Abstract
Objective
Hyperinsulinemia, which often coexists with obesity and type 2 diabetes, is a major risk factor for cardiovascular disease and thought to promote hypertension through the sympathetic effects of insulin. Here, we examined the effect of insulin on regional sympathetic nerve activity (SNA) in obesity.
Methods
Glucose and insulin tolerance tests were performed to examine insulin sensitivity in agouti obese mice. We used also multifiber recording to compare the regional SNA response to ICV insulin between lean and agouti obese mice.
Results
Agouti obese mice have significantly elevated levels of blood glucose and plasma insulin associated with glucose intolerance and insulin resistance. In lean mice, ICV administration of insulin (20 and 100 μU) caused a dose-dependent increase in SNA subserving hindlimb, kidney and brown adipose tissue (BAT). Of note, the regional SNA responses to insulin were differentially altered in agouti obese mice. While lumbar SNA response to insulin was intact in the obese mice, renal and BAT sympathetic activation to insulin were significantly attenuated in these agouti obese mice. Finally, we assessed the role of phosphoinositol-3 kinase (PI3K) signaling pathway in mediating sympathetic activation to insulin in obesity. Notably, ICV pre-treatment with a PI3K inhibitor (LY294002) blocked the increase in lumbar SNA induced by ICV insulin in lean and agouti obese mice.
Conclusions
Our data suggest a differential regulation by insulin of sympathetic outflow to peripheral tissues in obesity. Our findings also demonstrate the importance of PI3K in lumbar sympathetic activation to insulin in obesity.
Keywords: Sympathetic nervous system, obesity, insulin resistance, PI3K
Introduction
Insulin receptors are widely distributed throughout the central nervous system and insulin action in the brain produces broad spectrum physiological effects including sympathetic nerve activation [1–3]. Cerebroventricular administration of insulin increases sympathetic nerve activity to several beds including the hindlimb, brown adipose tissue (BAT), kidney and adrenal gland [4,5]. Such regional sympathetic nerve activation to insulin is consistent with the involvement of brain action of insulin in the regulation of a various physiological processes including body weight control, glucose homeostasis and cardiovascular function. The demonstration that lesions placed in the anteroventral third ventricle abolished insulin-induced sympathetic activation suggests the key role of the hypothalamus in the sympathoexcitatory effect of insulin [6].
Contrasting intracellular mechanisms appears involved in the regional sympathetic nerve activation to insulin [5]. Indeed, the increase in sympathetic outflow to thermogenic BAT is mediated by mitogen activated protein kinase (MAPK) while the sympathoexcitatory effect of insulin to hindlimb is phosphoinositol 3 kinase (PI3K)-dependent. However, neither of these signaling pathways appears to be involved in insulin-induced sympathetic activation to the kidney and adrenal gland. The critical role of PI3K and MAPK in the regulation of sympathetic outflow by insulin is consistent with the key role of these 2 intracellular pathways in insulin receptor signaling [7].
Obesity, type 2 diabetes and hypertension are common disorders that frequently cluster together leading to an increased overall cardiovascular risk profile. Insulin resistance that is common in obesity and type 2 diabetes is strongly associated with sympathetic activation, hypertension and cardiovascular mortality [1,8,9]. The sympathetic effects of insulin and chronic hyperinsulinemia have been suggested to link insulin resistance and cardiovascular disorders [9,10].
Agouti yellow mice are a genetic model of obesity in which the agouti protein is expressed ectopically in multiple tissues [11]. Blockade of hypothalamic melanocortin receptors by agouti protein is thought to be the main mechanism for obesity is this mouse model [11,12]. Of note, the obesity observed in these mice is associated with hypertension. Indeed, the obese mice exhibit higher arterial pressure than the wild type littermate controls [13–15].
In the present study, we sought to determine whether sympathetic nerve activation to central neural action of insulin is intact in obesity. For this, we examined the effect of ICV administration of insulin on the regional SNA in agouti obese mice as compared to littermate controls. We also assessed the molecular mechanism that mediates the sympathetic nerve response to insulin in obesity by determining the role of PI3K.
