Abstract
In larval lamprey, partial lesions were made in the rostral spinal cord to determine which spinal tracts are important for descending activation of locomotion and to identify descending brain neurons that project in these tracts. In whole animals and in vitro brain/spinal cord preparations, brain-initiated spinal locomotor activity was present when the lateral or intermediate spinal tracts were spared but usually was abolished when the medial tracts were spared. We previously showed that descending brain neurons are located in eleven cell groups, including reticulospinal (RS) neurons in the mesenecephalic reticular nucleus (MRN) as well as the anterior (ARRN), middle (MRRN), and posterior (PRRN) rhombencephalic reticular nuclei. Other descending brain neurons are located in the diencephalic (Di) as well as the anterolateral (ALV), dorsolateral (DLV), and posterolateral (PLV) vagal groups. In the present study, the Mauthner and auxillary Mauthner cells, most neurons in the Di, ALV, DLV, and PLV cell groups, and some neurons in the ARRN and PRRN had crossed descending axons. The majority of neurons projecting in medial spinal tracts included large identified Müller cells and neurons in the Di, MRN, ALV, and DLV. Axons of individual descending brain neurons usually did not switch spinal tracts, have branches in multiple tracts, or cross the midline within the rostral cord. Most neurons that projected in the lateral/intermediate spinal tracts were in the ARRN, MRRN, and PRRN. Thus, output neurons of the locomotor command system are distributed in several reticular nuclei, whose neurons project in relatively wide areas of the cord.
Keywords: Locomotor, Command systems, Reticulospinal, Descending pathways, Brain, Locomotor regions
Introduction
In vertebrate animals, “command systems” in the brain activate spinal central pattern generators (CPGs) and initiate locomotor behavior (reviewed in Grillner, 2003; McClellan, 1996). In a wide variety of vertebrates, brain locomotor command systems are thought to consist of neurons in several similar locations in the brain and appear to have a similar organization. First, in a wide range of vertebrates, spinal locomotor activity can be initiated by electrical or pharmacological microstimulation in similar brain locomotor areas (reviewed in Shik and Orlovsky, 1976; Grillner, 1976; Armstrong, 1988; Jordan, 1998; Jordan et al., 2008; Jahn et al. 2008): (1) lateral hypothalamus; (2) “subthalamic locomotor region” (SLR); (3) “mesencephalic locomotor region” (MLR); (4) “pontomedullary locomotor strip” (PLS); and (5) cerebellar locomotor region (CLR).
Second, neurons in some of the above higher-order brain locomotor areas project directly to reticular nuclei and activate RS neurons. For example, lesions or blocking activity in medullary reticular nuclei can abolish MLR-initiated locomotor activity (Shefchyk et al., 1984; Garcia-Rill and Skinner, 1987a; Bernau et al., 1991; Noga et al., 1991, 2003) or raise the threshold for evoking locomotion (Marlinskii and Voitenko, 1992; also see Gorska et al., 1995). Stimulation in the MLR or SLR elicits synaptic responses in RS neurons (Orlovsky, 1970; Garcia-Rill and Skinner, 1987b; Skinner et al., 1990), and anatomical experiments indicate that neurons in these higher-order locomotor areas project to reticular nuclei (Garcia-Rill et al., 1983; Steeves and Jordan, 1984; Garcia-Rill et al., 1986; Bernau et al., 1991). Finally, stimulation in reticular nuclei can activate spinal CPGs and initiate locomotor activity (Steeves et al., 1987; Noga et al., 1988; Livingston and Leonard, 1990; Kinjo et al., 1990; Bernau et al., 1991).
Third, the ventral or ventrolateral spinal tracts are important for transmitting descending command signals to spinal CPGs and initiating locomotion in a number of vertebrates: monkey (Eidelberg et al. 1981b); cat (Windle et al., 1958; Afelt 1974; Steeves and Jordan, 1980; Eidelberg et al., 1981a; Noga et al., 1991, 2003); rat (Harris et al., 1994; Schucht et al., 2002; You et al., 2003); bird (Jacobson and Holliday, 1982; Sholomenko and Steeves, 1987); and stingray (Livingston and Leonard, 1990; Williams et al., 1984) (reviewed in Eidelberg, 1981; McClellan, 1986; Rossignol et al., 1996, 1999). For example, in mammals, RS neurons in medial reticular nuclei project in the ventral (VF) and ventrolateral funiculi (VLF), and lesioning these tracts abolishes brain-evoked locomotor activity (Noga et al., 1991, 2003). In addition, stimulation of the lateral or ventrolateral tracts in the cervical spinal cord can initiate locomotion (Jacobson and Holliday, 1982; Williams et al., 1984; Yamaguchi 1986; Magnuson and Trinder, 1997). However, other studies suggest that rostral descending spinal tracts that support locomotion are more diffuse than the VLF, and other tracts, including the dorsolateral funiculi (DLF), may contribute (Yamaguchi, 1986; Stein, 1978; also see Bem et al., 1995, Gorska et al., 1996, Jiang and Drew, 1996 and Loy et al., 2002a,b for similar studies involving partial spinal cord lesions at thoracic levels). Finally, although acute lesions of the ventral or ventrolateral tracts of the thoracic cord usually abolish or disrupt locomotor activity below the lesion, the locomotor system can adapt during long recovery times, and this results in some return of voluntary locomotor function in cats (Gorska et al. 1990, 1993a,b; Brustein and Rossignol, 1998), monkeys (Vilensky et al. 1992), and even humans (Nathan 1994) (reviewed in Rossignol et al., 1996, 1999).
In the lamprey, a lower vertebrate, the relative simplicity of locomotor behaviors, a large and expanding literature concerning the neurons and networks responsible for locomotor behavior (reviewed in Grillner, 2003), and the relative simplicity of the brain and spinal cord combine to make this preparation a model system for investigating the control of locomotion and the anatomical substrates that are involved. In adult and larval lamprey, the lateral spinal tracts are important for the descending initiation of locomotion (McClellan, 1988; also see Ullen et al., 1997). In addition, sustained pharmacological stimulation in reticular nuclei initiates and drives swimming movements and locomotor activity in semi-intact larval lamprey preparations (Jackson et al., 2007).
Although much is known about descending brain neurons in lamprey, a detailed study of the brain neurons that project in the lateral and other spinal tracts and that are important for locomotor initiation has not been made. To begin to address the above issues, in the present study, partial lesions were made in the rostral spinal cord of normal larval lampreys to spare various tracts. In whole animals and in vitro brain/spinal cord preparations, brain-initiated locomotor activity was used to confirm and extend the importance of the lateral spinal tracts for the initiation of locomotion. In whole animals, retrograde labeling with HRP as well as double labeling with fluorescent tracers was used to determine the identity of descending brain neurons, particularly RS neurons, that project in various spinal tracts. Because descending axons regenerate in lamprey following spinal cord injury (reviewed in McClellan, 1998; Selzer, 2003), the results from normal lamprey in the present study lay the foundation for determining the descending brain–spinal cord projections that are important for behavioral recovery. Preliminary accounts of this work have appeared in abstract form (McClellan and Davis, 1992; Shaw et al. 2001).
Methods
Animal care
Larval sea lampreys (Petromyzon marinus) were used for both whole animal and in vitro experiments (Table 1) and were maintained in ~10 l aquaria at 23–25 °C. The procedures in this study have been approved by the Animal Use and Care Committee at the University of Missouri.
Table 1.
