003).
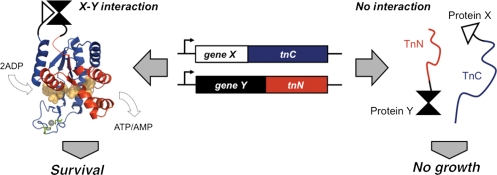
To establish an assay for studying protein complex formation within a living thermophile, we split Thermotoga neapolitana adenylate kinase (AKTn) into fragments that can be used as a protein-fragment complementation assay (PCA) in Thermus thermophilus (Fig. 1). AKTn has many characteristics that make it suitable for designing a split enzyme that reports on protein–protein interactions at extreme temperatures. AKTn is monomeric and extremely thermostable, exhibiting maximal phosphotransferase activity at 80°C (ATP + AMP ⇔ 2ADP) and having a melting temperature of 99.7°C (Vieille et al., 2003). Furthermore, AKTn can be split to generate fragments that spontaneously associate and cooperatively function within a mesophilic bacterium growing at 40°C (Nguyen et al., 2008). Polypeptides corresponding to residues 1–79 (TnN) and 80–220 (TnC) complemented the growth defect of Escherichia coli CV2, a strain that has a temperature-sensitive AK. In addition, structurally related fragments of a Bacillus subtilis AK with lower thermostability could not complement CV2 growth unless they were fused to polypeptides that associate (Nguyen et al., 2008). This suggested that TnN and TnC may be used to report on protein–protein interactions at higher temperatures where the residual structures of these polypeptides are more destabilized, such as within thermophiles that have a temperature-sensitive AK (Counago and Shamoo, 2005).
Fig. 1.
Schematic of an AK-based PCA. TnN (red) and TnC (blue) fragments are engineered to only complement thermophile growth when they are fused to proteins (X and Y) that drive their association. The AKTn fragments are mapped onto the structure of an AK ortholog (PDB ID, 1zin) (Bae and Phillips, 2004) with bound P1,P5-di(adenosine-5) pentaphosphate (peach) and zinc (gray) shown as space filling models. The side chains of the cysteines mutated in AKTn (C133 and C156) are shown in green.
Materials and methods
Materials
Agar was from Difco, Gelrite was from Research Products International and all other bacterial growth media components were from BD Biosciences and Sigma-Aldrich. QuikChange mutagenesis reactions were performed using PfuTurbo DNA polymerase from Stratagene, genes were amplified for cloning using VentR DNA Polymerase from New England Biolabs, and restriction endonucleases were obtained from Roche Biochemical, New England Biolabs and Promega. Synthetic oligonucleotides were from Operon Biotechnology, pET vectors were from EMD Biosciences, Escherichia coli XL1-Blue cells used for plasmid amplification and cloning were from Stratagene, and kits for DNA purification were from Zymo Research and Qiagen.
Thermus strains, transformations and growth media
Thermus thermophilus HB8 (ATCC #27634) was used as the parent strain for adk gene replacement. Liquid growth was performed in Evian-Thermus Medium (EvTM), which contains 8 g tryptone, 4 g yeast extract and 3 g NaCl per liter of Evian mineral water. EvTM-agar plates containing 3% agar were used for growth at temperatures below 75°C, whereas EvTM-Gelrite plates containing 1.5% Gelrite were used for growth at higher temperatures to minimize desiccation. Electrocompetent HB8 cells were prepared using a protocol similar to that previously described (de Grado et al., 1999). Cells (500 ml) were grown to mid-logarithmic phase in EvTM medium (A600 ≈ 0.7) at 65°C, concentrated by centrifugation at 5000 rpm for 30 min, washed with 10% glycerol (250 ml) two times at room temperature, resuspended in 10% glycerol (2 ml) and frozen at −80°C in aliquots (100 µl). Transformations were performed by mixing 3 µg of vector (100–800 ng/µl) and 100 µl of electrocompetent cells, incubating the mixture on ice for 1 h, and electroporating the cells with a pulse of 12.5 kV/cm in 0.1 cm cuvettes using a BioRad MicroPulser. Cells were immediately transferred to EvTM medium (5 ml) that had been pre-warmed to 60°C and incubated for 4 h at 60°C in a 50 ml flask shaking at 150 rpm. Cells transformed with pJJS-derived expression vectors (100 µl) were plated onto EvTM-agar plates containing 15 µg/ml bleocin (Calbiochem) and incubated at 65°C for 72 h. Cells transformed with the adk gene replacement vectors were plated onto EvTM-agar plates containing 250 µg/ml kanamycin and incubated at 60°C for 72 h, and colonies identified as inviable at 80°C were cured of their plasmid by re-streaking them twice onto EvTM-agar plates lacking antibiotic at 60°C. Desiccation was minimized during incubation by placing agar plates into Ziploc bags, removing extra air from the bags, and sealing all but one inch of the bag. To avoid condensation on plates during incubation, freshly poured plates were incubated with the agar facing up at 37°C for 1–2 h prior to use.
Constructing a gene replacement vector
Supplementary data, Figure S1 illustrates how the vector used for adk gene replacement was built. A 1600 bp segment of T.thermophilus HB8 genomic DNA including approximately 1000 bp adjacent to the adk start codon, the adk gene and 45 bp after the adk stop codon was PCR amplified from genomic DNA and cloned into pUC18 using hindIII and xbaI. In addition, approximately 1000 bp of genomic DNA adjacent to the stop codon and the last 121 bp of the adk gene and sequence adjacent to the adk stop codon was PCR amplified from genomic DNA and cloned into the pUC18-derived vector containing the first amplicon using xbaI and ecoRI to create pTT1. The gene encoding a highly thermostable kanamycin nucleotidyltransferase (htk) (Hoseki et al., 1999) with only a ribosomal binding site (RBS) was PCR amplified from pMK-18 (de Grado et al., 1999) and cloned into pTT1 using xbaI to obtain pTT2. This plasmid was modified by two QuikChange mutagenesis reactions to create pTT2-BK, a plasmid that has a unique bmtI site 8 bp prior to the adk start codon and a kpnI site 147 bp before the adk stop codon. The adk gene from Geobacillus stearothermophilus (adkGs) was PCR amplified from genomic DNA and cloned into pTT2-BK using bmtI and kpnI to obtain pTT-GsteAK. Selection of HB8 transformed with pTT-GsteAK on EvTM-agar plates containing 250 µg/ml kanamycin yielded multiple colonies. After curing these strains of their plasmid by sequentially streaking them onto two EvTM-agar plates lacking antibiotic, PCR amplification with primers complementary to the HB8 adk gene revealed that the native adk had not been removed from the chromosome (data not shown), suggesting that the small amount of genomic DNA separating the adk and htk genes in pTT-GsteAK had facilitated off-pathway recombination. To remove this DNA, the htk resistance cassette was PCR amplified and cloned into the kpnI and xbaI sites in pTT-GsteAK to create pTTΔ200-GsteAK, which was the vector successfully used for gene replacement.
