Abstract
High-throughput generation of antibodies against cellular components is currently a challenge in proteomics, therapeutic development and other biological applications. It is particularly challenging to raise antibodies that target membrane proteins due to their insolubility in aqueous solutions. To address these issues, a yeast display library of human single-chain antibody fragments (scFvs) was efficiently screened directly against detergent-solubilized and biotinylated lysates of a target cell line, thereby avoiding issues with membrane protein insolubility and eliminating the need for heterologous expression or purification of antigens. Antibody clones that specifically bind plasma membrane proteins or intracellular proteins were identified, depending on the biotinylation method applied. Antibodies against a predetermined target could also be identified using cell lysate as an antigen source as demonstrated by selecting an scFv against the transferrin receptor (TfR). When secreted from yeast and purified, the selected scFvs are active under physiological conditions in the absence of detergents. In addition, this method allows facile characterization of target antigens because it is compatible with yeast display immunoprecipitation. We expect that this method will prove useful for multiplex affinity reagent generation and in targeted antibody screens.
Keywords: antibody, cell lysate, library selection, yeast display
Introduction
Affinity reagents such as antibodies that specifically bind target proteins are an essential tool in studying key protein properties such as expression level, cellular localization and post-translational modification. In addition, antibodies have become an important class of therapeutics in the past decade, as exemplified by a number of FDA approved monoclonal antibodies for disease treatment (Schrama et al., 2006). Therefore, major efforts to establish a comprehensive set of well-characterized affinity reagents are under way (Taussig et al., 2007), along with efforts to identify those that may specifically target disease (Schrama et al., 2006; Kurosawa et al., 2008).
For systematic generation of antibody-based affinity reagents, high-throughput in vitro technologies (Marks et al., 1991; Kieke et al., 1997; Daugherty et al., 1998; Sheets et al., 1998) offer several advantages over conventional immunization approaches, since they allow rapid identification of targeting antibodies and are not limited by the response of the immune system (Marks et al., 1991). To date, there have been several studies using phage display to perform proteome-wide generation of affinity reagents (Liu et al., 2002; Schofield et al., 2007). In these cases, proteins or protein fragments are immobilized on a matrix after being separated in a 2D gel (Liu et al., 2002) or after being heterologously expressed (Schofield et al., 2007). However, such an immobilization step can cause denaturation of proteins, and 2D gel separation can lead to aggregation of membrane proteins (Santoni et al., 2000), which are especially important targets in therapeutic applications. Moreover, several screening methods, applying phage (Marks et al., 1993) and yeast (Richman et al., 2006; Wang et al., 2007) display in whole-cell panning formats have been used for isolation of affinity reagents. Although these techniques have been successful, they have mostly focused on screening antibodies against cell surface exposed antigens. Therefore, for proteome-wide identification of antibodies targeted to membrane proteins and other subcellular compartments, methods capable of subcellular, antibody-based membrane proteomics would be desirable.
Previously, we have demonstrated that yeast displayed scFvs allow immunoprecipitation of target antigens from detergent-solubilized cell lysates (Cho et al., 2009). The so-called yeast display immunoprecipitation (YDIP) method allows facile antigen characterization and identification, as well as quantitative detection of scFv–antigen interactions in detergent-containing solutions by flow cytometry (Cho et al., 2009). These findings suggested the possibility of isolating antibodies from yeast display libraries using detergent-solubilized cell lysates directly as antigen sources. Importantly, such an approach using whole-cell lysates obviates the need for heterologous expression and/or purification of antigens and detergent use mitigates the insolubility issues inherent to membrane proteins. In addition, since screens would be performed in the presence of competing species, cross-specificity and non-specificity can be easily assessed and avoided. Here, we demonstrate that a library of non-immune human scFvs (Feldhaus et al., 2003) can be efficiently screened using fluorescence-activated cell sorting (FACS) and biotinylated and detergent-solubilized whole-cell lysates from a mammalian cell line to yield target-specific antibodies. By manipulating biotinylation conditions, antibody screening could be performed against whole-cell proteins or focused towards plasma membrane proteins, illustrating the capability for subcellular antibody targeting. Moreover, additional specific selection criteria can be imposed during the screening process since antigens captured on the yeast cell surface can subsequently be assayed with other secondary agents. As an example, we exploited this unique advantage to demonstrate the capability to perform antibody screening against a predetermined target, namely transferrin receptor (TfR), using whole-cell lysates as the antigen source. Antibodies arising from all of these proof-of-concept screens are functional under physiological conditions when produced as soluble proteins and can be directly applied in YDIP, for characterization of specificity, antigen size and epitope location (intracellular/extracellular).
Materials and methods
Cells, media and plasmids
Saccharomyces cerevisiae strain EBY100 (Kieke et al., 1997) and YVH10 (Shusta et al., 1998) were used for surface display and secretion of scFvs, respectively. Surface display plasmid pCT201-D1.3 (VanAntwerp and Wittrup, 2000) was used for the display of anti-hen egg lysozyme D1.3 scFv. The non-immune human scFv library (Feldhaus et al., 2003) was a kind gift from Dr. K. Dane Wittrup at MIT. Yeast cells were grown in the SD-CAA medium (20.0 g/l dextrose, 6.7 g/l yeast nitrogen base, 5.0 g/l casamino acids, 10.19 g/l Na2HPO4·7H2O, 8.56 g/l NaH2PO4·H2O) at 30°C to reach an OD600nm of approximately 1.0 and induced in the same volume of SG-CAA medium (dextrose replaced by galactose in SD-CAA) for 16–18 h at 20°C for scFv display. The rat brain endothelial cell line (RBE4) was a kind gift from Dr. Roux et al. (1994). RBE4 cells were grown at 37°C in 5% CO2, in 45% alpha minimum essential medium, 45% Ham's F10 medium and 10% heat inactivated fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) supplemented with 100 µg/ml streptomycin, 100 units/ml penicillin G (Invitrogen, Carlsbad, CA), 0.3 mg/ml geneticin (Fisher Scientific, Pittsburgh, PA) and 1 µg/l basic fibroblast growth factor (Roche Diagnostics, Indianapolis, IN).
Biotinylation and lysis of RBE4 cells
For biotinylation and generation of whole-cell lysates, approximately 5 × 106 RBE4 cells were washed in 10 mM phosphate-buffered saline (PBS, pH 7.4) supplemented with 1 mM CaCl2, 0.5 mM Mg2SO4 (PBSCM) and incubated with 0.5 mg/ml sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) in PBSCM for 30 min with rocking at room temperature. After the incubation, the biotinylation solution was removed and cells were lysed by scraping the cells into 1 ml of PBS supplemented with a protease inhibitor cocktail (Calbiochem, Gibbstown, NJ), 2 mM EDTA and containing one of the following detergents: 1% (w/v) Triton X-100 (TX) (Sigma, St. Louis, MO), 1% (w/v) n-octyl-β-d-glucopyranoside (OG) (Anatrace, Maumee, OH) and 0.5% (w/v) CHAPS (Fisher Scientific). Since residual biotinylation reagents were not been quenched, intracellular proteins were biotinylated after detergent lysis. The cell lysates were then centrifuged at 18 000 × g for 15 min at 4°C to remove insoluble debris. After incubating the cleared lysate for 1 h at 4°C, 1 M Tris at pH 7.6 was added to a final concentration of 10 mM to quench the reaction prior to screening so as to prevent yeast cell biotinylation.