Parameters of muscle locomotor burst activity in whole animals.
| Group | T (ms) | BP | ϕINT | ϕRT-LT |
|---|---|---|---|---|
| Normal (8/774)a | ||||
| 351±75b (130–654)c | 0.270±0.085 (all) | 0.0066±0.0021 | 0.478±0.067 (all) | |
| 0.286±0.077 (rostral) | 0.458±0.062 (rostral) | |||
| 0.221±0.110 (caudal) | 0.498±0.066 (caudal) | |||
| Hemi-transectiond (9/822) | ||||
| 365±68 (185–689) | 0.250±0.081 (right) | 0.0070±0.0026 (right) | 0.477±0.068 | |
| 0.253±0.076 (left) | 0.0072±0.0017 (left) | |||
| Lateral tracts spared (8/792) | ||||
| 436±87 (230–774) | 0.252±0.087 (all) | 0.0070±0.0019 | 0.500±0.062 (all) | |
| 0.285±0.079 (rostral) | 0.494±0.054 (rostral) | |||
| 0.219±0.082 (caudal) | 0.505±0.069 (caudal) | |||
| Medial tracts sparede (5/337) | ||||
| 575±143* (178–967) | 0.320±0.118 (all) | 0.0067±0.0023 | 0.502±0.079 (all) | |
| 0.366±0.116 (rostral) | 0.505±0.061 (rostral) | |||
| 0.273±0.103 (caudal) | 0.499±0.095 (caudal) | |||
| Intermediate tracts spared (6/594) | ||||
| 608±289** (193–2112) | 0.214±0.079 (all) | 0.0081±0.0026 | 0.498±0.083 (all) | |
| 0.239±0.078 (rostral) | 0.462±0.078 (rostral) | |||
| 0.189±0.072 (caudal) | 0.535±0.072 (caudal) |
— p≤0.05,
— p≤0.01; compared to corresponding value in normal animals (ANOVA, Dunnett multiple comparison post-test).
n1/n2=number of animals /number of cycles.
Mean±SD.
Range of cycle times.
Hemi-transection on left side of spinal cord (parameters for right hemi-transections were transposed; see Methods).
Only for animals with the “smaller” lateral spinal lesions (see “1” in Fig. 2A) that could initiate locomotion.
Rationale
In the present study, several approaches were used to identify the spinal tracts and descending brain neurons that are important for the initiation of locomotion in larval lamprey. First, in whole animals various partial lesions were made in the rostral spinal cord (see below) to interrupt descending brain–spinal cord projections in specific regions of the cord. Locomotor behavior was videotaped and muscle activity was recorded to assess the locomotor capabilities of the lesioned animals (Figs. 1A, 2, 4-6). Since there was very little intact spinal cord rostral to the partial lesions, the locomotor capabilities very likely were due to descending brain neurons that projected in spared parts of the spinal cord. Second, horseradish peroxidase (HRP) was applied caudal to the partial lesions to retrogradely label descending brain neurons (Fig. 1B) that projected in the spared parts of the spinal cord (Fig. 3). Approximately 80% of descending brain neurons are RS neurons (Davis and McClellan, 1994a). Third, in in vitro brain/spinal cord preparations, similar partial lesions were made in the rostral spinal cord to test whether specific spinal tracts could support the initiation of locomotor activity from the brain in the absence of mechanosensory inputs (Figs. 1C, 7, 8). Fourth, double labeling with fluorescent tracers was used to determine if individual descending brain neurons project in multiple spinal tracts (Fig. 9). Fifth, partial spinal cord lesions and HRP labeling were used to determine if spinal axons of individual descending brain neurons switch tracts or cross the midline (Figs. 10, 11).
Fig. 1.
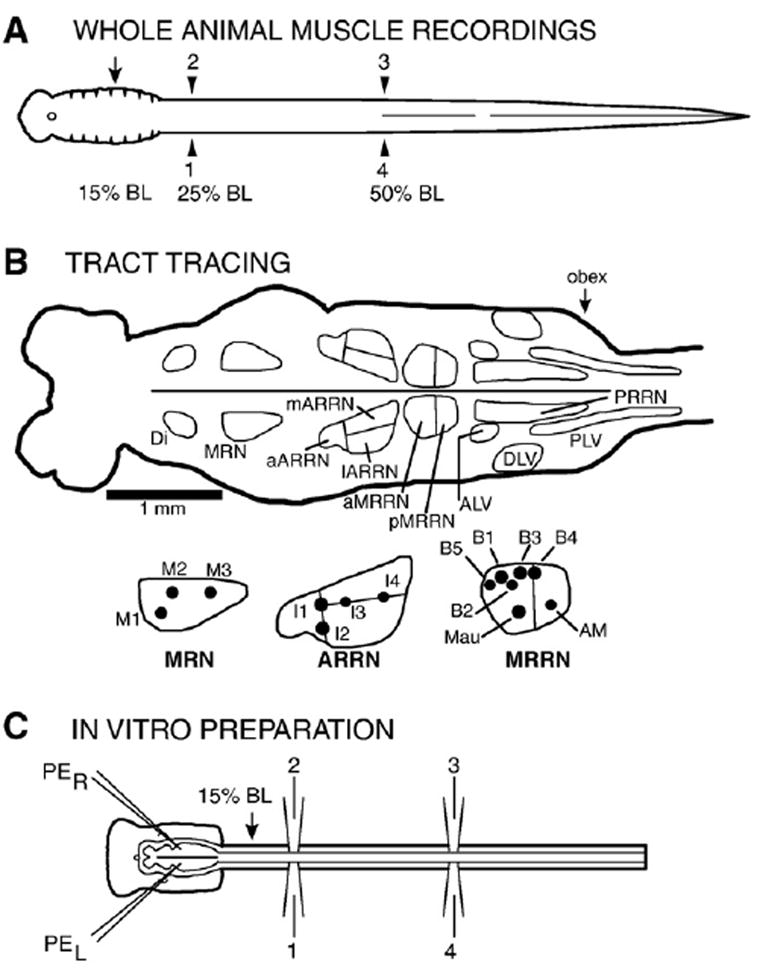
(A) Diagram of whole animal showing the locations of muscle recording electrodes (1–4, arrowheads) at 25% body length (BL, normalized distance from the anterior tip of the head) and 50% BL. Partial spinal cord lesions were made at 15% BL (arrow), which is located an average of ~7–8 mm below the obex at approximately segment 8. (B) Diagram of the brain (rostral left) and initial spinal cord (far right). Locations of cell groups containing descending brain neurons that project to the spinal cord in normal animals (see List of Abbreviations): Di; MRN; ARRN; MRRN; PRRN; ALV; DLV; and PLV. Some of the cell groups were further subdivided: aARRN, lARRN, and mARRN, and aMRRN and pMRRN. (Inset) Large identified Müller and Mauthner cells in the MRN (M1–M3 cells), ARRN (I1–I4 cells), and MRRN (B1–B5 cells, Mau and AM cells). (C) Diagram of in vitro brain/spinal cord preparation showing pressure ejection micropipettes (PE), ventral root recording electrodes at ~25% (1, 2) and ~50% BL (3, 4), and site of partial spinal cord lesions at 15% BL (arrow).
Fig. 2.