Growth rate measurements
Cultures of HB8 and PQN1 cells grown overnight at 60°C in EvTM were used to inoculate 50 ml flasks of pre-warmed EvTM. Cells were grown over a range of temperatures (60–85°C), and the optical density at 600 nm was measured to acquire growth curves until the stationary phase. Each data trace was fit in Kaleidagraph to a modified Gompertz equation (Zwietering et al., 1990), ln(N/N0) = A*(exp(−exp((μm*(2.71828183)/A)*(λ− t) + 1))), where N is the population size as determined by A600 measurements, N0 is the initial A600 upon inoculation, A is the asymptotic value of the curve, λ is the lag period and μm is the maximum specific growth rate. For each strain and temperature, we report the mean and standard error for the maximum specific growth rate calculated from three independent measurements.
Protein expression vectors
The E.coli–T.thermophilus shuttle vector pWUR112 (Brouns et al., 2005) containing a thermostable bleomycin selectable marker (shble) was modified through QuikChange mutagenesis to remove the unique ecoRI and xbaI restriction sties and create pWUR112-ΔEX (Supplementary data, Figure S2A). An ampicillin resistance cassette (bla) PCR amplified with primers that incorporated flanking kpnI–ecoRI–notI–xbaI and speI–notI–pstI–kpnI sites was cloned into the kpnI site of pWUR112-ΔEX to create pJJS. The strong constitutive slpA promoter (Faraldo et al., 1992) was PCR amplified from genomic DNA and subcloned into pJJS using xbaI and pstI, replacing the bla gene, to create pJJS-Pro (Supplementary data, Figure S2B). In addition, the slpA transcriptional terminator TslpA (Faraldo et al., 1992) was PCR amplified from T.thermophilus HB8 genomic DNA using primers that incorporate flanking ecoRI–notI–xbaI and speI–notI–pstI sites, digested with ecoRI and pstI, and cloned into pJJS digested with ecoRI and pstI to yield pJJS-Term. The pJJS, pJJS-Pro and pJJS-Term vectors are all compatible with a modular subcloning assembly strategy (Shetty et al., 2008), which was used for the construction of the majority of protein expression vectors.
The Thermotoga neapolitana adk gene was PCR amplified from pET21d-TnAK (Vieille et al., 2003) using primers that incorporate flanking ecoRI–notI–xbaI and speI–notI–pstI restriction sites, digested with xbaI and pstI, and cloned into pJJS-Pro that had been digested with speI and pstI to create pJJS-TnAK. In addition, fragments of the T.neapolitana adk gene that encode residues 1–79 (TnN) and 80–220 (TnC) were PCR amplified from pET21d-TnN and pET24d-TnC (Nguyen et al., 2008), respectively, using primers that incorporate flanking ecoRI–notI–xbaI and speI–notI–pstI restriction sites and cloned into pJJS-Pro using a similar protocol to create pJJS-TnN and pJJS-TnC, respectively. A vector for coexpressing TnN and TnC, pJJS-N+C, was created by digesting pJJS-TnC with xbaI and pstI, and subcloning the excised gene into pJJS-TnN that had been digested with speI and pstI. QuikChange mutagenesis was used to modify pJJS-TnC to create vectors that express TnC with C133A (pJJS-TnC133) and C156A (pJJS-TnC156) mutations. These plasmids were then used to create the coexpression vectors pJJS-N+C133 and pJJS-N+C156, using a cloning scheme similar to that described for pJJS-N+C.
The strategy used to create vectors that express AK fragment fusions from a single polycistronic transcript is shown in Supplementary data, Figure S3. The genes encoding the P1 and P2 domains of CheA (CheAP1P2; residues 1–264), full-length CheX, and full-length CheY were PCR amplified from Thermotoga maritima MSB8 genomic DNA (ATTC #43589D-5) and pJJS-Pro vectors were generated that encode each of these genes fused in frame to the N-terminus of TnN and TnCC156A mutation through a linker that is predicted to be flexible (GASGGGSSGGHM). These gene fusions were PCR amplified using primers that incorporate ecoRI–notI–xbaI sites upstream of the RBS and speI–notI–pstI restriction sites adjacent to the stop codon. The RBS in the amplified gene fusions is identical in sequence to that found adjacent to the T.thermophilus HB8 slpA promoter (Faraldo et al., 1992), and it includes 20 nucleotides upstream of the annotated RBS. PCR amplified gene fusions encoding the TnN fragments were cloned into pJJS, whereas the gene fusions encoding TnCC156A were cloned into pJJS-Term. Upon sequence verification, the rbs–cheA/X/Y–tnCC156A–TslpA gene fusions were excised from their vectors using XbaI and PstI and subcloned into the vectors harboring the rbs–cheA/X/Y–tnN gene fusions that had been digested with SpeI and PstI. Supplementary data available at PEDS online, Table S1 lists all vector intermediates, and Supplementary data, Table S2 lists the vectors used for complementation analysis.