Methods
Animals
Male 12–16 weeks old agouti obese (KKAY) and wild type littermate control (C57BL/6J) mice, obtained from the Jackson Laboratories (Bar Harbor, ME), were used. Mice were housed in a temperature- and light (12-h light-dark cycle)-controlled facility with free access to standard chow and water. ICV cannulation was performed at least one week before the studies as described previously (14). All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by The University of Iowa Animal Research Committee.
Glucose and insulin tolerance tests
Glucose tolerance test was performed in overnight fasted mice (n=10 and 9 lean and obese mice, respectively). Blood samples were taken from the tail in conscious mice to measure blood glucose and plasma insulin at baseline and then mice were injected intraperitoneally (IP) with glucose (D-glucose, anhydrous; Sigma–Aldrich, St. Louis, MO) (2 mg/g body weight). To test for the glucose reducing effect of insulin mice were fasted for 3 hours (n=7 and 8 lean and obese mice, respectively). After baseline glucose levels were measured mice were treated with insulin (IP, 1 U/kg BW; Novolin; Novo Nordisk). Blood glucose was measured 15, 30, 60 and 120 min after injection of glucose or insulin.
Blood glucose levels were determined by a glucometer (One Touch Ultra 1, LifeScan, Inc., Milpitas, CA). Plasma insulin was measured in lean and agouti obese mice (n=4 each) by radioimmunuassay using a commercially available kit (Crystal Chem Inc.).
Recording of sympathetic nerve activity
SNA to hindlimb, BAT and kidney was measured by multi-fiber recording. Using a dissecting microscope, the nerve subserving the hindlimb, BAT or left kidney was identified and carefully dissected free and placed on a bipolar 36-gauge platinum-iridium electrode (Cooner Wire Co., Chatsworth, CA). When optimum recording of SNA was obtained from each nerve, the electrode was covered with silicone gel (Kwik-Sil; World Precision Instruments Inc, Sarasota, FL).
Nerve electrodes were attached to a high impedance probe (HIP-511, Grass Instruments Co., Quincy, MA). The nerve signal was amplified 105 times with a Grass P5 AC pre-amplifier, filtered at a low and high frequency cutoff of 100 Hz and 1000 Hz respectively. The amplified, filtered nerve signal was directed to speaker system and to an oscilloscope (model 54501A, Hewlett-Packard Co., Palo Alto, CA) for auditory and visual monitoring of the nerve activity and finally to a resetting voltage integrator (model B600C, University of Iowa Bioengineering) that sums the total voltage output in units of 1V * sec before resetting to zero. The resetting voltage integrator and the amplified, filtered neurogram were continuously routed to a MacLab analogue-digital converter (model 8S, AD Instruments Castle Hill, New South Wales, Australia) for permanent recording and data analysis on a Macintosh computer. To ensure that background electrical noise was excluded in the assessment of sympathetic outflow in the integrated voltage, SNA was corrected for post-mortem background activity.
Effect of insulin on regional SNA
Each mouse was anesthetized with an IP injection of ketamine (91mg/kg) and xylazine (9.1 mg/kg) and instrumented for measurement of SNA as described above. Anesthesia was maintained with intravenous (IV) delivery of α-chloralose (initial dose; 25 mg/kg, sustaining dose; 6 mg/kg/h). In one cohort of lean mice (n=5), anesthesia was induced and maintained with intraperitoneal injection of pentobarbital (60 mg/kg). Mice were intubated and allowed to spontaneously breathe oxygen-enriched air throughout the experimental procedure. Body temperature was maintained constant at 37.5°C with a surgical heat lamp and a heating pad. SNA to the hindlimb, BAT or kidney was recorded from each mouse. Each mouse received one ICV injection of vehicle (2 μl) or insulin (20 or 100 μU). SNA was recorded for the next 4 hours after each treatment. The number of mice treated with vehicle, 20 or 100 μU of insulin, respectively, was as follow: Lumbar SNA (lean mice: 10, 10 and12; obese mice: 10, 10 and 12), BAT SNA (lean mice: 10, 9 and 10; obese mice: 10 each) and renal SNA (lean mice: 10, 12 and11; obese mice: 10, 10 and 12).