For biotinylation of plasma membrane proteins, the above procedure was followed except that 0.5 mg/ml sulfo-NHS-LC-Biotin in PBSCM is incubated for 30 min with rocking at 4°C to prevent internalization of reagent (Bayer and Wilchek, 1990). After the biotinylation, cells were washed three times with PBS containing 100 mM glycine (Fisher Scientific) at 4°C to completely quench remaining NHS groups. Lysis and centrifugation were then identical to that described for whole-cell biotinylation. The total protein concentration remaining in the supernatant was determined using the BCA assay per manufacturer's instructions (Pierce, Rockford, IL).
Screening of scFv library in cell lysates using FACS
Screening against whole-cell lysates or plasma membrane proteins
A 5 × 107 subset of the human non-immune scFv library was used for screening. All of the following washing and labeling steps were performed at 4°C. For the first round of scFv screening against cell lysates (either whole cell or plasma membrane biotinylated), 108 yeast cells were incubated overnight with 1 ml (approximately 2 mg/ml total lysate protein) of freshly prepared biotinylated detergent-solubilized cell lysates supplemented with 1 mM biotin (Fisher Scientific). Excess free biotin was added to avoid isolation of biotin-binding scFvs. For subsequent rounds, the number of yeast cells and the volume of lysates were scaled accordingly. The incubated yeast cells were washed two times with the corresponding detergent solution and once with PBS containing 0.1% (w/v) bovine serum albumin (PBS-BSA). The secondary detection reagents were alternated to avoid the isolation of scFvs against these molecules (Chao et al., 2006). For the first and third rounds, a mouse anti-c-myc antibody (9E10, 30 µg/ml, Covance, Berkeley, CA) followed by goat anti-mouse IgG-Alexa488 conjugate (αM488, 1:500 dilution, Invitrogen, Carlsbad, CA) was used to detect the full-length scFv expression. Streptavidin-phycoerythrin conjugate (SA-PE, 1:80 dilution, Sigma) was used to detect the scFv–antigen binding. In the second round, a polyclonal rabbit anti-c-myc antibody (1:100 dilution, Fisher Scientific) and goat anti-rabbit IgG-allophycocyanin conjugate (αRAPC, 1:1000 dilution, Invitrogen, Carlsbad, CA) was used to monitor the expression. For scFv–antigen detection in the second round, an anti-biotin monoclonal antibody (1 µg/ml, clone BTN.4, Labvision, Fremont, CA) followed by αM488 was used. Yeast cells that show both scFv expression and antigen binding were isolated using Becton Dickinson FACSVantage SE flow cytometric sorter (University of Wisconsin Comprehensive Cancer Center). Recovered yeast cells were grown in SD-CAA pH 4.0 (0.1 M sodium citrate, 0.1 g/L kanamycin) for two passages to avoid bacterial growth.
Isolated scFv sequences were analyzed according to previous methods (Wang et al., 2007). Briefly, the scFv gene was PCR amplified from yeast colonies on SD-CAA plates using primers PNL6 forward (5′-GTACGAGCTAAAAGTACAGTG-3′) and PNL6 reverse (5′-TAGATACCCATACGACGTTC-3′). Amplified DNA was analyzed either by restriction digest with BstNI (New England Biolabs, Ipswich, MA) or sequencing (University of Wisconsin-Madison Biotech Center). To verify individual clones, each plasmid was recovered from yeast using a miniprep kit (Zymed, Carlsbad, CA) and re-transformed into the parent yeast display strain.
Targeted antibody screening: identification of TfR-associated scFv
To demonstrate the capability of targeted antibody screens, a TfR-associated scFv was identified. The second round pools of plasma membrane biotinylation screens in TX and OG lysates were subjected to additional three rounds of screening for TfR binding. The membrane binding pools were first incubated with freshly prepared plasma membrane biotinylated cell lysates overnight at 4°C. After incubation, yeast cells were washed three times with corresponding lysis buffer and incubated with a monoclonal anti-TfR antibody (1:100 dilution, Zymed, clone H68.4, Carlsbad, CA) for 1 h at 4°C. The cells were washed and subsequently incubated with SA-PE and αM488. Yeast cells that show both TfR binding and cell lysate binding were isolated using Becton Dickinson FACSVantage SE flow cytometric sorter (University of Wisconsin Comprehensive Cancer Center).
To verify the TfR binding of the isolated antibody clone, the antigen that was captured on yeast cells was probed with a mouse monoclonal anti-TfR antibody (1:100 dilution, clone OX26, Serotec) that is different from the antibody that was used in screening. To further assess TfR association, YDIP was performed as described below. Eluted antigen was probed with the anti-biotin antibody (0.5 µg/ml, clone BTN.4, Labvision) or the anti-TfR antibody (1:500 dilution, OX26).
Identification of extracellular epitope-binding scFvs
To identify scFvs that bind extracellular epitope, plasma membrane proteins of RBE4 cells were biotinylated and digested using trypsin. After plasma membrane biotinylation, RBE4 cells were incubated with 1 µg/ml of trypsin (sequencing grade, Promega, Madison, WI) in PBS at 4°C for 15 min. followed by 10 min at 37°C. The digestion reaction was stopped by adding protease inhibitor cocktail (Sigma) and 10 mM EDTA. 0.1% BSA was also added to prevent the loss of digested fragments. The digested fragments were incubated with the isolated yeast clones for 2 h at 4°C to determine the binding of extracellular protein fragment with the isolated scFvs. As above, labeled yeast cells were sequentially incubated with a mouse anti-c-myc antibody followed by αM488 mixed with streptavidin-phycoerythrin conjugate. The fluorescence intensities were quantified using the FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Immunolabeling using purified scFvs
The isolated scFv sequences were subcloned into secretion vector pRS316-GAL4-4-20 (Hackel et al., 2006) as described previously (Wang et al., 2007). In brief, the scFv ORFs were amplified from the display vector using PNL6 primers and were subcloned into the secretion vector as an NheI-HindIII fragment using standard techniques. Yeast cells transformed with scFv secretion vector were grown in 200 ml batches of minimal SD medium (2% dextrose, 0.67% yeast nitrogen base) supplemented with 2X SCAA amino acid (190 mg/l Arg, 108 mg/l Met, 52 mg/l Tyr, 290 mg/l Ile, 440 mg/l Lys, 200 mg/l Phe, 1260 mg/l Glu, 400 mg/l Asp, 480 mg/l Val, 220 mg/l Thr, 130 mg/l Gly, 20 mg/l tryptophan lacking leucine and uracil) at 30°C for 72 h. Subsequently, scFv secretion was induced at 20°C for 72 h in SG-SCAA (dextrose substituted by galactose) with 0.1% (w/v) BSA. The secreted scFv residing in the supernatant was purified using Ni-NTA columns (Qiagen) as described previously (Hackel et al., 2006). Protein purity was determined by SDS-PAGE and concentration was measured using BCA assay.