PQN1 complementation
Complementation analysis involving monocistronic constructs was assessed by streaking colonies obtained from transformations on solid medium in glass Petri dishes, and incubating these plates at temperatures (≥78°C) where PQN1 cannot grow like parental HB8. Complementation studies involving the polycistronic constructs were performed by spotting a defined titer of cells grown at 65°C onto EvTM-Gelrite plates, and evaluating growth after 24 h at 78°C. In the spotting experiments, 5 ml EvTM liquid cultures containing 5 µg/ml bleocin were inoculated with colonies obtained from transformations and cultured overnight at 65°C while shaking at 150 rpm. The optical density of each culture was measured after 24 h, and each culture was diluted to an A600 of 0.5, pelleted by centrifugation and resuspended in 25% glycerol so that it was concentrated 4-fold. Serial dilutions (1×, 10×, 100× and 1000×) of the resuspended cells (10 µl each) were spotted onto EvTM-Gelrite plates. After incubation at 78°C for 24 h, growth at each spot was analyzed using a FluorChem 5500 imager (Alpha-Innotech), and the program ImageJ was used to quantify the relative growth of spots (Abramoff et al., 2004).
Chemotaxis protein purification
Thermotoga maritima MSB8 CheAP1P2, CheX and CheY were overexpressed in E.coli Rosetta cells (Novagen) using plasmids kindly provided by the Crane Lab (Park et al., 2004a,b) that express these proteins with N-terminal (His)6 tags. Cells transformed with these plasmids were grown at 37°C in LB supplemented with 25 µg/ml kanamycin, protein expression was induced at an A600 of ∼0.5 by adding 1 mM isopropylthio-β-D-galactoside (IPTG), cells were harvested by centrifugation after 3 h of growth, and cells were lysed by resuspending them in PSI buffer (50 mM phosphate pH 7.5, 150 mM NaCl and 10 mM imidazole) containing 1 mM MgCl2, 300 µg/ml lysozyme and 2 U/ml DNase I. Cells were frozen at −80°C, thawed and centrifuged at 15 000×g for 1 h. Cleared lysate was filtered through a sterile 0.2 micron syringe filter, applied to a 2 ml nickel talon affinity column (Qiagen) equilibrated with PSI buffer, washed with 10 column volumes of PSI buffer, and bound protein was eluted using PSI containing 250 mM imidazole. The elution containing CheAP1P2 (theoretical pI = 4.6) was diluted 100-fold into 50 mM phosphate pH 7.5 buffer, applied to a 5 ml HiTrap-Q HP anion exchange column (GE Healthcare) and eluted using a linear gradient from 0 to 500 mM NaCl in 50 mM phosphate pH 7.5. All purified proteins were dialyzed overnight into PS buffer (50 mM phosphate pH 7.5 and 150 mM NaCl), and stored at −80°C.
Analytical methods
An ÄKTA FPLC was used for all protein purification. Protein concentrations were determined by measuring their absorbance with a Cary 50 spectrophotometer, using the extinction coefficients ε280(CheAP1P2) = 5120 M−1 cm−1 and ε280(CheY) = 2560 M−1cm−1, calculated from the primary sequence by the PEPSTATS algorithm (Rice et al., 2000). Dynamic light scattering was performed with a Malvern Instruments Zetasizer Nano ZS using samples that contained 115 µM of each protein in PS buffer (50 mM Phosphate pH 7.5 and 150 mM NaCl). These samples were analyzed in triplicate at 78°C using a 1 cm quartz cuvette at a backscattering angle of 173° after a 5-min incubation at 78°C. Data processing was performed using the high resolution analysis model provided with Zetasizer software v6.01.
Results
Replacing T.thermophilus adk
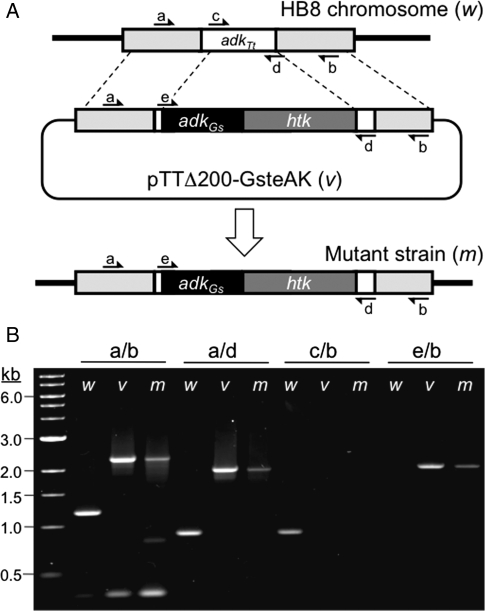
To generate a Thermus thermophilus HB8 mutant for evaluating AK fragment complementation above 75°C, homologous recombination was used to replace its adk gene with adkGs, an ortholog that encodes a protein that is not functional above 70°C in vitro (Bae and Phillips, 2004). Gene replacement was performed by transforming HB8 cells with the pTTΔ200-GsteAK plasmid and selecting for growth at 60°C on EvTM-agar plates containing kanamycin as previously described (Cameron et al., 2004). Since HB8 cells cannot replicate this plasmid, they are only able to grow if the htk gene is integrated into the chromosome as illustrated in Fig. 2A. Kanamycin-resistant colonies were screened for growth at 80°C in EvTM-liquid culture, and strains identified as nonviable at this temperature were cured of their plasmid. PCR analysis of genomic DNA from one of the strains obtained (designated PQN1) confirmed integration of adkGs into the targeted chromosome locus (Fig. 2B), and DNA sequencing of these amplicons revealed that PQN1 expresses an AKGs with an E70V mutation.
Fig. 2.