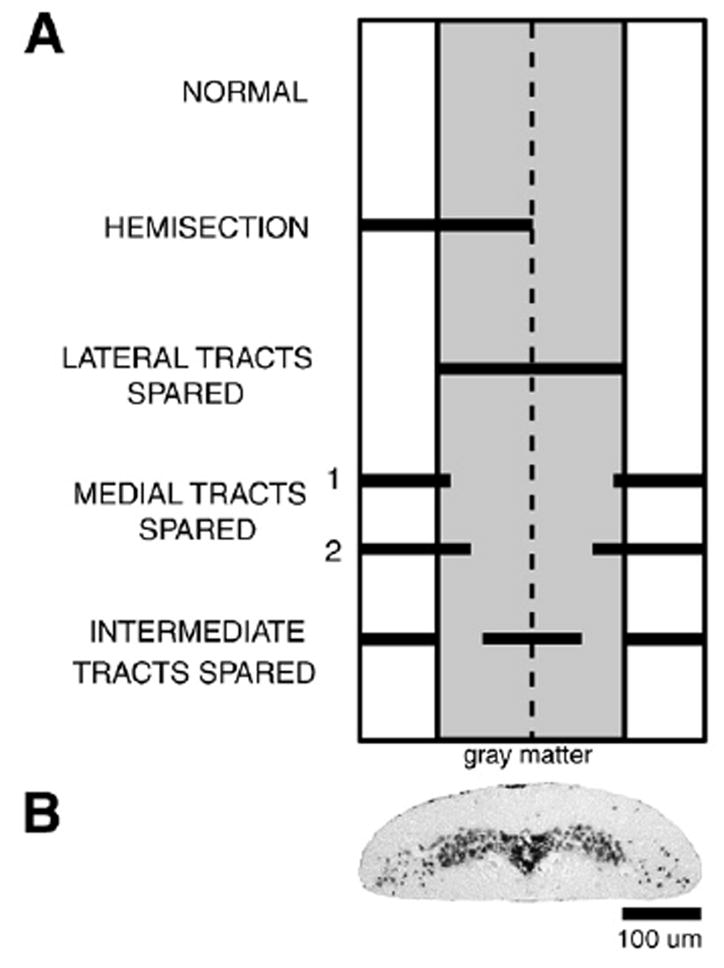
(A) Diagram of a dorsal view of the ribbon-like lamprey spinal cord (~100–150 μm thick×~1 mm wide) at 15% BL showing medial region that corresponds to the width of the “gray matter” (shaded area), “white matter” (lateral areas of cord), and midline (vertical dashed line). For comparison purposes, the different types of partial spinal cord lesions (solid horizontal bars; see Methods) are simultaneously displayed on the same diagram. (B) Photomicrograph of a cresyl violet-stained Paraffin transverse section of the spinal cord of a larval lamprey (80 mm) at ~15% BL (top is dorsal), showing central “gray matter” surrounded by “white matter”.
Fig. 4.
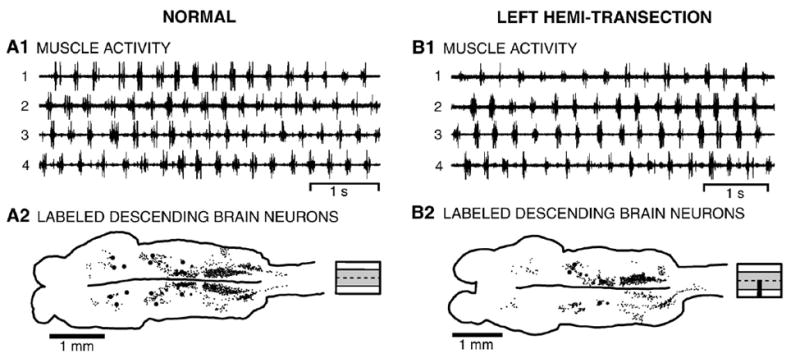
(A) Normal whole animal. (A1) Locomotor muscle burst activity was characterized by left–right alternation of muscle burst activity at the same segmental level (1↔2 and 3↔4) and a rostrocaudal phase lag of ipsilateral burst activity (1→4 and 2→3) (see Fig. 1A, Table 1). (A2) Brain (left) and rostral spinal cord showing the distributions of labeled descending brain neurons (small dots are unidentified neurons; large dots are Müller and Mauthner cells; see Fig. 1B) resulting from application of HRP to the spinal cord at 25% BL (diagram to right shows unlesioned spinal cord). (B) Whole animal with a left spinal cord hemi-transection at 15% BL (see Fig. 2A). (B1) Locomotor burst activity consisted of left–right alternation and a rostrocaudal phase lag (see Table 1). (B2) Brain (left) and spinal cord showing distributions of descending brain neurons that were labeled by application of HRP to the spinal cord at 25% BL, caudal to a hemi-transection at 15% BL (diagram to right shows partial spinal lesion).
Fig. 6.
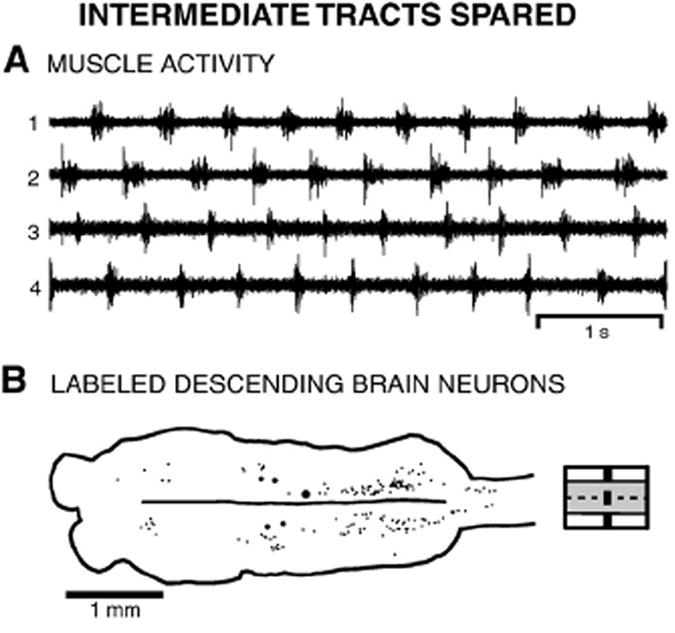
Whole animal in which the intermediate spinal tracts were spared (see Fig. 2A). (A) Locomotor activity consisted of left–right alternation and a rostrocaudal phase lag (see Table 1). (B) Brain (left) and rostral spinal cord showing the distributions of descending brain neurons that were labeled by application of HRP to the spinal cord at 25% BL, caudal to lesions at 15% BL (diagram to right shows partial spinal lesion).
Fig. 3.