Immunocytochemistry of RBE4 cells was performed as described previously (Wang et al., 2007) with minor modifications. Purified scFvs were pre-dimerized with anti-c-myc antibodies (9E10) by incubating at a molar ratio of 2:1 (scFv:antibody) in PBSCM with 1% BSA for 1 h at 4°C. The concentrations of 9E10 antibody and scFv were ∼70 and 140 nM, respectively. To label the permeabilized RBE4 cells, cells were first fixed with 4% paraformaldehyde (Fisher Scientific) for 10 min on ice and permeabilized with 0.1% TX in PBS for 10 min. For unpermeabilized cells, labeling was done without cell fixation. The pre-dimerized scFv were incubated with RBE4 cells for 1 h at 4°C followed by a goat anti-mouse IgG-Alexa555 conjugate (αM555, 1:500 dilution, Invitrogen). Labeled cells were post-fixed with 4% paraformaldehyde and examined with a fluorescence microscope (Olympus IX70) or scraped off the plate in PBS-BSA for flow cytometry.
Affinity measurement using purified scFvs
To determine the affinity of selected scFvs, RBE4 cells were labeled with varying concentrations of purified scFvs and the binding signal was quantified using flow cytometry. RBE4 cells grown on a 24-well plate were fixed, permeabilized (TX) and then incubated with purified scFvs at concentrations ranging between 10 nM to 2 µM. Incubated cells were washed and labeled with ∼100 nM of 9E10 epitope tag antibody for 30 min at 4°C, followed by a goat anti-mouse IgG-Alexa555 conjugate (αM555, 1:500 dilution) for 1 h at 4°C. Labeled cells were scraped off from the plate in PBSCM with 0.1% BSA and examined using the FACSCalibur flow cytometer. The monomeric dissociation constant (Kd) of each clone was determined by fitting the titration curve with a bimolecular equilibrium binding model.
YDIP of antigens
YDIP of antigens were performed with yeast cells displaying isolated scFv clones as previously described (Cho et al., 2009). Briefly, 108 yeast cells were incubated with 500 µl of biotinylated cell lysate made under the same detergent condition in which the scFv had been screened for 2 h at 4°C. After the incubation, yeast cells were washed three times in 1 ml of the corresponding detergent solution at 4°C. Antigens were eluted by resuspending yeast cells in 30 µl of 0.2 M glycine–HCl solution (pH 2.0) for 10 min. Eluted antigens were separated in either a non-reducing or reducing SDS-PAGE (8% separating gel), and blotted onto a nitrocellulose membrane (BioRad, Hercules, CA). Western blotting was performed with an anti-biotin monoclonal antibody (0.5 µg/ml, clone BTN.4, Labvision).
Results and discussion
Antibody screening against mammalian whole-cell lysates
The antibody library screening process is shown schematically in Fig. 1. The target cellular proteins of interest are biotinylated to enable the detection of scFv–antigen interaction. The biotinylation step can be controlled either to biotinylate all cellular proteins or to selectively biotinylate plasma membrane proteins (Fig. 1, Step 1). To biotinylate all proteins, the cells are lysed in the presence of biotinylation reagent. In contrast, for plasma membrane focused screens, a membrane-impermeable biotinylation reagent is used before cell lysis to selectively biotinylate plasma membrane proteins. This approach enables subcellular screening (e.g. plasma membrane-binding antibodies) of antibodies that would be rare in the context of whole-cell screens due to the relatively low expression level of membrane proteins. Next, detergents are used to disrupt and solubilize the target cell membranes, releasing cytosolic proteins as well as solubilized membrane proteins to create cell lysates. The solubilized cellular proteins residing in such cell lysates are then used as sources of antigens to screen an antibody library (Fig. 1, Step 2). Therefore, antibodies against a target cell type of interest can be identified without purification or heterologous expression of antigens. In addition, since antibodies are screened directly from a background consisting of all cellular proteins, antibodies isolated using this method can be specifically mined to identify those that have low or no cross-reactivity towards other non-target cellular proteins. In the final step, yeast cells displaying antibody clones that bind to biotinylated cell lysate proteins are then screened using FACS (Fig. 1, Step 3).
Fig. 1.
Overview of the antibody screening method. In Step (1), target cell proteins are biotinylated and the cells are lysed using detergents. In this process, the biotinylation conditions can be modified to biotinylate either whole-cell lysate or plasma membrane proteins. In Step 2, a yeast display scFv library is combined with the biotinylated cell lysate from (1) to allow scFv-target protein combinations to form. In Step 3, yeast cells with scFvs that interact with a biotinylated protein from cell lysate are screened using FACS. SA-PE is indicated as the means to detect the scFv–antigen interaction. For scFv screening against a predetermined target such as TfR, a monoclonal anti-TfR antibody indicated as ‘known antibody’ can be used to distinguish only those scFvs that bind to the desired target.
To maximize the diversity of isolated antibodies, it is important to use a variety of detergents that have different solubilization characteristics. In this study, we have used TX, OG and CHAPS that are known to have different solubilization properties. TX is known to have high solubility for proteins (Banerjee et al., 1995) but cannot solubilize lipid rafts (Kurzchalia et al., 1995), unlike OG, which efficiently solubilize lipid rafts (Garner et al., 2008). CHAPS is a zwitterionic detergent, which tends to prevent non-specific aggregation of proteins better than non-ionic detergents (Hjelmeland, 1980) and has been known to solubilize certain membrane protein receptors better than other detergents (Stephenson and Olsen, 1982). All three of these detergents are also non-denaturing, a property that will help preserve high fidelity scFv–antigen interactions in the absence of detergent.
Thus, as a proof-of-concept demonstration of the entire lysate-based screening procedure, we used a 5 × 107 clone subset of a previously described human naïve scFv library (Feldhaus et al., 2003) combined with three rounds of FACS screening against lysates generated from an RBE4 cell line. Although the original library diversity was 5 × 108, we used a fraction of the available clones such that each screening round was amenable to flow cytometry, thus allowing exploration of each of the different screening modalities described above, each in multiple detergents. If desired, established magnetic bead enrichment protocols (Yeung and Wittrup, 2002) could be performed in the first round of screening followed by subsequent rounds of screening using flow cytometry in order to search larger antibody repertoires.
