Abstract
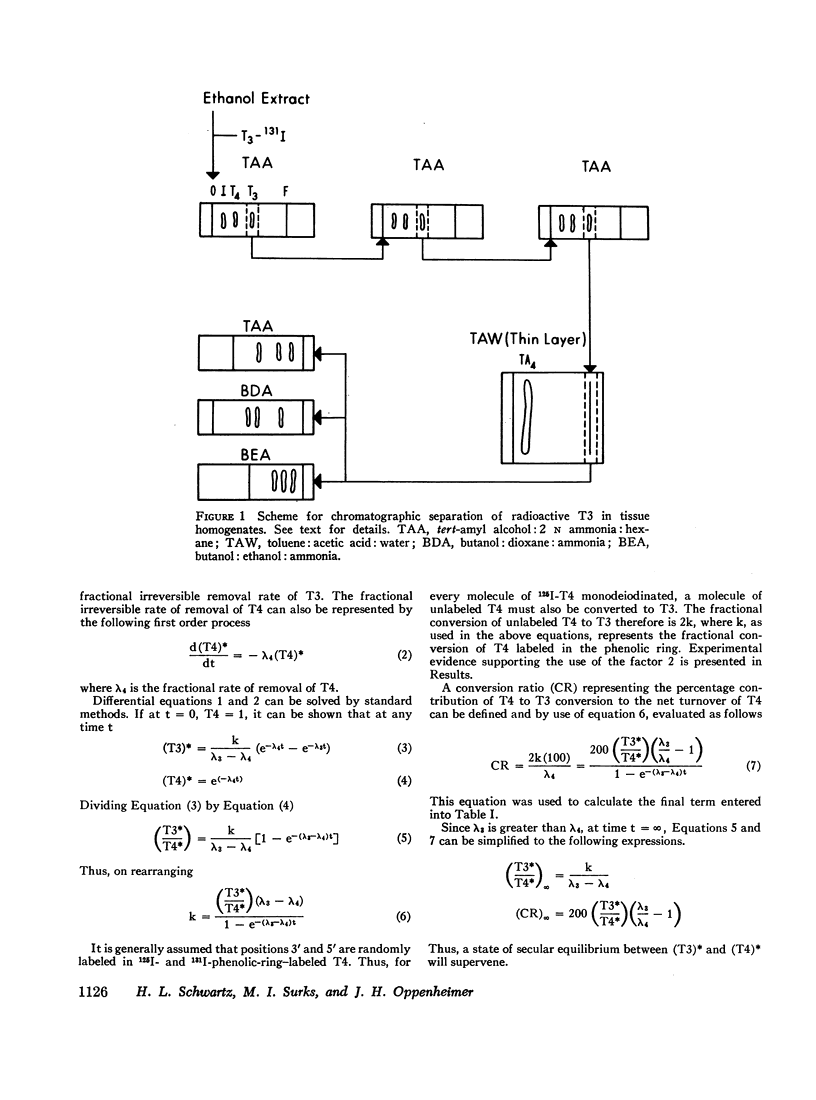
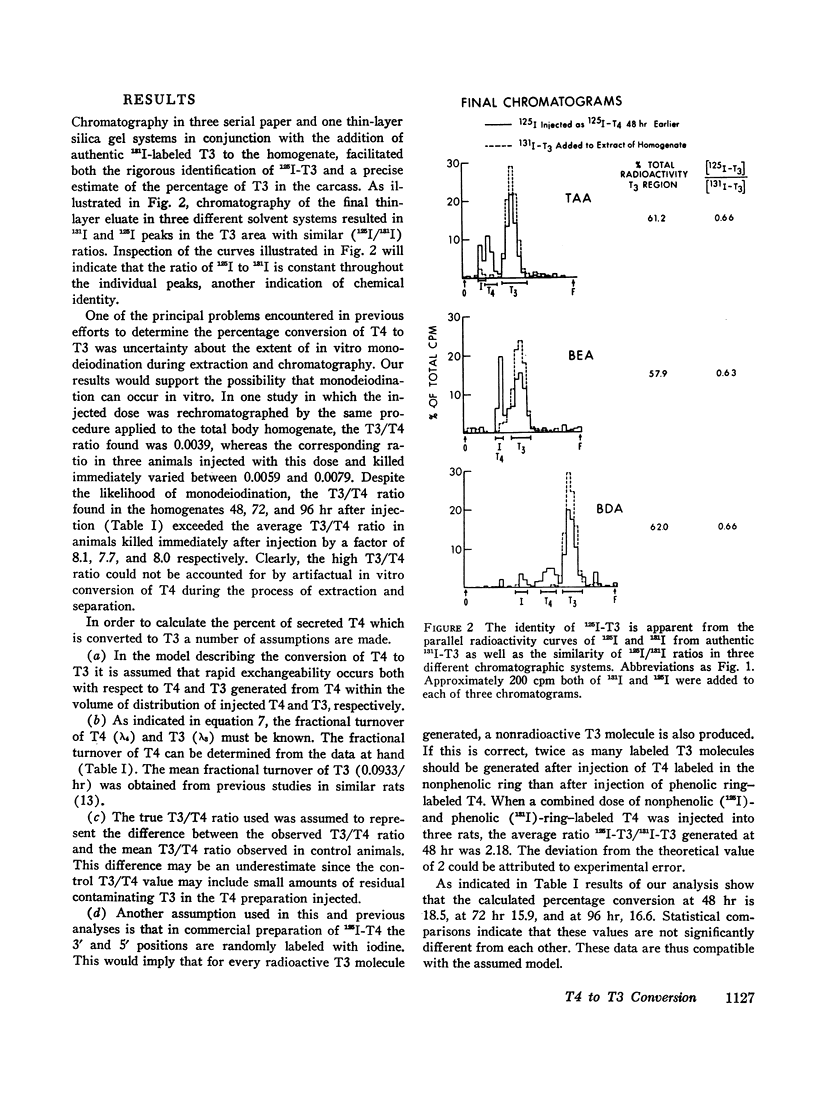
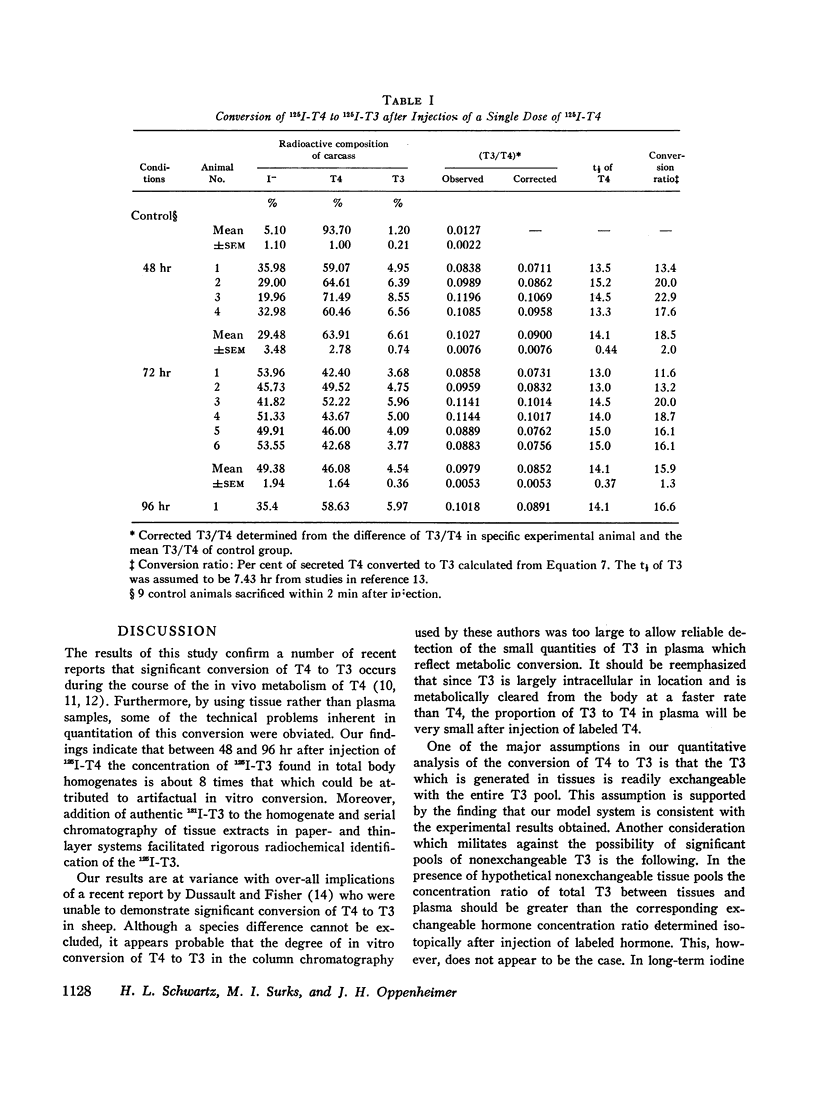
Studies of the rate of extrathyroidal conversion of thyroxine (T4) to 3,5,3′-triiodo-L-thyronine (T3) were carried out in rats. Total body homogenates were prepared and extracted with ethanol 48, 72, and 96 hr after the intravenous injection of 125I-T4. 131I-T3 was added, and the paper chromatographic purification of T3 was effected by serial elution and rechromatography in three paper and one thin-layer cycles. The ratio of 131I-T3 and 125I-T3 counting rates in the final chromatograms, which was identical in three different paper chromatography systems, was used to calculate the proportion of 125I-T3 to 125I-T4 in the original homogenates. In order to discount the effects of in vitro monodeiodination of T4 during extraction and chromatography, we killed control animals immediately after injection of 125I-T4 and processed them in a similar fashion to the experimental groups. The average ratio of 125I-T3 to 125I-T4 in carcass extracts of animals killed between 48 and 96 hr after isotopic injection was 0.08 whereas the average ratio of 125I-T3 to 125I-T4 in chromatograms of control animals was 0.01. On the basis of the proposed model, calculations indicated that about 17% of the secreted T4 was converted to T3. Assuming values cited in the literature for the concentration of nonradioactive T3 in rat plasma, these findings would suggest that about 20% of total body T3 is derived by conversion from T4. Moreover, since previous estimates have suggested that in the rat, T3 has about 3 to 5 times greater biologic activity than T4, these results also raise the possibility that the hormonal activity of T4 may be dependent in large part on its conversion to T3.