Creation of the temperature-sensitive T.thermophilus strain PQN1. (A) Chromosomal replacement of T.thermophilus adkTt was induced by transformation of HB8 with pTTΔ200-GsteAK, which contains a fusion of the G.stearothermophilus adk gene and the htk selectable marker flanked by 982 bp of genomic DNA that is upstream of the adkTt start codon and 1121 bp of genomic DNA that includes the last 121 bp of adkTt and 1000 bp following the gene. A portion of adkTt was included to ensure that the promoter that drives transcription of the predicted downstream methionine aminopeptidase gene was not disrupted upon recombination. (B) Wild-type HB8 genomic DNA (w), the pTTΔ200-GsteAK vector (v) and PQN1 mutant (m) genomic DNA were used as template for PCR reactions involving pairs of primers that bind to genomic DNA flanking adkTt (a and b) and sequences within the different adk genes (c, d and e).
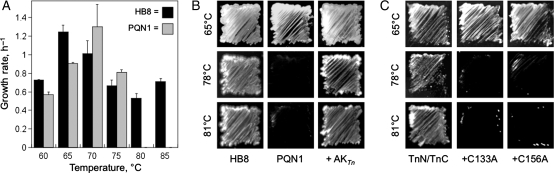
To better characterize the temperature-sensitive phenotype of PQN1, we compared its specific maximal growth rate in liquid medium to the parental strain used for recombination (Zwietering et al., 1990). Figure 3A shows that T.thermophilus HB8 grows over a broad temperature range (60–85°C) as previously reported (Oshima and Imahori, 1974). In contrast, PQN1 only displayed detectable growth up to 75°C, 10° lower than the maximal growth temperature of the parental HB8 strain. We also investigated the effect of temperature on the growth of PQN1 on solid medium over a range of temperatures (Fig. 3B). As in liquid medium, PQN1 did not grow above 75°C under these conditions. However, this temperature-sensitive phenotype could be rescued by transformation with a vector (pJJS-TnAK) that constitutively expresses the hyperthermophilic AKTn using the strong constitutive slpA promoter (Faraldo et al., 1992).
Fig. 3.
AKTn complementation of PQN1. (A) Single colonies of PQN1 and HB8 cells grown at 65°C on EvTM-agar plates were used to inoculate 5 ml EvTM cultures. After an overnight incubation at 60°C while shaking at 150 rpm, 1 ml of each culture was used to inoculate fresh 50 ml EvTM cultures. These cultures were grown at the indicated temperatures, the change in optical density at 600 nm was measured until the stationary phase was reached (≥12 h) and the growth rate was calculated. For each strain and temperature, the mean and standard error were calculated from three independent measurements. (B) AKTn complementation of PQN1 on solid medium. Colonies of HB8, PQN1 and PQN1 transformed with a plasmid expressing full-length AKTn streaked on EvTM-Gelrite plates after incubation at 65, 78 and 81°C for 48 h. (C) PQN1 complementation by AKTn fragments. Colonies of PQN1 transformed with plasmids that coexpress TnN and TnC, TnN and TnCC133A and TnN and TnCC156A streaked on EvTM-Gelrite plates after incubation for 48 h at 65, 78 and 81°C. Representative streaks are shown from selections that were performed in triplicate.
AKTn fragment complementation
To be useful as a PCA, fragments of a split protein must exhibit impaired function in the absence of protein tags (Tarassov et al., 2008). To evaluate whether this occurs with the AKTn fragments TnN and TnC at 78°C as observed with homologous fragments of B.subtilis AK at 40°C (Nguyen et al., 2008), we evaluated the growth of PQN1 transformed with pJJS-N+C, a vector that constitutively coexpresses monocistronic transcripts encoding each fragment. Figure 3C shows that PQN1 growth was complemented by TnN and TnC above 75°C without the assistance of protein tags that drive their association. We next examined whether point mutations within the tetracysteine motif that coordinates zinc (Glaser et al., 1992) abrogate the association and cooperative function of TnC and TnN sufficiently to allow them to be used as a PCA in PQN1. These cysteines were targeted for mutagenesis since previous studies have shown zinc binding by the tetracysteine motif in the LID domain enhances AK thermostability (Perrier et al., 1998; Vieille et al., 2003). We found that coexpression of TnN with TnC fragments harboring either a Cys133Ala (TnCC133A) or Cys156Ala (TnCC156A) mutation could not complement PQN1 growth at 78°C. This suggests that disruption of zinc binding in the TnCC133A and TnCC156A polypeptides causes a decrease in the association with TnN and/or a decrease in the thermostability of AKTn-fragment complexes, as observed previously upon loss of zinc from full-length AKTn (Vieille et al., 2003).
Assisted protein-fragment complementation
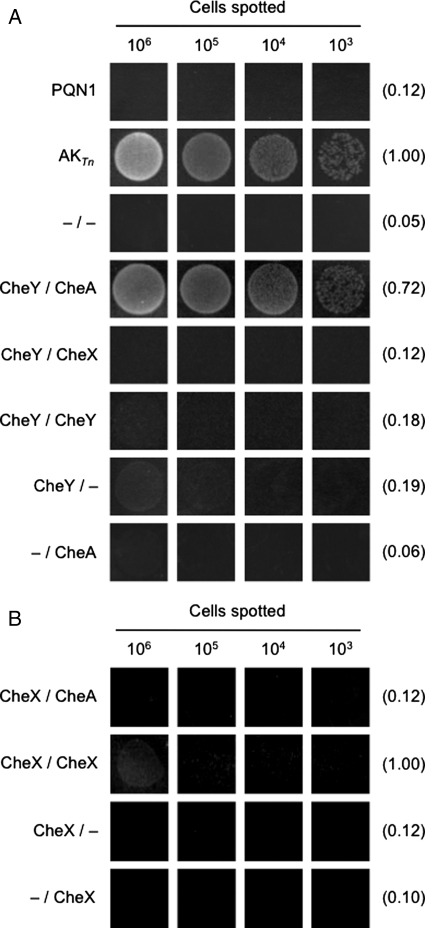
To test whether TnN and TnCC156A can be used with PQN1 to report on protein–protein interactions, we examined whether PQN1 growth could be complemented at 78°C by vectors that express these polypeptides fused to different combinations of the Thermotoga maritima MSB8 chemotaxis proteins (CheAP1P2, CheX and CheY). These proteins were chosen because biochemical and biophysical studies have provided evidence for interactions between CheAP1P2 and CheY (Park et al., 2004a,b), CheX and CheY (Park et al., 2004a,b) and CheX and CheX (Park et al., 2004a,b). Among these pairwise interactions, only the CheAP1P2 and CheY interaction has been studied at a temperature where thermophiles grow, although the maximum temperature where this interaction has previously been analyzed (70°C) is lower than the minimum temperature (78°C) where our assay can be performed (Park et al., 2004a,b).