When the scFv library was screened using whole-cell biotinylated lysates, binding antibodies were enriched in two to three rounds of FACS using any of the three detergents (Fig. 2A). For all isolated pools, antibody binding was mediated by selective interactions with cell lysate proteins since no reactivity against secondary reagents or biotin was detected due to the alternative use of detection reagents applied in each round of screening and the addition of excess biotin (1 mM) in lysates (see Materials and Methods). Notably, pools isolated from TX and OG lysates yielded highly enriched binding populations in two rounds, while three rounds were required for CHAPS lysate, which might result from varying effects of each detergent on scFv–antigen interactions and/or protein solubility in the respective detergents. Non-exhaustive sampling of a combined 40 scFv sequences from the three detergent screening pools revealed 11 unique scFv sequences that when analyzed on a single clone basis by FACS showed binding activity to biotinylated cell lysates (Fig. 2C, Table I, whole-cell binding). As suggested by the results of the whole pool analyses, none of the tested clones showed binding activity for secondary detection reagents (anti-biotin antibody or SA-PE) nor did they bind irrelevant biotinylated proteins (biotinylated BSA or hen egg lysozyme) (data not shown), indicating selective interactions with cell lysate components. Of these 11 clones, 6 originated from the TX screen, 3 were from the OG screen and 2 were from the CHAPS screen. The antibody germline gene family usage distribution for the isolated scFv clones was similar to a previous report that screened the same library under physiological conditions using soluble antigens with VH6 and VκIII/ Vλ2 being most frequent (Feldhaus et al., 2003) (Table I), indicating there is no particular bias in antibody stability or binding in the presence of detergents. Complementarity determining region analysis of the 11 scFvs did not reveal significant homology, indicating reasonable diversity of isolated antibodies given the non-exhaustive sampling of the isolated pools and pilot library size of 5 × 107 scFv clones. Moreover, the results suggested that it is beneficial to perform the screening in various detergents to maximize the diversity of isolated antibodies, and likely, target diversity as well. Finally, the sequence diversity of isolated scFvs (11 unique sequences out of 40) compared favorably with previous antibody-endothelial cell screening studies that used biopanning with phage display (Mutuberria et al., 2004) (17 unique sequences out of 132) or yeast display (Wang et al., 2007) (34 of 2000).
Fig. 2.
Screening of scFvs against (A) biotinylated whole-cell RBE4 lysates or (B) selectively biotinylated plasma membrane proteins residing in RBE4 cell lysates by FACS. Flow cytometric density plots illustrating scFv clone enrichment are shown after each indicated screening round with gates used for quantitative population analysis drawn in. Yeast cells that show both scFv expression (x-axis, detected using an anti-c-myc antibody) and lysate binding (y-axis, detected using SA-PE) were collected in each round. Density plots from TX screens are shown. The percentages of antibody-displaying yeast cells that bind to biotinylated lysate proteins are noted in each density plot for each detergent tested. (C) Assessment of cell lysate binding of individual clones by flow cytometry. Representative density plots of individual clones identified from either whole-cell screening (2T5, 2O1) or plasma membrane focused screening (3mO11) are shown. An anti-hen egg lysozyme scFv D1.3 was used as a negative control. The quantitative binding data for all individual clones are quantified in Table I.
Table I.
Isolated scFv clones and their properties
| Detergenta | Clonesb | Human germline familyc |
Number of hitsd | Whole-cell bindinge | Plasma membrane bindingf | Extracellular bindingg | Soluble scFv production (mg/l) | Soluble scFv bindingh (flow) P, UP | Soluble scFv bindingi (microscopy) P, UP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Triton X-100 | scFvA | VH3 | Vλ1 | – | – | 35.6 ± 2.2 | 14.1 ± 1.2 | – | – | – |
| scFvJj | VH3 | – | – | – | 27.1 ± 2.5 | 17.6 ± 1.3 | – | – | – | |
| D1.3 (NC) | – | – | – | 5.3 ± 0.2 | 5.1 ± 0.3 | 4.5 ± 0.2 | – | – | – | |
| 4420 (NC) | – | – | – | – | – | – | 2.0 | 18.8, 2.7 | ND, ND | |
| 2T1j | – | Vλ1 | 1 | 26.3 ± 3.4 | ND | ND | – | – | – | |
| 2T5 | VH6 | Vλ2 | 1 | 16.8 ± 3.0 | ND | ND | 3.4 | 75.2, ND | ++, ND | |
| 2T6 | VH6 | VκIII | 3 | 28.6 ± 9.2 | ND | ND | <0.2 | – | – | |
| 2T7 | VH6 | VκIII | 1 | 17.7 ± 1.5 | ND | ND | 0.9 | 32.0, ND | ND, ND | |
| 2T8 | VH6 | Vλ2 | 6 | 11.9 ± 0.5 | ND | ND | 3.1 | ND, ND | ND, ND | |
| 2T16 | VH6 | Vλ2 | 1 | 26.5 ± 3.8 | ND | ND | 4.8 | ND, ND | ND, ND | |
| 3mT23 | VH6 | Vλ1 | 2 | – | 137.5 ± 6.2 | 184.4 ± 4.3 | 2.7 | 53.1, 5.4 | ++, + | |
| 3mT25 | VH1 | Vλ6 | 1 | – | 34.7 ± 3.0 | ND | 1.9 | 36.2, ND | +, ND | |
| Octyl-glucoside | 2O1 | VH1 | Vλ2 | 14 | 30.6 ± 3.8 | 23.3 ± 4.4 | 38.4 ± 0.8 | 1.2 | 47.0, ND | ++, ND |
| 2O7 | VH6 | VκIII | 1 | 36.3 ± 2.5 | ND | ND | <0.2 | – | – | |
| 2O9 | VH4 | Vλ2 | 1 | 31.5 ± 4.0 | ND | ND | 3.1 | ND, ND | ND, ND | |
| 3mO11 | VH3 | Vλ6 | 3 | – | 129.2 ± 6.1 | 147.1 ± 3.6 | 1.8 | 46.7, 6.9 | ++, + | |
| 3tO4j | VH4 | – | 8 | – | 25.9 ± 3.1 | ND | – | – | – | |
| CHAPS | 3C1 | VH3 | VκIII | 5 | 31.9 ± 2.7 | ND | ND | 1.9 | 34.8, ND | ND, ND |
| 3C3 | VH6 | Vλ2 | 3 | 66.1 ± 3.0 | ND | ND | 2.8 | ND, ND | ND, ND | |
–, not determined; ND, not detected.
aDetergent in which the scFvs have been screened from.
bName of clones. Clones names that have ‘m’ indicate that the scFv was isolated from plasma membrane-specific screening. Clone 3tO4 was screened for TfR binding.
cHuman germline family was classified according to IgBLAST classifications (http://www.ncbi.nlm.nih.gov/igblast).
dNumber of times each clone was identified in sequence analysis.
e,f,gWhole celle, plasma membranef, extracellular bindingg were assessed by yeast display of indicated scFv clone and measured using flow cytometry. The indicated values are in arbitrary units proportional to fluorescence. Standard deviations from three samples are also shown. Only data for those scFvs with binding having statistically significant differences from the negative control (NC) are listed. Those with no difference are so indicated (ND).
h,iP, permeabilized; UP, unpermeabilized. RBE4 cells were labeled with pre-dimerized soluble scFvs at saturating concentrations followed by a secondary antibody. hThe indicated values are in arbitrary units proportional to fluorescence and represent putative differences in antigen expression level. iPlus signs indicate qualitative staining intensities.
jSingle-domain scFv; NC, negative control.