A necessary assumption in calculating T4 to T3 conversion in this and other studies is that the 3′ and 5′ positions are randomly labeled with radioiodine in phenolic-ring iodine-labeled T4. Evidence supporting this assumption was obtained in the rat by comparing the amount of labeled T3 produced after injection of phenolic and nonphenolic-ring iodine-labeled T4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRIGHT E. C., LARSON F. C. Metabolism of L-thyroxine by human tissue slices. J Clin Invest. 1959 Nov;38:1899–1903. doi: 10.1172/JCI103967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER S. B. Metabolic actions of thyroxine derivatives and analogs. Endocrinology. 1956 Nov;59(5):548–554. doi: 10.1210/endo-59-5-548. [DOI] [PubMed] [Google Scholar]
- BECKER D. V., PRUDDEN J. F. The metabolism of I 131-labeled thyroxine, triiodothyronine and diiodotyrosine by an isolated perfused rabbit liver. Endocrinology. 1959 Jan;64(1):136–148. doi: 10.1210/endo-64-1-136. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOCK E. V., BOLLMAN J. L., GRINDLAY J. H., STOBIE G. H. Partial deiodination of 1-thyroxine. Endocrinology. 1961 Sep;69:626–637. doi: 10.1210/endo-69-3-626. [DOI] [PubMed] [Google Scholar]
- GLEASON G. I. Some notes on the exchange of iodine with thyroxine homologues. J Biol Chem. 1955 Apr;213(2):837–841. [PubMed] [Google Scholar]