Effect of PI3K blockade on lumbar SNA response to insulin
Anesthetized mice were instrumented for direct multifiber recording of lumbar SNA as described above. After baseline recording of lumbar SNA was obtained, each mouse received 2 ICV injections: first, LY294002 (0.01 [n=7 each, lean and obese mice] or 0.1 μg [n=15 each, lean and obese mice]) or vehicle (DMSO; 2 μl [n=7 each, lean and obese mice]) followed 15 min later by insulin (100 μU, n=22 each, lean and obese mice) or vehicle (n=14 and 13 for lean and obese mice, respectively). In one cohort of lean mice (n=4), wortmannin (0.01 μg) was administered 15 min before ICV insulin (100 μU). After last ICV injection SNA, measurements were made every 15 min for 4 hours.
Statistical analysis
All results are expressed as means±SEM. The data for SNA are expressed as percentage change from baseline. Data were analyzed using Student’s t-test, one- or two-way analysis of variance (ANOVA) with or without repeated measure. When ANOVA reached significance, a post-hoc comparison was made using Tukey test. A value of P < 0.05 was considered to be statistically significant.
Results
Agouti obese mice are insulin resistant
Agouti mice were obese, weighing about 38% more than the littermate controls (Table 1). Fat mass was markedly increased in agouti obese mice as demonstrated by the significant increase in BAT, epididymal and peri-renal fat pads in agouti mice as compared to lean counterparts (Table 1).
Table 1.
Body weight and fat pads weight of lean controls and agouti obese mice.
| Parameter | Lean controls | Agouti obese |
|---|---|---|
| Body weight (g) | 28.2±0.2 | 39.1±0.4* |
| Brow Adipose Tissue (g) | 0.11±0.003 | 0.25±0.07* |
| Epididymal fat (g) | 0.53±0.02 | 1.57±0.03* |
| Peri-renal fat (g) | 0.16±0.01 | 0.52±0.02* |
Data are means±SEM. Abbreviation: BAT, brown adipose tissue.
P<0.05 vs. lean controls mice.
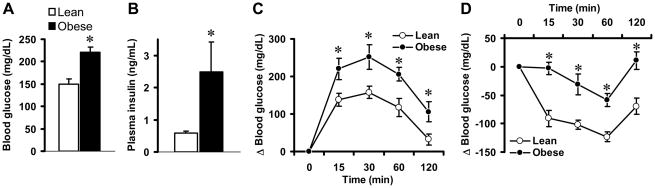
We tested whether the agouti obese mice are insulin resistant. Compared to lean controls, agouti obese mice have significantly higher levels of fasting blood glucose (Fig. 1A) and plasma insulin (Fig. 1B) indicating loss of insulin action in these agouti obese mice. To test this further, we performed glucose- and insulin-tolerance tests which revealed that agouti obese mice are glucose intolerant and insulin resistant. Following acute intraperitoneal glucose challenge (2 g/kg body weight), the plasma glucose levels in lean mice started to decline after peaking at 15–30 min. The plasma blood glucose levels returned to near baseline value after 120 min in these lean mice. In contrast, in obese mice the post-challenge plasma glucose levels continued to rise and peaked at 30 min (Fig. 1C) indicating that agouti obese mice had markedly impaired glucose excursion from blood.
Fig. 1.
Insulin resistance in agouti obese mice. (A–B) Comparison of blood glucose (A) and plasma insulin (B) between lean controls and agouti obese mice. (C–D) Glucose (C) and insulin (D) tolerance tests in lean controls and agouti obese mice. Data represent means±SEM; n=4–10 mice per group. There was a significant difference (P<0.05) between lean and obese mice in the effects of glucose and insulin on blood glucose. * P<0.05 vs. lean control mice.
The ability of insulin to stimulate glucose mobilization was also significantly blunted in obese mice (Fig. 1D). Indeed, in lean mice, insulin treatment caused a robust decrease in the blood glucose. This effect was attenuated in the agouti obese mice. Altogether, these results demonstrate that agouti obese mice are insulin resistant.