Antibody screening focused on plasma membrane proteins
Since antibodies that bind plasma membrane proteins have various applications in targeted drug delivery and diagnostics, we next demonstrated lysate-based screening of antibodies targeting plasma membrane proteins. To screen antibodies that bind membrane proteins, two separate approaches were taken. First, antibody pools isolated after two (TX and OG) or three (CHAPS) rounds against whole-cell biotinylated lysates (Fig. 2A) were subjected to two additional rounds of FACS with selectively membrane-biotinylated cell lysates (see Materials and Methods for details). This allowed sub-fractionation of membrane protein-binding antibodies from amongst all binding antibodies. After two rounds of screening against membrane proteins, a membrane protein-binding population was only found in the OG lysate and not in TX and CHAPS lysates (data not shown). Moreover, even in the enriched pool against OG lysate, only one unique clone 2O1, which was also identified in the screen against whole-cell lysates (Table I, compare whole-cell binding to plasma membrane binding), was identified when 20 clones were sequenced. This could be a result of the fact that the concentration of intracellular protein is much higher than that of plasma membrane proteins (Santoni et al., 2000), biasing the screening towards more abundant intracellular proteins in the first few rounds of screening using whole-cell lysates. Alternatively, it could also be partially a result of the reduced library size (5 × 107) used in the screens.
Thus, rather than simply subfractionating for membrane protein-binding clones after 2 and 3 rounds of whole lysate selections, we employed a second approach where the initial unselected antibody pool was screened using membrane-biotinylated cell lysates for every round. We applied the initial pool of 5 × 107 scFvs to three rounds of screening using TX and OG cell lysates that were selectively biotinylated at the plasma membrane. After three rounds of screening, binding populations were successfully enriched in both detergents (Fig. 2B). Evaluating 10 clones from each detergent screen, TX clones 3mT23 and 3mT25, and OG clones 2O1 and 3mO11 were identified (Fig. 2C, Table I plasma membrane binding). As before, no secondary reagent or irrelevant biotinylated protein binding was detected for any of the clones tested (data not shown), suggesting specific antibody interaction with a lysate component (as further verified in the cellular immunofluorescence and YDIP sections below).
Through the isolation of plasma membrane protein-binding antibodies, we have demonstrated that the screening scheme is versatile and can be modified per application. In this example, we adjusted the biotinylation condition to narrow down the target antigen pool to plasma membrane proteins. In principle, the screening procedure could also be combined with various cell fractionation techniques (Stasyk and Huber, 2004; Yates et al., 2005; de Araujo et al., 2008) to identify antibodies that target proteins in specific cellular components and organelles such as cytoplasm, nuclei, mitochondria and microsomes. In addition, cells directly isolated from tissue could be used as antigen sources, which would allow antibody screenings against in vivo samples.
Targeted antibody screening: identification of TfR-associated scFv
As another example of adjusting the screening procedure to control the nature of scFvs isolated, it is possible to raise antibodies against a specific target residing in detergent-solubilized cell lysates. In the antibody screening process, antigens are captured on the yeast surface by scFvs (Fig. 1, Step 3). Therefore, it is possible to obtain more information regarding the captured antigen using interactions with other molecules as the basis for another set of screening criteria. As one example, scFvs that are associated with a specific target of interest can be identified by incubating antigen-captured yeast cells with a known antibody against the target. Such an approach allows the isolation of a panel of antibodies recognizing a given biomarker such as a tumor-specific antigen without purification or heterologous expression of the antigens, which is often difficult for membrane proteins (Grisshammer, 2006). This can be important in the development of antibody therapeutics since it has been shown that antibodies that bind to different epitopes of a single biomarker such as epidermal growth factor receptor 2 can give rise to highly divergent pharmacodynamics due to varying mechanism of action (Baselga and Swain, 2009).
To demonstrate this capability, we have identified an scFv capable of binding the TfR or its associated proteins. The TfR is a membrane protein present at the plasma membrane and known to be expressed in the RBE4 cells used here at a relatively high level of approximately 71 000 molecules per cell (Huwyler et al., 1999). The plasma membrane protein-binding pools isolated after two rounds of sorting in TX and OG lysates (Fig. 2B) were first incubated with plasma membrane biotinylated cell lysate to capture their antigens and subsequently incubated with a monoclonal anti-TfR antibody that recognizes the cytosolic tail of the TfR to selectively identify TfR-associated scFvs (Fig. 1, Step 3). Using this approach, scFvs that possess the dual characteristics of lysate binding and anti-TfR binding were enriched. After three additional rounds of sorting from the second round plasma membrane restricted pool, a single clone 3tO4 capable of pulling the TfR out of cell lysates was identified from the OG binding pool (Fig. 3A, Table I, plasma membrane binding). To further confirm the result, a second monoclonal antibody that was not used in the screening procedure was used to probe TfR captured by yeast cells displaying scFv 3tO4 (Fig. 3B). Both the anti-TfR antibody used in the screen and the additional anti-TfR used for the confirmation indicated the presence of captured TfR on the surface of yeast displaying scFv 3tO4. An irrelevant anti-hen egg lysozyme scFv D1.3 and scFv 3mO11, which binds to a non-TfR plasma membrane protein were used as negative controls to further verify the specificity of 3tO4 (data not shown). Thus, it is possible to isolate an scFv (3tO4) against a specific target of interest (TfR) by taking advantage of the yeast display format's capability to capture antigen. Since this particular scFv was not identified in the cursory sequencing of 10 clones as described in the previous section, the diversity of the plasma membrane screens was likely quite good. However, given only a single TfR-associated scFv clone was identified, scaling up to a full antibody library size and applying the anti-TfR screening criterion from the beginning as opposed to the second round from a generalized plasma membrane screening would likely expand the diversity.
Fig. 3.
Identification of TfR-associated scFv. (A) Screening results after each round of FACS. The second round plasma membrane binding pool shown in Fig. 2B was subject to an additional three rounds of screening using an anti-TfR antibody as a secondary screening criterion. X-axis indicates anti-TfR binding (anti-TfR clone H68.4 recognizing a cytosolic epitope (White et al., 1992)) and y-axis indicates cell lysate binding (SA-PE). Gates used for quantitative population analysis are drawn in. Single clones from the double positive population were analyzed after round 3. (B) Assessment of TfR association of scFv 3tO4. Biotinylated antigen was captured from TX solubilized lysates onto 3tO4 displaying yeast and probed with negative control antibody against rat glyceraldehyde 3-phosphate dehydrogenase (left panel), the H68.4 antibody used in the screening (center panel), or OX-26, another anti-TfR antibody recognizing a different epitope (extracellular) (Friden et al., 1991) (right panel). Note shift to the right of the lysate-binding population upon incubation with anti-TfR antibodies indicating the capture of TfR on the yeast surface.
Discriminating epitope localization for plasma membrane targeting scFvs
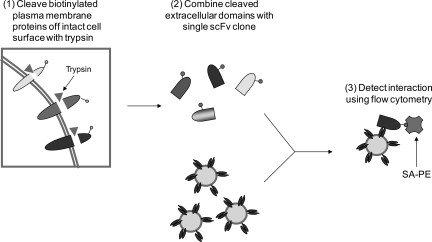
In principle, the isolated plasma membrane protein-binding antibodies can bind to any part of their target proteins including extracellular or intracellular epitopes. Although antibodies that bind to intracellular epitopes have value such as in the generation of intrabodies (Marasco et al., 1993) or cellular/tissue localization studies, antibodies that bind to extracellular epitopes can have additional utility as targeted therapeutics. Therefore, we developed an experimental scheme to rapidly identify which of the isolated plasma membrane antibodies targeted an extracellular epitope (Fig. 4). Here, the plasma membrane proteins are biotinylated and the extracellular portions of biotinylated proteins are subjected to trypsin digestion, releasing them into the solution (Fig. 4, Step 1). The trypsin digestion is quenched using protease inhibitors and the solution-phase extracellular fragments of plasma membrane proteins are used to assess whether or not the isolated scFvs recognize extracellular epitopes as defined by interaction with tryptic fragments (Fig. 4, Step 2).