Figure 4A compares the growth of untransformed PQN1 with cells harboring vectors that coexpress TnN and TnCC156A fused to different combinations of chemotaxis proteins via a 12 amino acid linker. For these experiments, both TnN and TnCC156A fragments were expressed from a single polycistronic transcript with the thermostable bleomycin selectable marker. We found that PQN1 coexpressing CheY-TnN and CheAP1P2-TnCC156A grew after 24 h of incubation at 78°C at all titers analyzed, similar to that observed with PQN harboring a vector that expresses full-length AKTn. The average magnitude of the growth was calculated for the highest titers analyzed in each experiment by integrating the intensity of replicate spots using ImageJ. This analysis revealed that PQN1 coexpressing CheY-TnN and CheAP1P2-TnCC156A grows 72% as dense as cells expressing full-length AKTn. In contrast, the growth detected with PQN1 expressing AK fragments fused to only CheY (CheY-TnN and TnCC156A) or CheAP1P2 (TnN and CheAP1P2-TnCC156A) is ≥4-fold lower than that of cells expressing AK fragments fused to CheY and CheAP1P2 at the highest titer and undetectable at the lowest titer analyzed. Similarly, little growth was observed after 24 h with PQN1 transformed with vectors that express TnN and TnCC156A fused to nothing, CheY and CheX, and CheY and CheY. Taken together, these findings provide direct evidence that the complementation observed with PQN1 coexpressing CheY-TnN and CheAP1P2-TnCC156A arises because the association of these chemotaxis proteins drives the cooperative function of the AKTn fragments at the physiological growth temperatures of T.maritima as predicted (Park et al., 2004a,b). CheY and CheX are also predicted to interact in T.maritima (Park et al., 2004a,b). However, the lack of growth with CheY and CheX protein fusions is consistent with studies which find that CheY must be phosphorylated to associate strongly with CheX (Muff et al., 2007).
Fig. 4.
Detection of chemotaxis protein–protein interactions. (A) Analysis of CheAP1P2 and CheY binding using AKTn-fragment complementation. Relative growth after 24 h at 78°C of PQN1 alone (PQN1) and PQN1 transformed with vectors that coexpress TnN and TnCC156A (–/–), CheY-TnN and CheAP1P2-TnCC156A (CheY/CheA), CheY-TnN and CheX-TnCC156A (CheY/CheX), CheY-TnN and CheY-TnCC156A (CheY/CheY), CheY-TnN and TnCC156A (CheY/–), and TnN and CheAP1P2-TnCC156A (–/CheA). The relative integrated densities are reported in parenthesis relative to that of PQN1 cells expressing AKTn. (B) Analysis of CheX self-association using AKTn-fragment complementation. Relative growth after 24 h at 78°C of PQN1 cells transformed with plasmids that coexpress CheX-TnN and CheAP1P2-TnCC156A (CheX/CheA), CheX-TnN and CheX-TnCC156A (CheX/CheX), CheX-TnN and TnCC156A (CheX/–), and TnN and CheX-TnCC156A (–/CheX). The integrated densities are reported relative to that of PQN1 cells expressing CheX-TnN and CheX-TnCC156A. The spots in both panels represent different titers of cells aliquoted onto EvTM-Gelrite plates, which were obtained from liquid cultures incubated at 65°C in the presence of 5 µg/ml bleocin. ImageJ was used to quantify the density of cell growth relative to background levels for the spots corresponding to the highest titer. The number of cells spotted was calculated by assessing colony counts on EvTM plates incubated at 65°C for 48 h.
Since CheX is a dimer in its crystal structure (Park et al., 2004a,b), we also investigated whether two CheX molecules could enhance AKTn-fragment complementation of PQN1 at 78°C such as CheAP1P2 and CheY. Figure 4B shows that PQN1 cells coexpressing CheX-TnN and CheX-TnCC156A display detectable growth after a 24 h incubation at 78°C. At the highest titer analyzed, this growth is ≥5.5-fold higher than that of PQN1 cells harboring vectors that only express one of the two AKTn fragments as a fusion with CheX, i.e. CheX-TnN and TnCC156A or TnN and CheX-TnCC156A. In addition, PQN1 cells coexpressing CheX-TnN and CheX-TnCC156A grow to a density that is ∼8-fold higher than that of cells expressing CheX-TnN and CheAP1P2-TnCC156A, chemotaxis protein fusions that are not expected to interact and promote AK-fragment function. However, PQN1 were not complemented by CheX-TnN and CheX-TnCC156A to the same extent as with CheY-TnN and CheAP1P2-TnCC156A. Growth was only observed with cells expressing CheX protein fusions at the highest titer of cells analyzed even after incubation for an additional day, whereas growth could be detected with CheAP1P2 and CheY protein fusions at all titers analyzed after 24 h.