Regional sympathetic nerve responses to ICV insulin in obesity
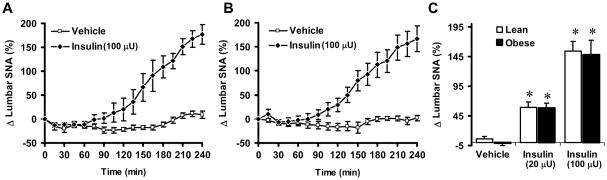
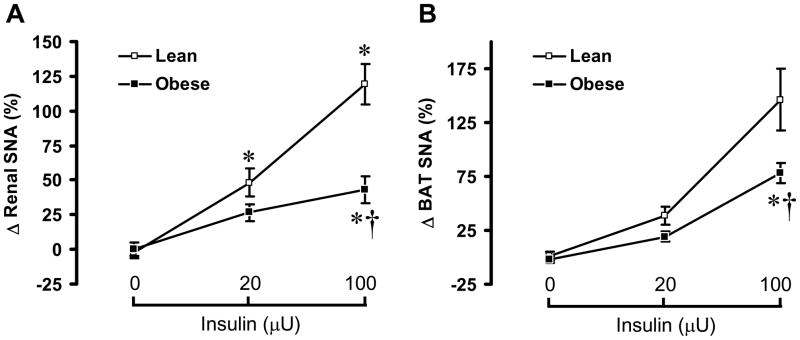
To examine the ability of insulin to increase the SNA, we compared the regional SNA response to insulin between agouti obese mice and lean controls. In α-chloralose anesthetized lean mice, ICV administration of insulin caused a significant (P<0.05) and dose-dependent increase in lumbar SNA (Fig. 2A), with 59 ± 9% and 154 ± 15% increases in the 4th hour at the doses of 20 μU and 100 μU, respectively. In lean mice, ICV insulin also produced a significant and dose-dependent increase in BAT SNA (Fig. 3B) and renal SNA (Fig. 3C).
Fig. 2.
Lumbar sympathetic nerve activity (SNA) responses to ICV insulin in lean controls and agouti obese mice. (A–B) Time-course of lumbar SNA response to ICV insulin (100 μU) vs. vehicle in lean controls (A) and agouti obese mice (B). (C) Comparison of the 4th h of lumbar SNA responses to ICV insulin (0 [vehicle], 20 and 100 μU) and vehicle between controls and agouti obese mice. Data represent means±SEM; n=10–12 mice per group. There was no significant difference between lean and obese mice in the effect of insulin on lumbar SNA (P=0.69). * P<0.05 vs. vehicle.
Fig. 3.
Renal and brown adipose tissue (BAT) sympathetic nerve activity (SNA) responses to ICV insulin in lean controls and agouti obese mice. Renal (A) and BAT (B) SNA responses, 4 h after ICV injection of insulin (0 [vehicle], 20 and 100 μU) was compared between controls and agouti obese mice. Data represent means±SEM; n=9–12 mice per group. There was a significant difference between lean and obese mice in the effects of insulin on renal (P=0.029) and BAT SNA (P=0.045). * P < 0.05 vs. vehicle; † P < 0.05 vs. lean control mice.
To ascertain that the sympathetic nerve activation to central insulin was not influenced by the anesthetic (α-chloralose), we measured the SNA responses to ICV insulin in other cohorts of mice that were anesthetized with a different regimen. In pentobarbital anesthetized mice, ICV insulin (100 μU) increased lumbar SNA and renal SNA by 141 ± 30% (n=6) and117 ± 48% (n=5), respectively (P=0.32 and 0.47 as compared to the same dose of insulin in mice anesthetized with α-chloralose).
Surprisingly, the regional SNA responses to insulin were differentially altered in agouti obese mice. Lumbar SNA response to insulin was intact in the obese mice: the increase in lumbar SNA induced by ICV insulin was of the same magnitude in agouti obese mice as compared lean controls (Fig 2B-C). In the agouti obese mice, in the 4th hour 20 μU and 100 μU ICV insulin increased lumbar SNA by 58 ± 8% and 148 ± 24%, respectively.
In contrast to lumbar SNA, renal and BAT sympathetic nerve activation to insulin were significantly less in the agouti obese mice as compared to the lean controls. In obese mice, renal SNA increased by 27 ± 6% and by 44 ± 10% in the 4th hour at the doses of 20 and 100 μU, respectively. Insulin-induced BAT sympathetic activation was also attenuated in agouti obese mice as compared to lean controls (Fig. 3A). These results indicate that the preserved lumbar sympathetic activation to central neural action of insulin is selective.