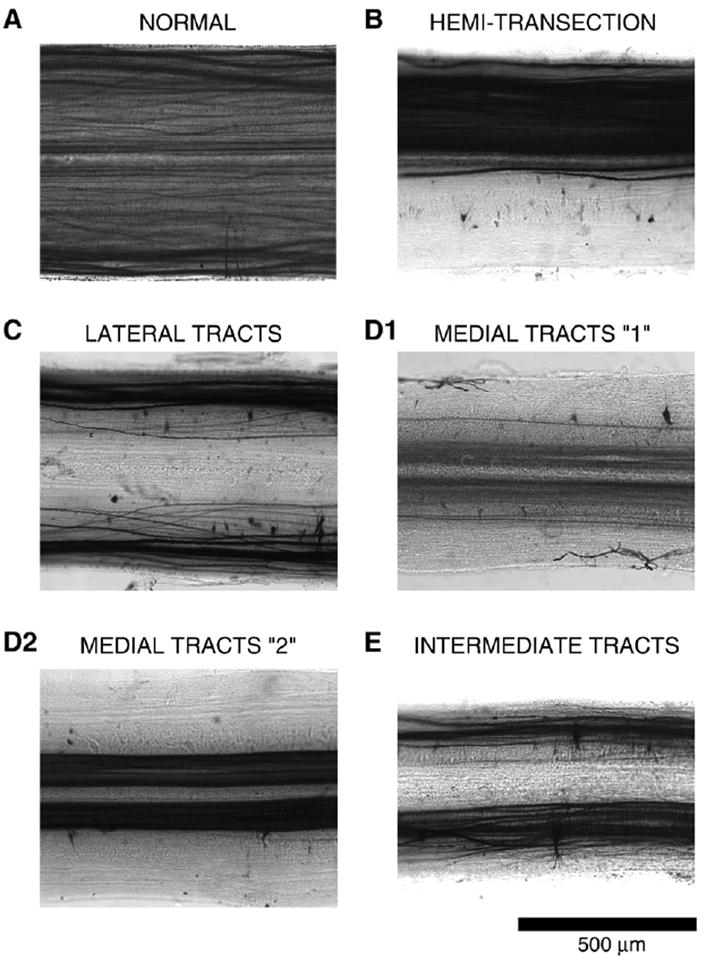
Pattern of labeling of descending axons at ~12–14% BL in the spinal cord, above the partial spinal cord lesions at 15% BL (see Fig. 2), in the following animal groups: (A) normal; (B) left spinal cord hemi-transection; (C) lateral tracts spared; (D1) medial tracts spared (#1 in Fig. 2); (D2) medial tracts spared (#2 in Fig. 2); and (E) intermediate tracts spared.
Fig. 7.
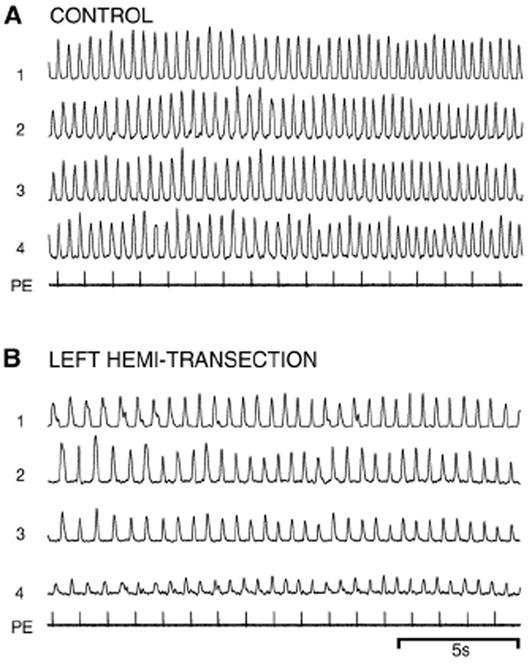
In vitro locomotor activity before and after a hemi-transection of the spinal cord at 15% BL (Figs. 1C, 2A). (A) Prior to the lesion, pharmacological microstimulation in brain locomotor areas (PE=pressure ejection pulses; see Methods) initiated well-coordinated locomotor burst activity consisting of left–right alternation (1↔2 and 3↔4) and a rostrocaudal phase lag (1→4 and 2→3) (see Table 3). (B) Following a left hemi-transection, spinal locomotor activity could still be initiated below the lesion by brain stimulation.
Fig. 8.
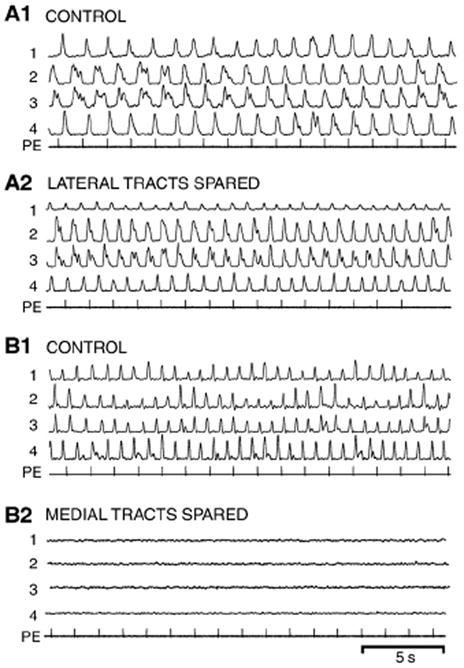
In vitro locomotor activity before and after lesions that spared the (A) lateral or (B) medial spinal cord tracts (see Fig. 2A and legend in Fig. 7). (A1) Prior to medial spinal cord lesions, brain-initiated control locomotor activity consisted of left–right alternation and a rostrocaudal phase lag (see Table 3). (A2) Following a lesion of the medial spinal tracts at 15% BL, pharmacological microstimulation in brain locomotor areas still initiated well-coordinated spinal locomotor activity (Table 3). (B1) Brain-initiated control locomotor activity prior to spinal lesions. (B2) Following lesions of the lateral spinal cord tracts at 15% BL (see “1” in Fig. 2A), pharmacological microstimulation in the brain no longer elicited spinal locomotor activity (channel gains were increased by 2x to show lack of locomotor burst activity).
Fig. 9.
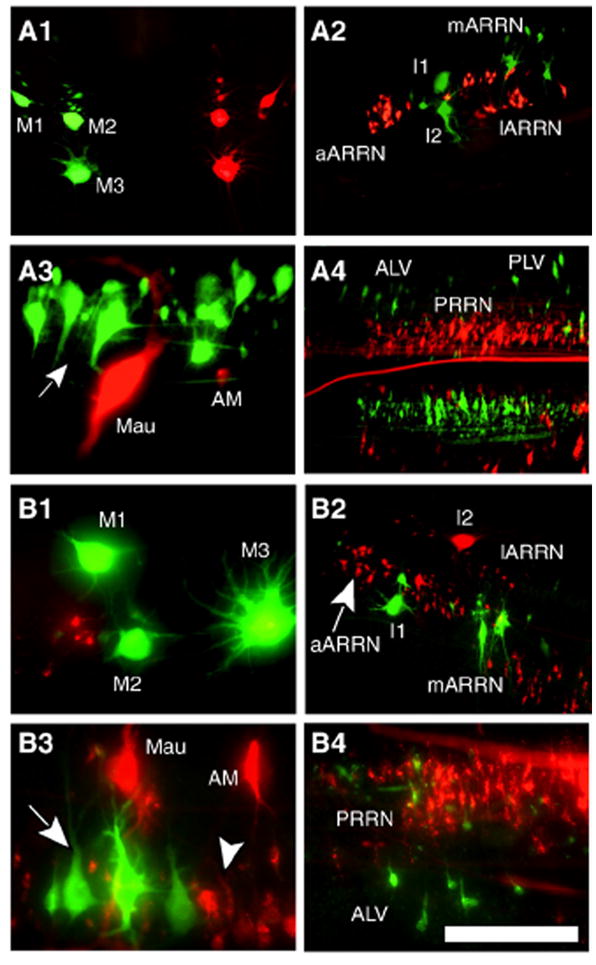
Double labeling experiments to test whether descending brain neurons have multiple axonal branches that project in different spinal tracts. (A) Descending brain neurons labeled by application of Alexa (green) to the left half of the spinal cord at 10% BL and TRDA (red) to the right half of the spinal cord at 20% BL (see Methods) (unless otherwise stated, rostral is left, caudal is right): (A1) right and left MRN (rostral is up, caudal is down); (A2) left ARRN; (A3) left MRRN (arrow, aMRRN); (A4) ALV, PLV, and PRRN. (B) Descending brain neurons labeled by application of Alexa to the medial spinal tracts at 10% BL and TRDA to the lateral spinal tracts at 20% BL (see Methods): (B1) right MRN; (B2) right ARRN (arrow, aARRN); (B3) right MRRN (arrow, aMRRN; arrowhead, pMRRN); (B4) left ALV and PRRN. In all experiments, no double-labeled descending brain neurons were observed. Scale bar: 200 μm (A3, B1, B3, B4), 400 μm (A1, A2, A4, B2).