In vitro analysis of CheAP1P2 and CheY binding
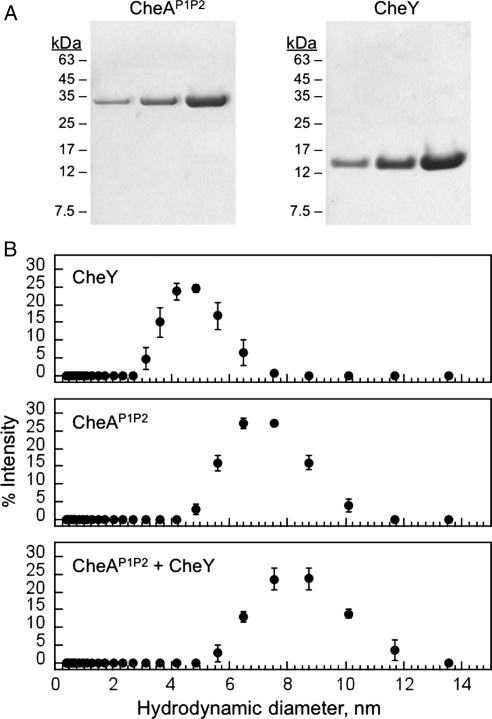
The results from our PQN1 complementation analysis extends the temperature where a CheAP1P2–CheY heterodimer has been observed from 70°C (Park et al., 2004a,b) to 78°C. To test whether this interaction can also be detected in vitro at the temperature where our assay is implemented (78°C), we purified CheAP1P2 and CheY to greater than 95% homogeneity (Fig. 5A) and evaluated their apparent molecular size using dynamic light scattering. Figure 5B shows the results from dynamic light scattering measurements performed at 78°C with samples containing CheAP1P2, CheY and a mixture of CheAP1P2 and CheY. This analysis revealed that an equimolar mixture of CheAP1P2 and CheY displays a hydrodynamic diameter (8.3±0.1 nm) that is greater than that observed with either CheAP1P2 (7.1±0.2 nm) or CheY (4.7±0.1 nm) alone.
Fig. 5.
In vitro analysis of CheAP1P2 and CheY binding at 78°C. (A) SDS–PAGE analysis of purified chemotaxis proteins. A comparison of 1, 3 and 10 µg of T.maritima CheAP1P2 and CheY indicate a high degree of purity for each protein. (B) The size distribution of CheAP1P2, CheY and a mixture of CheAP1P2 and CheY were measured at 78°C using dynamic light scattering after a 5-min incubation at that temperature. All proteins were dialyzed into PS buffer prior to analysis, and the data shown represent average values calculated from three separate experiments performed using 115 µM of each protein. The hydrodynamic diameters of CheY (4.7±0.1 nm), CheAP1P2 (7.1±0.2 nm) and a mixture of CheAP1P2 and CheY (8.3 ± 0.1 nm) were calculated using the Zetasizer software high-resolution analysis model.
Discussion
We provide evidence that AK fragment complementation in T.thermophilus is proportional to the thermostability of the protein being fragmented, as previously observed in E.coli (Nguyen et al., 2008). Whereas AKTn fragments alone could complement PQN1 at 78°C, fragments with mutations in the cysteines that coordinate a thermostabilizing Zn2+ (Glaser et al., 1992; Vieille et al., 2003) could not support PQN1 growth at this temperature. However, AKTn fragments harboring the destabilizing C156A mutation were able to complement PQN1 when they were fused to chemotaxis proteins that homodimerize (CheX) and heterodimerize (CheAP1P2 and CheY). This indicates that covalent linkage to associating proteins can drive the reassembly of the AKTn fragments harboring the C156A mutation at 78°C, extending the temperature range where a split AK can be used as a PCA from mesophile to thermophile growth temperatures (Nguyen et al., 2008). The finding that the TnN and TnC fragments cooperatively function at 78°C upon heterologous expression also reinforces the hypothesis that the ability of a protein to fold and function at extreme temperatures is intrinsically encoded by its amino acid sequence (Berezovsky and Shakhnovich, 2005; Zeldovich et al., 2007), and this suggests that these AKTn polypeptides may be useful for creating a PCA that functions at even higher temperatures.
Our studies examining the effects of T.maritima chemotaxis proteins on AKTn-fragment complementation increases the maximum temperature (78°C) where an interaction has been observed between the kinase CheAP1P2 and its target CheY (Huber et al., 1986). PQN1 growth was complemented by a vector that expresses AKTn fragments fused to CheAP1P2 and CheY but not by vectors that express AKTn fragments fused to only one of these chemotaxis proteins. This is consistent with our findings from light scattering measurements performed at 78°C, which revealed that a mixture of purified CheAP1P2 and CheY exhibits an average hydrodynamic diameter that is greater than either of the individual proteins. Measurements of CheX-assisted fragment complementation also provides evidence that the CheX phosphatase self-associates at the temperature where it functions in T.maritima (Huber et al., 1986). PQN1 growth was complemented at 78°C by a vector that coexpresses CheX-TnN and CheX-TnCC156A to a greater extent than vectors that express only one of the two AKTn fragments as a fusion with CheX. However, this growth was less pronounced than that observed with AKTn fragment fused to CheAP1P2 and CheY. This latter finding cannot be interpreted as necessarily arising from a weaker interaction between two CheX molecules, since several issues could influence the magnitude of complementation observed in our assay. Homodimerization is expected to decrease the amount of CheX-TnN and CheX-TnCC156A that is available to heterodimerize and exhibit AK activity in PQN1. In addition, CheX protein fusions could accumulate to a lesser extent than CheAP1P2 and CheY fusions in PQN1, associate to a greater extent with T.thermophilus protein(s) in a manner that competes with self-association, and be less compatible with our high-temperature PCA due to the proximity of their termini fused to TnN and TnCC156A (Park et al., 2004a,b). Additional studies will be required to establish the detection limit of our high-temperature PCA.
The high-temperature PCA described herein is expected to have several advantages in discovering interactions among natural and engineered proteins compared with previously described approaches, such as mesophilic two-hybrid (Usui et al., 2005) and PCA screens (Michnick et al., 2007). The AKTn-based PCA is implemented within a T.thermophilus HB8 strain growing at a temperature where many thermophiles and hyperthermophiles grow (Rothschild and Mancinelli, 2001), making it better suited for discovering interactions among proteins that have evolved to fold and function at extreme temperatures (Abd Rahman et al., 1997; Siddiqui et al., 1998; Goda et al., 2005). In addition, this selection is expected to be more sensitive than existing assays at detecting interactions among proteins whose free energy of binding increases as temperature decreases below the physiological growth range of thermotolerant microbes (Ogasahara et al., 2003; Esue et al., 2005). Unfortunately, the fraction of hyperthermophilic proteins that exhibit this behavior is not known because screens have not yet been performed at temperatures near the boiling point of water. Furthermore, the high-temperature PCA will be capable of assessing protein complex formation in the presence of specialized solutes that accumulate within thermolerant microbes (Empadinhas and da Costa, 2006), which have been proposed to affect the equilibrium binding of protein complexes (Ellis, 2001).