Fig. 4.
Schematic of strategy for classifying scFvs capable of binding extracellular epitopes using tryptic fragments derived from intact cells. In Step 1, biotinylated plasma membrane proteins are digested with trypsin to release extracellular fragments. The cleaved fragments are incubated with an isolated scFv displaying yeast in Step 2. In Step 3, the interaction between extracellular fragment and scFv is quantified using a flow cytometer. If binding is retained using the tryptic fragments, an scFv is classified as binding to an extracellular epitope (Table I).
To first validate this approach, two scFvs previously selected for extracellular binding to RBE4 cells using a yeast biopanning method (Wang et al., 2007) (scFvA and scFvJ) were evaluated for their binding activity against tryptic plasma membrane fragments generated from RBE4 cells. Both scFvs bound to tryptic isolates at levels similar to that found with membrane-biotinylated cell lysates, while an irrelevant scFv did not show any binding (Table I, compare plasma membrane binding to extracellular binding). Among the four membrane protein-binding clones isolated in this study, 2O1, 3mO11 and 3mT23 showed binding activity against trypsin-digested fragments, indicating that they bind to extracellular epitopes (Table I), while no binding was detected for 3mT25. Since trypsin cleaves the peptide bond after lysine or arginine, it is possible that the antibody epitope becomes disrupted due to the digestion, thereby generating a false-negative reading in determining extracellular binding. In addition, it is possible that a conformational epitope is lost upon tryptic digest or the extracellular domain is inaccessible for enzymatic digestion as in multipass transmembrane proteins such as GPCRs. Therefore, negative results such as those for 3mT25 in this facile test do not preclude the possibility that an scFv binds an extracellular epitope. However, the power of the approach is that it can allow rapid mining of scFv pools directly on the surface of yeast for putative extracellular binding antibodies that warrant further exploration and validation as secreted scFv proteins. In addition, if desired more site-specific enzymes such as Lys-C or Asp-N could be used to reduce the epitope disruption, reducing the potential for false negatives. Yeast display compatibility with tryptic fragments also suggests the possibility for using protease-cleaved protein fragments directly in antibody screens focused on targeting extracellular domains of plasma membrane proteins.
Antibody activities under physiological conditions
Since the scFvs were screened in the presence of detergents, it is important to confirm that the isolated scFvs bind to their target under physiological conditions. Although the detergents used in the screens are known to be non-denaturing (Hjelmeland and Chrambach, 1984), it may still be possible that the detergent could affect the antibody or antigen conformation so that the interaction is abolished in the absence of detergent. Moreover, although it is known that antibody–antigen interaction is preserved in non-denaturing detergent solutions (Cho et al., 2009; Dimitriadis, 1979), the reverse question of whether antibodies isolated in detergent solutions would retain their binding activity in the absence of detergents has not been well studied. Therefore, we produced all of the full-length scFvs (excluding those that are single-domain, represented by ‘j’ in Table I) as soluble proteins and tested their capacity to bind to the target cell line in the absence of detergents.
Of the 13 scFvs, 11 were secreted at significant levels of 0.7–3 mg/l while 2 were below 0.2 mg/l (Table I). These expression levels are commensurate with what is expected from antibody libraries in general and this library in particular (Miller et al., 2005). Binding activity was assessed by immunolabeling RBE4 cells under physiological conditions followed by flow cytometry or fluorescent microscopy (Table I and Fig. 5). Significant binding was detected for 7 of the 11 well-secreted scFvs using flow cytometry and/or fluorescence microscopy (Table I and Fig. 5). This frequency was expected given that not all surface displayed scFvs retain their binding activity when produced as a soluble protein (Miller et al., 2005) and it may be possible that a small subset of scFvs are only active in the presence of detergents. To further characterize the binding of scFvs, the binding affinity was determined for three of the plasma membrane-binding clones 2O1, 3mO11 and 3mT23 by titrating soluble scFvs and quantifying the RBE4 cell binding using flow cytometry. The dissociation constants (Kd) were 34.8 ± 2.8 nM for 3mT23, 181 ± 28 nM for 3mO11 and 1200 ± 204 nM for 2O1 (Supplementary Fig. S1), which are within the expected range given results using the same library against purified soluble proteins (Feldhaus et al., 2003) and against the same target cells using a biopanning approach (Wang et al., 2007). When the labeling patterns of scFvs were analyzed under fluorescent microscope, distinct localization patterns such as nuclear (2T5) or punctate cell surface (2O1) were found. Importantly, scFvs 3mO11 and 3mT23 labeled unpermeabilized cells while 3mT25 labels only permeabilized cells (Fig. 5), corroborating the extracellular binding results using trypsin fragments (Table I). However, for scFv 2O1 we detected immunolabeling only in permeabilized cells (Fig. 5) while extracellular trypsin fragment labeling was detected using flow cytometry. For an scFv like 2O1 that binds small amounts of protein in discrete structures (Fig. 5), this discrepancy could be explained by the fact that in flow cytometry analysis, yeast cells that display scFv at relatively high levels (approximately 50 000 per yeast cell) are used to concentrate and detect antigens that may be expressed at undetectable levels at the extracellular surface of the target cell using conventional immunochemistry approaches with purified scFv.
Fig. 5.
Immunolabeling of RBE4 cells with purified scFvs. Immunofluorescence patterns generated by scFvs 2T5, 2O1, 3mO11 are shown as examples. ScFv labeling is indicated in green along with the corresponding DAPI nuclear staining in red. P denotes permeabilized cells and UP denotes unpermeabilized cells. An anti-fluorescein scFv was used as a negative control.
Characterization of target antigens by YDIP
Since the distinct localization patterns of scFvs suggest differences in their target antigens, the target antigens were characterized using YDIP (Cho et al., 2009). In YDIP, yeast cells displaying a given scFv clone are used as affinity reagents to immunoprecipitate the target antigen from cell lysates. For the screens described above (whole cell, plasma membrane and TfR focused), the entire scFv screening process essentially follows the YDIP process, except that an antibody library instead of a single scFv clone is used to immunoprecipitate the target antigen. Therefore, once isolated, the antibody clones can be directly applied in YDIP format without further optimization of target antigen solubilization or scFv–antigen interaction conditions.