Role of PI3K in lumbar SNA response to insulin in obesity
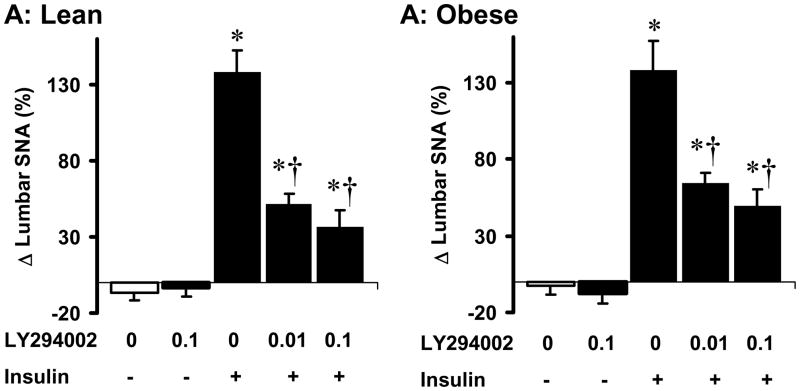
To gain insight into the molecular mechanism that mediates insulin-induced lumbar sympathetic activation in agouti obese mice, we tested the role of PI3K. We previously demonstrated the pivotal role of PI3K in mediating lumbar sympathetic activation to insulin (5). Consistent with this, ICV pre-treatment with the PI3K inhibitor (LY294002, 0.01 μg) attenuated the lumbar SNA response to ICV insulin in lean mice (Fig. 4A). Pre-treatment with a higher dose of LY294002 (0.1 μg) caused a slightly pronounced inhibition of lumbar sympathetic nerve response to ICV insulin. However, the lumbar sympathetic activation to insulin was not statistically different (P=0.26) in presence of 0.1 or 0.01 μg of LY294002. In a different group of mice (n=4), we used wortmannin instead of LY294002 to confirm the inhibition of insulin-induced lumbar sympathetic activation by PI3K blockade. Like LY294002, ICV pre-treatment with wortmannin (0.01 μg) substantially inhibited the lumbar SNA response to ICV insulin (8 ± 4%, P = 0.006 vs. vehicle-insulin group).
Fig. 4.
Effects of PI3K blockade on the lumbar sympathetic nerve activity (SNA) responses to ICV insulin in lean controls (A) and agouti obese mice (B). PI3K inhibitor LY294002 (0.01 and 0.1 μg) or vehicle were given ICV, 10 min before ICV insulin or vehicle. Data represent means±SEM; n=6–9 mice per group.
* P<0.05 vs. vehicle; † P<0.05 vs. vehicle-insulin group.
Interestingly, in agouti obese mice ICV pre-treatment with LY294002 (0.01 μg) also attenuated lumbar sympathetic activation induced by ICV insulin (Fig. 4B). Again, a higher dose of LY294002 (0.1 μg) caused a slightly, but not statistically significant (P=0.24), enhanced inhibition of lumbar sympathetic nerve response to ICV insulin. ICV administration of LY294002 alone had no effect on baseline lumbar SNA in lean and agouti obese mice. Thus, PI3K mediates the preserved lumbar SNA response to insulin in obesity.
Discussion
In the present study, we demonstrate that insulin-induced regional sympathetic nerve activation is differentially altered in the insulin resistant agouti obese mice. Indeed, we found that agouti obese mice are resistant to the stimulation by insulin of SNA to the kidney and brown adipose tissue. In contrast, insulin activation of lumbar SNA is intact in the obese mice. Preservation of the lumbar sympathetic nerve activation to insulin despite hyperinsulinemia and resistance to the glucose-lowering effect of insulin support the concept that insulin resistance associated with obesity is selective. Finally, our data demonstrate the key role of PI3K in mediating the preserved lumbar sympathetic activation to insulin.
Obesity increases cardiovascular morbidity and mortality in part by inducing hypertension. Clinical and animal studies have confirmed a strong relationship between obesity and hypertension [16–18]. Although the mechanisms of obesity-induced hypertension are not fully understood, the heightened sympathetic nerve traffic has been suggested to play an important role. Using different approaches including direct measurement with microneurography, several groups have demonstrated an adrenergic cardiovascular overdrive in obese patients [9;19]. In addition, compelling evidence have been obtained implicating the activation of the sympathetic nervous system in the development of hypertension-related target organ damage, such as kidney abnormalities, cardiac dysfunction and arterial remodeling and hypertrophy [18,19]. Potential mediators of sympathetic overdrive in obesity include circulating humoral factors such as insulin [9].