Our AKTn-based PCA should also aid protein design efforts working to create artificial protein complexes that are stable at the temperatures where thermophiles and hyperthermophiles grow. This novel PCA can be used as a high-throughput screen to evaluate the association of proteins created through rational design (Huang et al., 2007) and laboratory evolution (Huang et al., 2008). In addition, this PCA can be used to investigate the robustness of protein complexes to different mutational processes (Bloom et al., 2005; Drummond et al., 2005) and to examine why some hyperthermophilic protein complexes weaken in affinity as temperature is decreased below the physiological growth range of hyperthermophiles (Ogasahara et al., 2003), whereas others do not (Park et al., 2004a,b). The latter problem can be addressed by mining libraries of engineered (or natural) proteins for interactions that occur at 78°C and rescreening discovered variants for decreased association at 40°C, using a structurally related PCA that was developed for use in E.coli at 40°C (Nguyen et al., 2008). By comparing the sequences of the protein complexes that support protein complementation to different extents at high and low temperatures, hypotheses about the biophysical origin of these observations can be tested (Sheinerman et al., 2000).
Supplementary data
Funding
This work was supported by the National Aeronautics and Space Administration (grant number NNX08AO20G to J.J.S.), the Robert A. Welch Foundation (grant number C-1614 to J.J.S.) and the National Institutes of Health Biotechnology Training Grant (grant number 2T32-GM008362 to P.Q.N.).
Supplementary Material
Acknowledgements
We thank Shirley Liu for expert technical support, Kevin G. Hoff and the S3 group for critical feedback, and Stan J.J. Brouns, Brian R. Crane, Albert E. Dahlberg, Yousif Shamoo and Claire Vieille for providing vectors.
Footnotes
Edited by Jacques Fastrez
References
- Abd Rahman R.N., Fujiwara S., Takagi M., Kanaya S., Imanaka T. Biochem. Biophys. Res. Commun. 1997;241:646–652. doi: 10.1006/bbrc.1997.7850. [DOI] [PubMed] [Google Scholar]
- Abramoff M.D., Magelhaes P.J., Ram S.J. Biophot. Int. 2004;11:36–42. [Google Scholar]
- Bae E., Phillips G.N., Jr J. Biol. Chem. 2004;279:28202–28208. doi: 10.1074/jbc.M401865200. doi:10.1074/jbc.M401865200. [DOI] [PubMed] [Google Scholar]
- Bajpai P., Anand A., Bajpai P.K. Biotechnol. Annu. Rev. 2006;12:349–378. doi: 10.1016/S1387-2656(06)12010-4. doi:10.1016/S1387-2656(06)12010-4. [DOI] [PubMed] [Google Scholar]
- Berezovsky I.N., Shakhnovich E.I. Proc. Natl Acad. Sci. USA. 2005;102:12742–12747. doi: 10.1073/pnas.0503890102. doi:10.1073/pnas.0503890102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J.D., Silberg J.J., Wilke C.O., Drummond D.A., Adami C., Arnold F.H. Proc. Natl Acad. Sci. USA. 2005;102:606–611. doi: 10.1073/pnas.0406744102. doi:10.1073/pnas.0406744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer-Schuette S.E., Kataeva I., Westpheling J., Adams M.W., Kelly R.M. Curr. Opin. Biotechnol. 2008;19:210–217. doi: 10.1016/j.copbio.2008.04.007. doi:10.1016/j.copbio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Brouns S.J., Wu H., Akerboom J., Turnbull A.P., de Vos W.M., van der Oost J. J. Biol. Chem. 2005;280:11422–11431. doi: 10.1074/jbc.M413623200. doi:10.1074/jbc.M413623200. [DOI] [PubMed] [Google Scholar]
- Butland G., et al. Nature. 2005;433:531–537. doi: 10.1038/nature03239. doi:10.1038/nature03239. [DOI] [PubMed] [Google Scholar]
- Cameron D.M., Gregory S.T., Thompson J., Suh M.J., Limbach P.A., Dahlberg A.E. J. Bacteriol. 2004;186:5819–5825. doi: 10.1128/JB.186.17.5819-5825.2004. doi:10.1128/JB.186.17.5819-5825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.J., Jenney F.E., Jr, Adams M.W., Kelly R.M. Metab. Eng. 2008;10:394–404. doi: 10.1016/j.ymben.2008.06.007. doi:10.1016/j.ymben.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Counago R., Shamoo Y. Extremophiles. 2005;9:135–144. doi: 10.1007/s00792-004-0428-x. doi:10.1007/s00792-004-0428-x. [DOI] [PubMed] [Google Scholar]
- Crabb W.D., Mitchinson C. Trends Biotechnol. 1997;15:349–352. [Google Scholar]
- de Grado M., Castan P., Berenguer J. Plasmid. 1999;42:241–245. doi: 10.1006/plas.1999.1427. [DOI] [PubMed] [Google Scholar]
- Drummond D.A., Silberg J.J., Meyer M.M., Wilke C.O., Arnold F.H. Proc. Natl Acad. Sci. USA. 2005;102:5380–5385. doi: 10.1073/pnas.0500729102. doi:10.1073/pnas.0500729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R.J. Trends Biochem. Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. doi:10.1016/S0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- Empadinhas N., da Costa M.S. Int. Microbiol. 2006;9:199–206. [PubMed] [Google Scholar]
- Esue O., Cordero M., Wirtz D., Tseng Y. J. Biol. Chem. 2005;280:2628–2635. doi: 10.1074/jbc.M410298200. doi:10.1074/jbc.M410298200. [DOI] [PubMed] [Google Scholar]