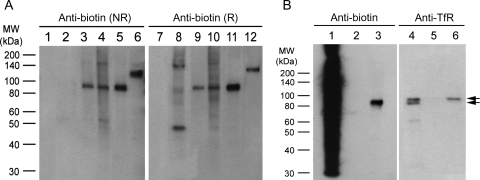
YDIP antigen characterization efforts were largely focused on clones identified from plasma membrane and TfR screens, since these clones were of particular interest in terms of their applicability, and also showed strong immunolabeling (Fig. 5). Thus, using YDIP, antigens for clones 2T6, 3mT23, 3mT25, 2O1, 3mO11 and 3tO4 were immunoprecipitated and subsequently detected by anti-biotin western blotting (Fig. 6). For each of these scFv clones, biotinylated proteins were immunoprecipitated from cell lysate. As an indication of the specificity that can be realized for isolated scFvs, single antigen bands were immunoprecipitated for 2O1, 3mT23 and 3mT25 from a complex cell lysate. Although the bands from 2O1, 3mO11 and 3mT23 have similar molecular weights (Fig. 6A), bands from 2O1 and 3mO11 could be detected only after YDIP from OG lysate, while the band from 3mT23 was detected in both TX and OG lysates (Fig. 6A and data not shown). This suggests that clone 3mT23 has a different antigen from clones 2O1 and 3mO11, which themselves have differing antigen localization patterns in cellular immunofluorescence assays (Fig. 5). For scFv 2T6, the antigen band could be only seen under reducing conditions (Fig. 6A), indicating that the antigen or antigen complex has a large molecular weight.
Fig. 6.
YDIP coupled with western blotting for antigen characterization. (A) Target antigens of scFvs from both whole-cell and plasma membrane screens were immunoprecipitated from biotinylated whole-cell RBE4 lysate and probed with a monoclonal anti-biotin antibody. Lanes 1–6 are under non-reducing (NR) conditions and lanes 7–12 are under reducing (R) conditions. scFvs used for YDIP were 2T6 (lanes 2 and 8), 2O1 (lanes 3 and 9), 3mO11 (lanes 4 and 10), 3mT23 (lanes 5 and 11) and 3mT25 (lanes 6 and 12). An anti-hen egg lysozyme scFv was used as a negative control (lanes 1 and 7). (B) The target antigen of 3tO4 was purified from plasma membrane biotinylated RBE4 cell lysate and probed with anti-biotin (lanes 1–3) or anti-TfR (lanes 4–6) antibodies. Lanes 1 and 4: raw plasma membrane biotinylated RBE4 cell lysate, lanes 2 and 5: YDIP from negative control scFv, lanes 3 and 6: YDIP from 3tO4. Top arrow indicates TfR isoform immunoprecipitated by 3tO4, bottom arrow indicates the size of the biotinylated protein pulled down by 3tO4. The lower band in lane 4 is an isoform recognized by multiple anti-TfR antibodies, but it is not pulled down by 3tO4 (not found in lane 6) and hence is not the biotinylated band seen in lane 3.
In addition, we were able to further confirm the association of 3tO4 and TfR using YDIP. The immunoprecipitated antigen of 3tO4 was isolated and probed with both anti-biotin and anti-TfR antibodies (Fig. 6B). TfR was indeed immunoprecipitated (Fig. 6B, lane 6), although a single, slower migrating band was present on the anti-biotin western blot (Fig. 6B, lane 3). This result suggests that 3tO4 either directly binds to non-biotinylated TfR that is in turn associated with another plasma membrane protein that is biotinylated (via a protein complex) or that 3tO4 binds to the associated protein that in turn pulls down the non-biotinylated TfR. Interestingly, TfR is biotinylated using the protocols employed here (Wang et al., 2007), so 3tO4 is selective for the non-biotinylated isoform. Since protein–protein complexes are preserved under the detergent conditions used here and many antigens function in protein complexes, targeted TfR-type screening schemes do not guarantee that every isolated antibody interacts directly with the specific target. We therefore anticipate that in addition to raising antibodies recognizing a specific antigen, concomitant raising of antibodies against the target protein complex could find broad application. For example, antibodies against components of protein complexes have been used to elucidate the function of each component in tumor cell migration (Sugiura and Berditchevski, 1999) or to block a complex cellular process such as T-cell mediated cytolysis (Sarmiento et al., 1980). Moreover, as demonstrated with 3tO4, by using YDIP and western blotting, one can rapidly mine through the antibody clones isolated against a specific target complex to identify those with the desired attributes whether it be binding to the complex or directly to the antigen of interest.
Conclusions
Here we have developed an antibody screening method that allows identification of antibodies and cognate antigen pairs in the context of the cellular environment by combining detergent solubilized cell lysates and a yeast display human scFv library. Since scFvs displayed on yeast cells are generally active in various detergent solutions commonly used in mammalian cell lysis (Cho et al., 2009), and yeast cells themselves are stable in various non-denaturing detergents (Navarrete and Serrano, 1983), yeast display provides a robust platform for antibody screening in detergent solutions. We have demonstrated that specific scFvs functional under physiological conditions can be isolated against diverse target proteins including plasma membrane proteins. One of the major advantages of this approach is that the target cell can be directly used as the antigen source, eliminating the need for antigen purification or heterologous expression, which can be particularly difficult for membrane proteins. In addition, since antibodies are screened from a background that includes all cellular proteins, antibodies isolated using this method are expected to have minimal cross-reactivity towards other cellular proteins and can be easily assayed for said cross-reactivity (as exemplified in Fig. 6).
Another main advantage of this antibody screening strategy is the direct study of the cognate antigens using a streamlined YDIP process. As demonstrated above, one can readily determine the molecular weight of antigens for isolated scFvs by western blotting. It is also possible to identify the amino acid sequence of the target antigens by applying YDIP in combination with tandem mass spectrometry as demonstrated previously (Cho et al., 2009). Therefore, this method enables the discovery and detailed analysis of target protein–antibody combinations using the cell proteome directly and this versatile tool could have wide application in the generation of affinity reagents.
Supplementary data
Funding
This work was supported by National Institutes of Health [NS052649 and EY018506]. Y.K.C. is a recipient of a Genomic Sciences Training Program Fellowship funded through the National Institutes of Health, 5T32HG002760.