Fig. 10.
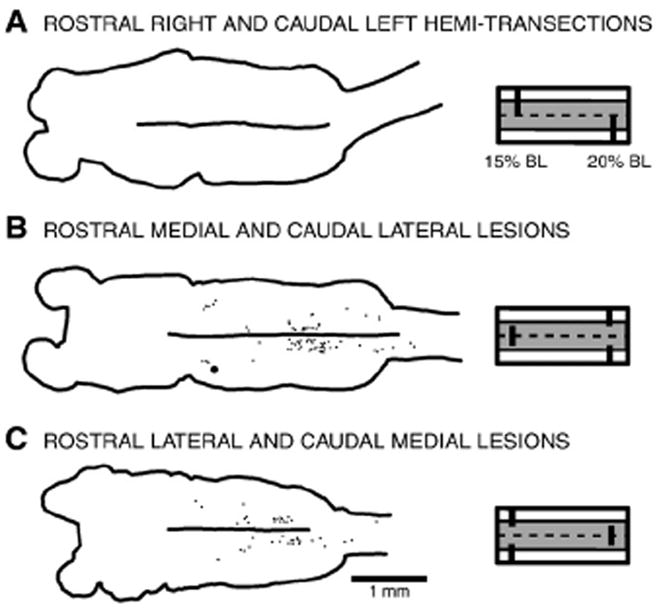
“Complementary partial lesion” experiments to test whether axons from descending brain neurons are relatively straight or can switch tracts. The following spinal cord lesions were used: (A) complementary right (15% BL) and left (20% BL) hemi-transections; (B) complementary medial (15% BL) and lateral (20% BL) lesions; or (C) complementary lateral (15% BL) and medial (20% BL) lesions. After the above spinal lesions, animals were unable to initiate locomotion (data not shown). Subsequently, HRP application to the spinal cord at 25% BL labeled either no descending brain neurons or labeled relatively few neurons, mostly in the PRRN, suggesting that, as a rule, descending axons of these neurons do not switch tracts, at least in the rostral cord.
Fig. 11.
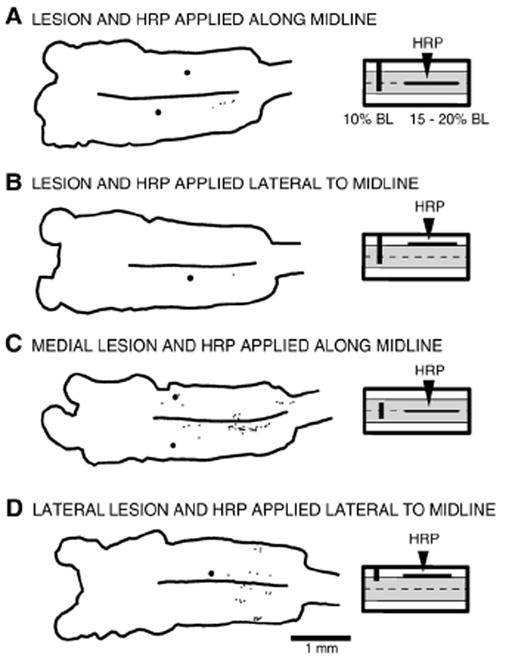
Lesion experiments to test whether axons from descending brain neurons cross the midline. (Right) Partial transverse lesions were made in the spinal cord at 10% BL (dark vertical bars), and then HRP was applied to a longitudinal lesion of the cord (horizontal lines, 15–20% BL; see Methods) to label descending brain neurons whose axons might have changed tracts or sent collaterals into adjacent regions of the spinal cord. (A) Left lateral tracts spared and HRP applied to the midline. (B) Left lateral tracts spared and HRP applied to the right lateral tracts. (C) Both lateral tracts spared and HRP applied to the midline. (D) Medial and left lateral tracts spared and HRP applied to the right lateral tract. In all of the above experiments, relatively few brain neurons were labeled, suggesting that, as a rule, most descending axons of these neurons do not switch tracts, at least in the rostral spinal cord.
Spinal cord tracts important for locomotor activity in whole animals
Partial spinal cord lesions
Larval sea lampreys (80–144 mm, n=100 animals) were anesthetized in tricaine methanesulphonate (~200 mg/l, MS-222, Sigma Chemical Co., St. Louis, MO). A small midline dorsal incision (~5 mm) was centered at 15% body length (BL, normalized distance from the anterior tip of the head; an average of ~7–8 mm below the obex at segment ~8), and the meninges were deflected to allow visualization of the spinal cord. Under the dissecting microscope, fine forceps and iridectomy scissors were used to make one of several types of partial spinal cord lesions (Fig. 2A): hemi-transections (n=27); medial lesions (lateral tracts spared) (n=21); lateral lesions (medial tracts spared) (n=35); or medial plus lateral lesions (“intermediate” tracts spared in a region about halfway between the midline and lateral edge of the spinal cord) (n=17). All of the different types of partial lesions encompassed the full dorsoventral thickness of the spinal cord, which is relatively flat and only ~100–150 μm thick in larval animals (Fig. 2B). The lateral edge of the medially located “gray” matter (shaded area in Fig. 2A), which is clearly visible under the dissecting microscope, was used as a reference for making the spinal cord lesions. For example, the width of the gray matter approximately corresponds to the width of the spinal cord referred to here as “medial tracts”. Hemi-transections were made on the left or right sides of the spinal cord, but for right side hemi-transections, the right–left representations of the results were “transposed” so that all data were expressed as left side lesions. Two types of lateral lesions were made: “smaller” lateral lesions that extended only slightly into the “gray” matter (“1” in Fig. 2A; n=23); and “larger” lateral lesions that extended well into the “gray” matter (“2” in Fig. 2A; n=12). Following partial spinal cord lesions, the incision was closed, and animals were returned to their home aquaria (~23 °C) to recover for seven days before first determining the behavioral capabilities of the lesioned animals and then applying HRP below the lesions to label descending brain neurons that projected in spared tracts of the spinal cord (see below). It should be noted that seven days after complete transections of the rostral spinal cord, transected axons have sealed (Davis and McClellan, 1994a), but there is very little axonal regeneration and no restoration of brain-initiated locomotor functions (Davis et al. 1993).
Video recordings and muscle activity
Normal lamprey (n=24) and experimental animals with partial spinal cord lesions (n=100) were tested in a 22 cm×40 cm plastic tank that contained 2–6 cm of aquarium water. Behavioral responses were observed as well as videotaped from above using an S-VHS camera (Panasonic PVS 770 camcorder; 30 frames/s, 8 ms shutter speed) and stored on tape. Locomotor behavior occurred spontaneously or was initiated by mild tactile stimulation or electrical stimulation (1–5 mA, 5 ms pulses at 20 Hz for 0.5 s) of the oral hood (anterior head) or tail. Animals with partial spinal cord lesions were stimulated multiple times over the course of 15–20 min, and the criteria for a positive “swimming response” was that animals had to execute at least 10 undulatory cycles as well as engage in forward progression.