- Faraldo M.M., de Pedro M.A., Berenguer J. J. Bacteriol. 1992;174:7458–7462. doi: 10.1128/jb.174.22.7458-7462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Presecan E., Delepierre M., Surewicz W.K., Mantsch H.H., Barzu O., Gilles A.M. Biochemistry. 1992;31:3038–3043. doi: 10.1021/bi00127a002. doi:10.1021/bi00127a002. [DOI] [PubMed] [Google Scholar]
- Goda S., Kojima M., Nishikawa Y., Kujo C., Kawakami R., Kuramitsu S., Sakuraba H., Hiragi Y., Ohshima T. Biochemistry. 2005;44:15304–15313. doi: 10.1021/bi050478l. doi:10.1021/bi050478l. [DOI] [PubMed] [Google Scholar]
- Hoseki J., Yano T., Koyama Y., Kuramitsu S., Kagamiyama H. J. Biochem. (Tokyo) 1999;126:951–956. doi: 10.1093/oxfordjournals.jbchem.a022539. [DOI] [PubMed] [Google Scholar]
- Huang P.S., Love J.J., Mayo S.L. Protein Sci. 2007;16:2770–2774. doi: 10.1110/ps.073125207. doi:10.1110/ps.073125207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Koide A., Makabe K., Koide S. Proc. Natl Acad. Sci. USA. 2008;105:6578–6583. doi: 10.1073/pnas.0801097105. doi:10.1073/pnas.0801097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Langworthy T.A., Koenig H., Thomm M., Woese C.R., Sleytr U.B., Stetter K.O. Arch. Microbiol. 1986;144:324–333. doi:10.1007/BF00409880. [Google Scholar]
- Kongjan P., Min B., Angelidaki I. Water Res. 2009;43:1414–1424. doi: 10.1016/j.watres.2008.12.016. doi:10.1016/j.watres.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Michnick S.W., Ear P.H., Manderson E.N., Remy I., Stefan E. Nat. Rev. Drug Discov. 2007;6:569–582. doi: 10.1038/nrd2311. doi:10.1038/nrd2311. [DOI] [PubMed] [Google Scholar]
- Muff T.J., Foster R.M., Liu P.J., Ordal G.W. J. Bacteriol. 2007;189:7007–7013. doi: 10.1128/JB.00896-07. doi:10.1128/JB.00896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P.Q., Liu S., Thompson J.C., Silberg J.J. Protein Eng. Des. Sel. 2008;21:303–310. doi: 10.1093/protein/gzn005. doi:10.1093/protein/gzn005. [DOI] [PubMed] [Google Scholar]
- Niehaus F., Bertoldo C., Kahler M., Antranikian G. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. doi:10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- Ogasahara K., Ishida M., Yutani K. J. Biol. Chem. 2003;278:8922–8928. doi: 10.1074/jbc.m210893200. doi:10.1074/jbc.M210893200. [DOI] [PubMed] [Google Scholar]
- Oshima T., Imahori K. Int. J. Syst. Bacteriol. 1974;24:102–112. doi:10.1099/00207713-24-1-102. [Google Scholar]
- Park S.Y., Beel B.D., Simon M.I., Bilwes A.M., Crane B.R. Proc. Natl Acad. Sci. USA. 2004a;101:11646–11651. doi: 10.1073/pnas.0401038101. doi:10.1073/pnas.0401038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Chao X., Gonzalez-Bonet G., Beel B.D., Bilwes A.M., Crane B.R. Mol. Cell. 2004b;16:563–574. doi: 10.1016/j.molcel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Perrier V., Burlacu-Miron S., Bourgeois S., Surewicz W.K., Gilles A.M. J. Biol. Chem. 1998;273:19097–19101. doi: 10.1074/jbc.273.30.19097. doi:10.1074/jbc.273.30.19097. [DOI] [PubMed] [Google Scholar]
- Rain J.C., et al. Nature. 2001;409:211–215. doi: 10.1038/35051615. doi:10.1038/35051615. [DOI] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. doi:10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Rothschild L.J., Mancinelli R.L. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. doi:10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- Shaw A.J., Podkaminer K.K., Desai S.G., Bardsley J.S., Rogers S.R., Thorne P.G., Hogsett D.A., Lynd L.R. Proc. Natl Acad. Sci. USA. 2008;105:13769–13774. doi: 10.1073/pnas.0801266105. doi:10.1073/pnas.0801266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman F.B., Norel R., Honig B. Curr. Opin. Struct. Biol. 2000;10:153–159. doi: 10.1016/s0959-440x(00)00065-8. doi:10.1016/S0959-440X(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Shetty R.P., Endy D., Knight T.F., Jr J. Biol. Eng. 2008;2:5. doi: 10.1186/1754-1611-2-5. doi:10.1186/1754-1611-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M.A., Fujiwara S., Takagi M., Imanaka T. FEBS Lett. 1998;434:372–376. doi: 10.1016/s0014-5793(98)00998-3. doi:10.1016/S0014-5793(98)00998-3. [DOI] [PubMed] [Google Scholar]
- Tarassov K., Messier V., Landry C.R., Radinovic S., Serna Molina M.M., Shames I., Malitskaya Y., Vogel J., Bussey H., Michnick S.W. Science. 2008;320:1465–1470. doi: 10.1126/science.1153878. doi:10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- Usui K., et al. Genome Biol. 2005;6:R98. doi: 10.1186/gb-2005-6-12-r98. doi:10.1186/gb-2005-6-12-r98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieille C., Zeikus G.J. Microbiol. Mol. Biol. Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. doi:10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieille C., Krishnamurthy H., Hyun H.H., Savchenko A., Yan H., Zeikus J.G. Biochem. J. 2003;372:577–585. doi: 10.1042/BJ20021377. doi:10.1042/BJ20021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldovich K.B., Berezovsky I.N., Shakhnovich E.I. PLoS Comput. Biol. 2007;3:e5. doi: 10.1371/journal.pcbi.0030005. doi:10.1371/journal.pcbi.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwietering M.H., Jongenburger I., Rombouts F.M., van 't Riet K. Appl. Environ. Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.