Our study adds to the large body of evidence implicating insulin in the sympathetic activation commonly associated with obesity. Indeed, insulin stimulation of sympathetic neural outflow has been suggested as a key mechanism for the tonic activation of the sympathetic nervous system, hypertension and other cardiovascular complications associated with obesity and insulin resistance [9,10]. Our finding of preserved insulin-induced sympathetic nerve activation to hindlimb in obese mice is consistent with the clinical reports regarding the positive correlation between circulating insulin and muscle SNA in obese patients [20]. In addition, induction of insulin resistance in lean subjects by free fatty acid infusion does not alter the muscle sympathetic nerve response to insulin [21]. However, our data differs from a previous study where systemic infusion of insulin to obese patients caused only a modest increase in muscle sympathetic nerve activity suggesting an impairment of insulin-induced muscle sympathoexcitation in obesity [22]. In this study, the ability of insulin to increase heart rate which is sympathetically-mediated was found to be unaltered in the obese patients as compared to lean controls demonstrating that in these obese patients the resistance to the sympathetic effects of insulin was not uniform [22].
The loss of renal SNA response to insulin in agouti obese mice is in keeping with other studies showing a lack of correlation in the obese patients between serum insulin and renal sympathetic tone as determined by norepinephrine spillover [23]. These data argue against a role for hyperinsulinemia in the increased baseline renal SNA found in obese patients and animal models of obesity.
Consistent with our previous results in rats [5], we found that in lean mice ICV insulin caused a dose-dependent increase in the sympathetic tone subserving thermogenic BAT. Insulin action in the brain is known to promote weight loss by decreasing food intake and increasing energy expenditure [2,22]. Furthermore, insulin action in the central nervous system stimulates the level of uncoupling protein in the brown adipose tissue [24]. This effect is mediated by the sympathetic nervous system [25]. Additional evidence for the importance of the brain action of insulin in energy homeostasis derives from studies showing that genetic ablation of the insulin receptor in the central nervous system leads to higher fat mass in mice [26]. Our data regarding the attenuated thermogenic BAT SNA response to ICV insulin in the agouti obese mice is in line with other studies demonstrating that animal models of obesity have reduced ability of insulin action in the brain to decrease food intake and body weight [27–29].
PI3K pathway is critical in the transduction of insulin receptor signaling in the brain. The insulin receptor activates PI3K via the phosphorylation of insulin receptor substrates (IRS-1 to IRS-4) [7]. Once activated PI3K stimulates protein kinase C and a serine/threonine protein kinase, Akt/protein kinase B. In the central nervous system, PI3K appears to mediate the modulation by insulin of neuronal firing as well as neuropeptide expression [3,30]. This molecular mechanism was shown to mediate the anorectic effect of central action of insulin [31]. We previously demonstrated the importance of PI3K in mediating the lumbar SNA response to insulin [5]. In rats, we found that insulin’s ability to increase lumbar SNA was blocked by pre-treatment with PI3K inhibitors. Here, we extended these findings by demonstrating that PI3K blockade reverses the lumbar sympathetic activation to ICV insulin in lean mice. More importantly, we demonstrate that this pathway is mediating the preserved lumbar sympathetic nerve response to ICV insulin in the agouti obese mice.
There are several potential limitations in the current studies that need to be addressed. First, the metabolic actions of insulin were studied in conscious mice, whereas the sympathetic effects of insulin were assessed in anesthetized mice. The presence of anesthesia in the SNA studies may have influenced the findings. However, we showed that 2 different regimens of anesthetics yielded comparable lumbar and renal sympathoexcitation in response to insulin. In addition, in a previous study, we found that, compared to conscious rats, anesthesia as used in our current studies does not affect the sympathetic nerve responses induced by various stimuli such as baroreflex activation or hemorrhage [32]. Second, our technique of measuring SNA does not let us compare absolute values of SNA between different groups of animals. However, this technique is considered as state-of-the-art to study the SNA response to stimuli. Third, we have not measured insulin concentrations in the cerebrospinal fluid following ICV injection of insulin. The doses of insulin injected ICV may have increased significantly insulin concentrations in the cerebrospinal fluid. However, our data indicate that the sympathetic nerve responses to ICV insulin are within the physiological range. In addition, the responses caused by 20 and 100 μU were dose-related indicating that we have not saturated the insulin receptors in the central nervous system. Fourth, the region in the brain where PI3K inhibitors act to block the lumbar SNA response to insulin was not determined. We previously shown that ICV insulin activate hypothalamic PI3K [5]. Intra-hypothalamic injection of the PI3K inhibitors could uncover the role of this region in the lumbar sympathetic activation to insulin in the lean as well as the obese mice.