Supplementary Material
Footnotes
Edited by James Marks
References
- Banerjee P., Joo J.B., Buse J.T., Dawson G. Chem. Phys. Lipids. 1995;77:65–78. doi: 10.1016/0009-3084(95)02455-r. doi:10.1016/0009-3084(95)02455-R. [DOI] [PubMed] [Google Scholar]
- Baselga J., Swain S.M. Nat. Rev. Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. doi:10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- Bayer E.A., Wilchek M. Methods Enzymol. 1990;184:138–160. doi: 10.1016/0076-6879(90)84268-l. doi:10.1016/0076-6879(90)84268-L. [DOI] [PubMed] [Google Scholar]
- Chao G., Lau W.L., Hackel B.J., Sazinsky S.L., Lippow S.M., Wittrup K.D. Nat. Protoc. 2006;1:755–768. doi: 10.1038/nprot.2006.94. doi:10.1038/nprot.2006.94. [DOI] [PubMed] [Google Scholar]
- Cho Y.K., Chen I., Wei X., Li L., Shusta E.V. J. Immunol. Methods. 2009;341:117–126. doi: 10.1016/j.jim.2008.11.005. doi:10.1016/j.jim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty P.S., Chen G., Olsen M.J., Iverson B.L., Georgiou G. Protein Eng. 1998;11:825–832. doi: 10.1093/protein/11.9.825. doi:10.1093/protein/11.9.825. [DOI] [PubMed] [Google Scholar]
- de Araujo M.E., Huber L.A., Stasyk T. Methods Mol. Biol. 2008;424:317–331. doi: 10.1007/978-1-60327-064-9_25. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G.J. Anal. Biochem. 1979;98:445–451. doi: 10.1016/0003-2697(79)90165-9. doi:10.1016/0003-2697(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Feldhaus M.J., et al. Nat. Biotechnol. 2003;21:163–170. doi: 10.1038/nbt785. doi:10.1038/nbt785. [DOI] [PubMed] [Google Scholar]
- Friden P.M., Walus L.R., Musso G.F., Taylor M.A., Malfroy B., Starzyk R.M. Proc. Natl Acad. Sci. USA. 1991;88:4771–4775. doi: 10.1073/pnas.88.11.4771. doi:10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A.E., Smith D.A., Hooper N.M. Biophys. J. 2008;94:1326–1340. doi: 10.1529/biophysj.107.114108. doi:10.1529/biophysj.107.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisshammer R. Curr. Opin. Biotechnol. 2006;17:337–340. doi: 10.1016/j.copbio.2006.06.001. doi:10.1016/j.copbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Hackel B.J., Huang D., Bubolz J.C., Wang X.X., Shusta E.V. Pharm. Res. 2006;23:790–797. doi: 10.1007/s11095-006-9778-7. doi:10.1007/s11095-006-9778-7. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L.M. Proc. Natl Acad. Sci. USA. 1980;77:6368–6370. doi: 10.1073/pnas.77.11.6368. doi:10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L.M., Chrambach A. Methods Enzymol. 1984;104:305–318. doi: 10.1016/s0076-6879(84)04097-0. doi:10.1016/S0076-6879(84)04097-0. [DOI] [PubMed] [Google Scholar]
- Huwyler J., Froidevaux S., Roux F., Eberle A.N. J. Recept. Signal Transduct. Res. 1999;19:729–739. doi: 10.3109/10799899909036683. [DOI] [PubMed] [Google Scholar]
- Kieke M.C., Cho B.K., Boder E.T., Kranz D.M., Wittrup K.D. Protein Eng. 1997;10:1303–1310. doi: 10.1093/protein/10.11.1303. doi:10.1093/protein/10.11.1303. [DOI] [PubMed] [Google Scholar]
- Kurosawa G., et al. Proc. Natl Acad. Sci. USA. 2008;105:7287–7292. doi: 10.1073/pnas.0712202105. doi:10.1073/pnas.0712202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T.V., Hartmann E., Dupree P. Trends Cell Biol. 1995;5:187–189. doi: 10.1016/s0962-8924(00)88990-4. doi:10.1016/S0962-8924(00)88990-4. [DOI] [PubMed] [Google Scholar]
- Liu B., Huang L., Sihlbom C., Burlingame A., Marks J.D. J. Mol. Biol. 2002;315:1063–1073. doi: 10.1006/jmbi.2001.5276. doi:10.1006/jmbi.2001.5276. [DOI] [PubMed] [Google Scholar]
- Marasco W.A., Haseltine W.A., Chen S.Y. Proc. Natl Acad. Sci. USA. 1993;90:7889–7893. doi: 10.1073/pnas.90.16.7889. doi:10.1073/pnas.90.16.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J.D., Hoogenboom H.R., Bonnert T.P., McCafferty J., Griffiths A.D., Winter G. J. Mol. Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. doi:10.1016/0022-2836(91)90498-U. [DOI] [PubMed] [Google Scholar]
- Marks J.D., Ouwehand W.H., Bye J.M., Finnern R., Gorick B.D., Voak D., Thorpe S.J., Hughes-Jones N.C., Winter G. Biotechnology (N Y) 1993;11:1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- Miller K.D., Weaver-Feldhaus J., Gray S.A., Siegel R.W., Feldhaus M.J. Protein Expr. Purif. 2005;42:255–267. doi: 10.1016/j.pep.2005.04.015. doi:10.1016/j.pep.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Navarrete R., Serrano R. Biochim. Biophys. Acta. 1983;728:403–408. doi: 10.1016/0005-2736(83)90512-6. [DOI] [PubMed] [Google Scholar]
- Roux F., Durieu-Trautmann O., Chaverot N., Claire M., Mailly P., Bourre J.M., Strosberg A.D., Couraud P.O. J. Cell. Physiol. 1994;159:101–113. doi: 10.1002/jcp.1041590114. doi:10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Santoni V., Molloy M., Rabilloud T. Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. doi:10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A.L., Fitch F.W. J Immunol. 1980;125:2665–2672. doi:10.1038/nrd1957. [PubMed] [Google Scholar]
- Schofield D.J., et al. Genome Biol. 2007;8:R254. doi: 10.1186/gb-2007-8-11-r254. doi:10.1038/nrd1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrama D., Reisfeld R.A., Becker J.C. Nat. Rev. Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. doi:10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- Sheets M.D., et al. Proc. Natl Acad. Sci. USA. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. doi:10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusta E.V., Raines R.T., Pluckthun A., Wittrup K.D. Nat. Biotechnol. 1998;16:773–777. doi: 10.1038/nbt0898-773. doi:10.1038/nbt0898-773. [DOI] [PubMed] [Google Scholar]
- Stasyk T., Huber L.A. Proteomics. 2004;4:3704–3716. doi: 10.1002/pmic.200401048. doi:10.1002/pmic.200401048. [DOI] [PubMed] [Google Scholar]
- Stephenson F.A., Olsen R.W. J. Neurochem. 1982;39:1579–1586. doi: 10.1111/j.1471-4159.1982.tb07990.x. doi:10.1111/j.1471-4159.1982.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Berditchevski F. J. Cell Biol. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. doi:10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M.J., et al. Nat Methods. 2007;4:13–17. doi: 10.1038/nmeth1001. doi:10.1021/bp990133s. [DOI] [PubMed] [Google Scholar]
- VanAntwerp J.J., Wittrup K.D. Biotechnol. Prog. 2000;16:31–37. doi: 10.1021/bp990133s. doi:10.1021/bp990133s. [DOI] [PubMed] [Google Scholar]
- Wang X.X., Cho Y.K., Shusta E.V. Nat. Methods. 2007;4:143–145. doi: 10.1038/nmeth993. doi:10.1038/nmeth993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S., Miller K., Hopkins C., Trowbridge I.S. Biochim. Biophys. Acta. 1992;1136:28–34. doi: 10.1016/0167-4889(92)90081-l. [DOI] [PubMed] [Google Scholar]
- Yates J.R., III, Gilchrist A., Howell K.E., Bergeron J.J. Nat. Rev. Mol. Cell Biol. 2005;6:702–714. doi: 10.1038/nrm1711. [DOI] [PubMed] [Google Scholar]
- Yeung Y.A., Wittrup K.D. Biotechnol. Prog. 2002;18:212–220. doi: 10.1021/bp010186l. doi:10.1021/bp010186l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.