- GROSS J., PITT-RIVERS R. 3:5:3'-triiodothyronine. 2. Physiological activity. Biochem J. 1953 Mar;53(4):652–657. doi: 10.1042/bj0530652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., PITT-RIVERS R. The identification of 3:5:3'-L-triiodothyronine in human plasma. Lancet. 1952 Mar 1;1(6705):439–441. doi: 10.1016/s0140-6736(52)91952-1. [DOI] [PubMed] [Google Scholar]
- HEMING A. E., HOLTKAMP D. E. Calorigenic and antigoitrogenic actions of L-triiodothyronine and L-thyroxine in thyroidectomized and intact rats. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):875–879. doi: 10.3181/00379727-83-20520. [DOI] [PubMed] [Google Scholar]
- HENINGER R. W., LARSON F. C., ALBRIGHT E. C. IODINE-CONTAINING COMPOUNDS OF EXTRATHYROIDAL TISSUES. J Clin Invest. 1963 Nov;42:1761–1768. doi: 10.1172/JCI104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Grimm Y., Greer M. A. Quantitative studies on the iodinated components secreted by the rat thyroid gland as determined by in situ perfusion. Endocrinology. 1967 Nov;81(5):946–964. doi: 10.1210/endo-81-5-946. [DOI] [PubMed] [Google Scholar]
- LARSON F. C., TOMITA K., ALBRIGHT E. C. The deiodination of thyroxine to triiodothyronine by kidney slices of rats with varying thyroid function. Endocrinology. 1955 Sep;57(3):338–344. doi: 10.1210/endo-57-3-338. [DOI] [PubMed] [Google Scholar]
- LASSITER W. R., STANBURY J. B. The in vivo conversion of thyroxine to 3:5:3'triiodothyronine. J Clin Endocrinol Metab. 1958 Aug;18(8):903–906. doi: 10.1210/jcem-18-8-903. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Shapiro H. C., Bernstein G., Surks M. I. Differences in primary cellular factors influencing the metabolism and distribution of 3,5,3'-L-triiodothyronine and L-thyroxine. J Clin Invest. 