For some normal lamprey (n=8) and spinal cord-lesioned animals (n=30), locomotor behavior was videotaped simultaneously with recordings of muscle activity (EMGs) (Fig. 1A), as previously described (McClellan, 1990; Davis et al., 1993). Briefly, animals were anesthetized and pairs of copper wire (56 μm dia.), insulated except at the tip, were inserted into body musculature at rostral (25% BL) and caudal (50% BL) levels of the body (Fig. 1A) to record muscle activity (Model 1700; A-M Systems, Inc., Carlsborg, WA), which was amplified (1000x), filtered (100 Hz–5 kHz), and stored on tape (Neurodata DR-890, Cygnus Technologies, Delaware Water Gap, PA; 11 kHz sampling rate per channel). Following muscle recordings, the numbers of segments between ipsilateral muscle recording electrodes were counted in order to calculate intersegmental phase lags (see below).
Analysis of locomotor activity
Locomotor muscle activity from data tapes was acquired with custom data acquisition and analysis software, and the onsets and offsets of locomotor bursts were marked (Table 1). During episodes of relatively straight swimming at approximately constant velocity, the following parameters of locomotor activity were determined for each cycle (Davis et al., 1993; McClellan and Hagevik, 1997; see Fig. 1 in Boyd and McClellan, 2002): cycle times (T) were measured as the interval between the onsets of consecutive bursts; burst proportions (BP) were calculated as the ratio of burst duration (onset-to-offset) and cycle time; intersegmental phase lags (ϕINT) were calculated as the ratio of the delay between the midpoints of ipsilateral bursts and cycle time, divided by the number of intervening body segments; and right–left phase values (ϕRT–LT) were calculated as the relative timing of the midpoint of a burst on the right within the cycle defined by the midpoints of bursts on the left. For a given animal group, the composite mean±SD was determined for each locomotor parameter (see Table 1).
Projections of descending brain neurons in whole animals
Retrograde labeling with HRP
In normal and experimental animals, the spinal cord was exposed and transected at 25% BL, which was ~10 mm caudal to partial spinal lesions in experimental animals (see above). A Gelfoam (Upjohn, Kalamazoo, MI) pledget soaked in a solution of 40% HRP (type VI; Sigma, St. Louis, MO) and 1% dimethyl sulfoxide (DMSO) in water was immediately applied between the severed ends of the spinal cord to retrogradely label descending brain neurons with axons that projected to the spinal cord. The incision was manually closed and sealed with cyanoacrylate (Super Glue Gel; Loctite Company, Rocky Hill, CT). After ~14 days to allow for retrograde transport, brains and spinal cords, including the HRP application sites, were processed for HRP, as previously described in detail (Davis and McClellan, 1994a,b; Zhang et al., 2002). Briefly the tissue was reacted en bloc at 0 °C for 8–25 min in a modified Hanker–Yates solution, consisting of 0.10% catechol, 0.05% phenylenediamine, and 0.075% H2O2 in 0.1 M Tris buffer (pH 7.4). Subsequently, the whole-mount tissue was dehydrated in an ethanol series, cleared in methyl salicylate, and mounted on slides with Permount (Fisher Scientific, Fair Lawn, NJ). The pattern of labeling of descending axons just above the partial spinal cord lesion site was used to confirm the extent of the lesions (Fig. 3).
Cell counting and classification
Using a custom computer-based marking/tracing system, the outlines of the whole-mount brains were traced, and HRP-labeled brain neurons were marked at 400x and counted in eleven cell groups (Fig. 1B; Table 2), as previously described (Davis and McClellan, 1994a,b; Zhang et al., 2002). The marking/tracing system produced two-dimensional output plots (see Fig. 1B), as if all structures and neurons were in focus simultaneously. Definitions for cell groups containing descending brain neurons (see Fig. 1B) and criteria for cell labeling were identical to those described in detail in previous studies (Davis and McClellan, 1994a,b; Zhang et al., 2002). Briefly, the minimum criteria for labeling were the presence of at least three individual HRP granules within the visible outline of a neuronal cell body (Rouse and McClellan, 1997). Because cell counting was performed with whole-mount tissue, there was very little chance of double counting neurons. Histological reconstruction of spinal lesion sites is not very useful in the lamprey because descending spinal tracts are very poorly defined (Nieuwenhuys and Nicholson, 1998), unlike those in mammals. In addition, RS neurons and other descending systems appear to project in relatively broad areas of the spinal cord, unlike those in mammals.
Table 2.
Descending brain neurons labeled in normal and lesioned animals.
| Cell groups | Normal | Hemi-transection lesion b | Lateral tracts spared | Medial tracts spared (“smaller” lateral lesionsc) | Medial tracts spared (“larger” lateral lesionsd) | Intermediate tracts spared |
|---|---|---|---|---|---|---|
| n=17 | n=19 | n=14 | n=19 | n=11 | n=11 | |
| Di (RT) | 8.2±5.1a | 1.1±1.5 | 1.1±1.7 | 4.3±3.4 | 1.0±1.8 | 3.9±3.2 |
| (LT) | 9.6±4.1 | 6.4±4.2* | 1.2±2.6 | 2.4±2.9 | 0.8±2.4 | 3.9±3.6 |
| M cells | 3.0±0.0 | 2.2±1.0* | 0.1±0.5 | 2.5±0.8 | 2.4±1.1 | 0.1±0.3 |
| 3.1±0.2 | 0.2±0.5 | 0.0±0.0 | 2.1±1.1 | 2.2±1.0 | 0.0±0.0 | |
| MRN | 26.5±7.6 | 18.2±8.7* | 2.0±2.3 | 9.2±6.9 | 11.3±8.1 | 2.4±2.5 |
| 23.8±6.9 | 0.0±0.0 | 1.4±2.5 | 10.0±.7.4 | 7.4±7.4 | 3.8±3.5 | |
| I cells | 3.9±0.2 | 3.7±0.7* | 1.5±1.3 | 1.6±1.4 | 1.7±1.3 | 1.1±1.1 |
| 3.9±0.2 | 0.0±0.0 | 1.1±1.0 | 2.0±1.4 | 1.2±1.3 | 1.3±1.0 | |
| aARRN | 27.7±8.5 | 11.6±6.4 | 12.2±5.8 | 6.3±3.7 | 4.5±8.6 | 7.8±5.8 |
| 26.3±7.1 | 13.9±3.0 | 12.7±5.0 | 4.6±3.7 | 3.3±6.2 | 5.3±3.8 | |
| lARRN | 39.1±8.6 | 6.6±5.3 | 10.5±5.6 | 13.7±9.1 | 5.5±9.6 | 11.5±6.0 |
| 37.8±9.4 | 29.0±8.5* | 13.4±8.6 | 10.5±7.6 | 7.1±11.7 | 10.1±7.9 | |
| mARRN | 21.8±5.7 | 17.2±5.5* | 11.9±5.6 | 2.0±2.8 | 1.5±3.4 | 4.6±6.1 |
| 26.8±6.8 | 6.9±8.4 | 9.9±3.4 | 3.8±3.3 | 1.6±3.4 | 5.5±4.7 | |