In summary, our data demonstrate non-uniform resistance to insulin action in a mouse model of obesity. We found that agouti obese mice are resistant to the glucose lowering effect of insulin. In addition, we showed that these obese mice have diminished renal and BAT sympathetic nerve responses to ICV insulin. In contrast, agouti obese mice had intact lumbar sympathetic nerve response to ICV insulin indicating that hyperinsulinemia may be involved in the increased sympathetic tone commonly associated with insulin resistance and obesity. Finally, we defined the molecular mechanism underlying the preserved lumbar SNA response to insulin in agouti obese mice by implicating the PI3K signaling pathway.
Acknowledgments
Funding: Dr. Rahmouni is supported by grant HL084207 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of interest: None
References
- 1.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone - Implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation. 1997;96:4104–4113. doi: 10.1161/01.cir.96.11.4104. [DOI] [PubMed] [Google Scholar]
- 2.Porte D, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system - A critical role in metabolic homeostasis and disease from C-elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- 3.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntzel MS, Anderson EA, Johnson AK, Mark AL. Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens. 1995;17:39–50. doi: 10.3109/10641969509087053. [DOI] [PubMed] [Google Scholar]
- 5.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntzel M, Beltz T, Mark AL, Johnson AK. Anteroventral 3rd ventricle Lesions abolish lumbar sympathetic responses to insulin. Hypertension. 1994;23:1059–1062. doi: 10.1161/01.hyp.23.6.1059. [DOI] [PubMed] [Google Scholar]
- 7.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37 :1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 9.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 10.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lu DS, Willard D, Patel IR, Kadwell S, Overton L, Kost T, et al. Agouti Protein Is An Antagonist of the Melanocyte-Stimulating-Hormone Receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 12.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of malanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 13.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 14.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39:486–490. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 15.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26:S28–S38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 17.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 19.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 20.Monroe MB, Van Pelt RE, Schiller BC, Seals DR, Jones PP. Relation of leptin and insulin to adiposity-associated elevations in sympathetic activity with age in humans. Int J Obes Relat Metab Disord. 2000;24:1183–1187. doi: 10.1038/sj.ijo.0801364. [DOI] [PubMed] [Google Scholar]
- 21.Vollenweider L, Tappy L, Owlya R, Jequier E, Nicod P, Scherrer U. Insulin-induced sympathetic activation and vasodilation in skeletal muscle - Effects of insulin resistance in lean subjects. Diabetes. 1995;44:641–645. doi: 10.2337/diab.44.6.641. [DOI] [PubMed] [Google Scholar]
- 22.Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest. 1994;93:2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 24.Geloen A, Trayhurn P. Regulation of the Level of Uncoupling Protein in Brown Adipose-Tissue by Insulin. Am J Physiol. 1990;258:R418–R424. doi: 10.1152/ajpregu.1990.258.2.R418. [DOI] [PubMed] [Google Scholar]
- 25.Geloen A, Trayhurn P. Regulation of the Level of Uncoupling Protein in Brown Adipose-Tissue by Insulin Requires the Mediation of the Sympathetic Nervous-System. FEBS Lett. 1990;267:265–267. doi: 10.1016/0014-5793(90)80941-b. [DOI] [PubMed] [Google Scholar]
- 26.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 27.Carvalheira JBC, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, et al. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46:1629–1640. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- 28.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 30.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford MLJ. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosc. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 31.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus - A key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 32.Morgan DA, Thoren P, Staudt SM, Mark AL. Differential effects of anesthesia on heart rate (HR), renal (RSNA), and lumbar sympathetic nerve activity (LSNA) in the rat. FASEB J. 1988;2:A1690. (Abstract) [Google Scholar]