1970 May;49(5):1016–1024. doi: 10.1172/JCI106301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITT-RIVERS R., RALL J. E. Radioiodine equilibrium studies of thyroid and blood. Endocrinology. 1961 Feb;68:309–316. doi: 10.1210/endo-68-2-309. [DOI] [PubMed] [Google Scholar]
- PITT-RIVERS R., STANBURY J. B., RAPP B. Conversion of thyroxine to 3-5-3'-triiodothyronine in vivo. J Clin Endocrinol Metab. 1955 May;15(5):616–620. doi: 10.1210/jcem-15-5-616. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Newman E. S. Conversion of thyroxine to triiodothyronine in normal human subjects. Science. 1970 Sep 11;169(3950):1099–1100. doi: 10.1126/science.169.3950.1099. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Formation of iodoprotein during the peripheral metabolism of 3,5,3'-triiodo-L-thyronine-125I in the euthyroid man and rat. J Clin Invest. 1969 Apr;48(4):685–695. doi: 10.1172/JCI106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Shapiro H. C. Preparation of high specific activity nonphenolic-ring 125-I- and 14C-labeled thyroxine. J Clin Endocrinol Metab. 1969 Sep;29(9):1263–1265. doi: 10.1210/jcem-29-9-1263. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Weinbach S., Volpert E. M. On the identfication of 4-hydroxy-3,5-diiodophenylpyruvic acid in rat thyroid glands. Endocrinology. 1968 Jun;82(6):1156–1162. doi: 10.1210/endo-82-6-1156. [DOI] [PubMed] [Google Scholar]
- TAUROG A., PORTER J. C., THIO D. T. NATURE OF THE 131-I-COMPOUNDS RELEASED INTO THE THYROID VEINS OF RABBITS, DOGS AND CATS, BEFORE AND AFTER TSH ADMINISTRATION. Endocrinology. 1964 Jun;74:902–913. doi: 10.1210/endo-74-6-902. [DOI] [PubMed] [Google Scholar]