| B Cells | 5.0±0.0 | 4.3±0.9* | 1.5±1.1 | 2.4±1.7 | 2.8±1.5 | 0.6±1.1 |
| 4.8±0.4 | 0.2±0.5 | 1.1±0.9 | 2.5±1.4 | 2.1±1.6 | 0.4±0.8 | |
| Mau and AM | 1.9±0.4 | 0.0±0.0 | 0.4±0.7 | 0.2±0.4 | 0.3±0.7 | 0.8±0.6 |
| 2.0±0.4 | 2.0±0.0* | 0.7±0.9 | 0.2±0.4 | 0.3±0.7 | 0.5±0.5 | |
| aMRRN | 18.7±4.6 | 14.8±7.2* | 16.4±6.8 | 3.5±2.4 | 1.4±2.7 | 3.1±3.4 |
| 17.8±6.2 | 2.5±2.5 | 15.3±4.3 | 4.1±3.2 | 1.1±1.6 | 3.9±3.8 | |
| pMRRN | 37.5±7.0 | 31.3±9.0* | 21.9±6.0 | 7.1±6.2 | 6.4±6.6 | 4.4±4.1 |
| 37.1±7.8 | 0.9±1.7 | 21.8±6.9 | 6.0±3.9 | 3.6±6.4 | 6.9±4.5 | |
| PRRN | 284.4±38.0 | 275.3±44.6* | 147.9±51.2 | 81.0±53.8 | 44.7±43.1 | 77.6±43.0 |
| 290.4±64.2 | 44.7±17.3 | 133.4±29.3 | 88.8±39.3 | 46.3±57.8 | 85.3±52.3 | |
| ALV | 13.7±5.7 | 0.1±0.2 | 2.1±6.6 | 5.7±4.4 | 4.2±4.8 | 3.7±3.1 |
| 12.3±4.6 | 11.6±3.8* | 1.4±2.1 | 5.8±5.0 | 5.6±3.7 | 3.5±3.1 | |
| DLV | 24.5±9.0 | 1.6±2.6 | 0.5±0.9 | 13.6±5.8 | 9.6±7.8 | 4.7±4.5 |
| 24.8±11.5 | 14.3±8.8* | 2.6±4.0 | 11.5±8.1 | 11.2±9.0 | 5.4±5.4 | |
| PLV | 68.8±19.0 | 13.2±10.8 | 30.2±15.8 | 27.2±17.8 | 11.9±15.9 | 33.4±14.0 |
| 71.8±19.3 | 67.7±38.1* | 37.8±23.0 | 22.5±17.0 | 11.4±13.0 | 24.5±12.9 | |
| Total | 1177±159 | (RT) 401±68* | 514±138 | 357±154 | 214±209 | 317±162 |
| (LT) 200±56 |
—p≤0.05 (significantly greater labeling compared to the opposite side of the brain; t-test).
Mean±SD of labeled brain neurons; right (top number)/left (bottom) from HRP application to spinal cord at 25% BL.
Hemi-transection on left side of the spinal cord (left–right cell counts were transposed for right hemi-transections).
All animals were capable of locomotion (see spinal lesion “1” in Fig. 2A).
None of the animals were able to initiate locomotion (see spinal lesion “2” in Fig. 2A).
Diffusion controls
To ensure that significant tracer did not diffuse from the application site and be picked up by transected axons at the partial spinal cord lesion site (distance ~10 mm), the rostral spinal cord was completely transected at 10% BL, and 7 days later HRP was applied 5–10 mm caudal to the complete transection. Following histological processing, virtually no neurons were labeled in the brain (n=4; not shown), identical to the results of similar control experiments in a previous study (Davis and McClellan, 1994a). Thus, virtually all of the labeled descending brain neurons can be considered to be neurons with axons in spared parts of the spinal cord.
Effects of partial spinal cord lesions in in vitro brain/spinal cord preparations
Following the partial spinal cord lesions described above, mechanosensory inputs might contribute to the locomotor capabilities in whole animals. Therefore, in vitro brain/spinal cord preparations (Fig. 1C) were used to determine which spinal cord tracts could support the descending activation of spinal locomotor networks in the absence of mechanosensory inputs. Larval sea lampreys (92–130 mm; n=17) were anesthetized, and in vitro brain/spinal cord preparations (Fig. 1C) were set up as previously described in detail (Hagevik and McClellan, 1994; McClellan, 1994; Paggett et al., 2004). Briefly, the caudal body below the anus was discarded, and most of the body musculature was removed, except for some muscle and skin surrounding the cranium. The dorsal surface of the nervous system was exposed, and the preparation was transferred dorsal side up to a recording chamber containing cooled (6–9 °C), oxygenated lamprey Ringer’s solution (McClellan 1990) as well as 15 mg/l d-tubocurarine chloride to block possible contractions in remaining musculature around the cranium and notochord. Suction electrodes were placed on ventral roots at 20–25% BL and 40–50% BL to record spinal locomotor activity (1, 2 and 3, 4; Fig. 1C).
Pharmacological microstimulation
Spinal locomotor activity was initiated by bilaterally symmetrical pharmacological microstimulation in one of several brain locomotor areas, as previously described in detail (Jackson et al., 2005, 2007; see Fig. 1 in Paggett et al. 2004): ventromedial diencephalon (VMD); dorsolateral mesencephalon (DLM); or rostrolateral rhombencephalon (RLR). Briefly, two micropipettes were filled with a mixture of 5 mM d-glutamate and 5 mM d-aspartate in lamprey Ringer’s solution (pH=7.4), and Fast green was added to visualize the ejection bolus. The tips of the micropipettes were broken off (~2– 5 μm tip dia.) and then advanced ~25–50 μm into the dorsal surface of the brain in one of the above locomotor areas. Excitatory agents were pressure ejected (10–35 ms pulses at 1 Hz; 20 psi applied to both barrels) into brain locomotor areas, and spinal locomotor activity recorded from ventral roots was amplified (1000x), filtered (10 Hz–2 kHz), and stored on tape (Neurodata DR-890; 11 kHz sampling rate per channel). Following partial spinal cord lesions (see below), similar results were obtained for stimulation in the RLR, VMD, or DLM, suggesting that the specific brain locomotor area used to initiate spinal locomotor activity was not critical for these types of experiments.
Partial spinal cord lesions
For in vitro brain/spinal cord preparations, the following partial lesions were made in the rostral spinal cord at 15% BL with fine forceps and iridectomy scissors (Fig. 1C): hemi-transections (n=5); medial lesion (lateral tracts spared; n=7); or lateral lesions (medial tracts spared; n=5) (see Fig. 2B). Partial spinal cord lesions were performed similar to the method used in whole animals (see above). Lesions of the lateral spinal tracts were similar in extent to the “smaller” lateral lesions made in whole animals (“1” in Fig. 2A).
Analysis of locomotor patterns
In vitro locomotor activity was rectified and integrated (τ=50 ms) to better reveal burst activity, stored on tape, and later acquired using a custom data acquisition and analysis system. Locomotor parameters were calculated similar to the method used for locomotor muscle burst activity recorded from whole animals, as described above. For a given animal group, the composite mean±SD was determined for each locomotor parameter (see Table 3).
Table 3.
Parameters of locomotor activity in in vitro preparations.
| Group | T (ms) | BP | ϕINT | ϕRT–LT |
|---|---|---|---|---|
| Pre-lesion controlsa (17/355)b | ||||
| 889±337c (426–2822)d | 0.374±0.132 (all) | 0.0032±0.0024 | 0.482±0.270 (all) | |
| 0.379±0.129 (rostral) | 0.510±0.057 (rostral) | |||
| 0.369±0.136 (caudal) | 0.509±0.052 (caudal) | |||
| Hemi-transectione | ||||
| Pre-lesion (5/102) | 783±238 (426–1469) | 0.378±0.112 (right) | 0.0038±0.0019 (right) | 0.508±0.051 |
| 0.392±0.126 (left) | 0.0040±0.0020 (left) | |||
| Post-lesion (5/100) | 1208±504 (567–2410) | 0.313±0.110 (right) | 0.0019±0.0016 (right) | 0.488±0.089 |
| 0.312±0.110 (left) | 0.0049±0.0036 (left) | |||
| Lateral tracts spared | ||||
| Pre-lesion (7/141) | 889±275 (430–1584) | 0.395±0.138 (all) | 0.0025±0.0023 | 0.508±0.055 (all) |
| 0.404±0.138 (rostral) | 0.510±0.059 (rostral) | |||
| 0.386±0.139 (caudal) | 0.506±0.051 (caudal) | |||
| Post-lesion (7/139) | 1305±520 (636–2822) | 0.387±0.134 (all) | 0.0003±0.0089 | 0.498±0.092 (all) |
| 0.390±0.128 (rostral) | 0.496±0.065 (rostral) | |||
| 0.383±0.141 (caudal) | 0.500±0.116 (caudal) | |||
| Medial tracts spared | ||||
| Pre-lesion (5/112) | 987±442 (475–2492) | 0.338±0.131 | 0.0033±0.0028 | 0.512±0.056 |
| Post-lesion (5/0) | – | – | – | – |
In a given group of animals, the parameters of locomotor activity before and after partial spinal cord lesions were not significantly different (pN0.05; unpaired t-test).
Composite of all pre-lesion control data prior to making partial lesions.
n1/n2=number of animals /number of cycles.
Mean±SD.
Range of cycle times.
Hemi-transection on the left side of the spinal cord (parameters for right hemi-transections were transposed; see Methods).
Specific features of descending brain–spinal cord projections
Test for descending brain neurons with axons that branch
Descending brain neurons in certain brain cell groups were labeled when HRP was applied to the right or left sides of the spinal cord or to the medial or lateral regions of the spinal cord. Therefore, double labeling was performed to determine if individual neurons in these cell groups project in only one tract of the spinal cord or if they might have two or more branches that project in multiple spinal tracts.
Animals were anesthetized, the spinal cord was exposed at 10% BL, and one of two partial spinal cord lesions was made (Fig. 2A): hemi-transection on the left side (n=27); or medial lesion (n=11). A small pledget of Gelfoam soaked in a 6.25% solution of Alexa 488 dextran amine (Alexa, MW=10,000; Molecular Probes, Eugene, OR) or Fluorescein dextran amine (FDA, MW=3000; Molecular Probes) in 0.1 M Tris buffer was immediately applied to the partial lesion, and the incision was closed and sealed with cyanoacrylate. After 7 days for retrograde transport and sealing of severed axons (Davis and McClellan, 1994a), animals were re-anesthetized, the spinal cords were exposed at 20% BL, and a second partial lesion was made to complement the above lesions: hemi-transection on the right side; or “smaller” lateral lesions (see “1” in Fig. 2A). Small Gelfoam pledgets soaked in a 6.25% solution of Texas red dextran amine (TRDA, MW=10,000; Molecular Probes) in 0.1 M Tris buffer were immediately applied to the partial lesion sites, and the incision was closed and sealed with cyanoacrylate. The 10% BL and 20% BL tracer application sites were selected because they span the 15% BL location where partial spinal cord lesions were made in whole animals and in vitro preparations.
After an additional fourteen days for tracer transport, the brains and spinal cords were removed and processed, as previously described in detail (Zhang and McClellan, 1999). Fluorescence images were taken with a research microscope (Leitz Laborlux S) equipped with filters for fluorescence (TRDA filter: 530–580 nm excitation, 610–685 nm emission; Omega Optical, Brattleboro, VT; Alexa or FDA filter: 475–495 nm excitation, 515–545 nm emission; Chroma Technologies; Rockingham, VT) and a cooled CCD camera (Retiga 1300C; QImaging, Burnaby BC Canada). Due to the thickness of the whole-mount preparations, not all neurons were in focus simultaneously. In addition, only one excitation/emission filter set could be used at a time. Therefore, multiple images with different focal planes usually were taken of the same regions of the brain. For the purposes of illustration, the in-focus regions of the images were cut and pasted together, enhanced for contrast and brightness, false colored and merged so that both tracers were visible in the same images. The resultant images were carefully checked against the original images to ensure fidelity to the experimental findings.
Test for descending axons that switch tracts or cross the midline
First, in one group of animals, one type of partial spinal lesion was made at 15% BL, and a “complementary” partial lesion was made at 20% BL, which together encompassed the entire width of the spinal cord (see diagrams on right side of Fig. 10): rostral right plus caudal left hemi-transections (n=6); rostral medial plus caudal lateral lesions (n=6); or rostral lateral plus caudal medial lesions (n=9). Seven days later, after transected axons had sealed, HRP was applied to the spinal cord at 25% BL. After ~14 days for HRP transport, the brains were removed and processed for HRP (Davis and McClellan, 1994a; Zhang et al., 2002) to determine if axons from descending brain neurons switch tracts or if they remain in one area of the spinal cord.
Second, in another group of animals, partial lesions were made in the rostral spinal cord at 10% BL to interrupt the following regions of cord: ~75% of the spinal cord from the right side (Fig. 11A, B, n=10); the medial spinal cord tracts (Fig. 11C, n=17); or the lateral spinal tracts on the right side (Fig. 11D, n=7). After seven days to allow lesioned axons to seal, the spinal cord was exposed from 15% to 20% BL, and a longitudinal lesion was made with a microdissection blade (Beaver “mini-blade” #376500; Arista Surgical Supply, New York, NY) either at the midline (Fig. 11A, C) or along a sagittal plane approximately halfway between the midline and right edge of the cord (Fig. 11B, D). A Gelfoam pledget soaked in an HRP solution (see above) was immediately applied to the longitudinal spinal lesion, the incision was closed and sealed with cyanoacrylate, and animals were returned to their home aquaria. After ~14 days to allow transport of HRP, the brains were removed and histologically processed for HRP, as previously described in detail (Davis and McClellan, 1994a; Zhang et al., 2002). The purpose of the initial partial spinal cord lesion at 10% BL was to prevent direct retrograde labeling of descending brain neurons that projected through the HRP-application site but whose axons did not cross the midline. The above technique should only label descending brain neurons with axons or axonal branches that cross the midline in the rostral cord between 15% and 20% BL.
Results
Muscle activity and labeled descending brain neurons in whole animals
Normal animals
In normal larval lampreys, tactile stimulation of the oral hood usually initiated an escape response, consisting of a body flexure away from the stimulus, followed by locomotor movements, as previously described (McClellan 1984, 1990). During locomotion, body undulations propagated toward the tail with increasing amplitude (Davis et al., 1993), and locomotor muscle activity was characterized by two features (Fig. 4A1): left–right alternation of burst activity at the same segmental level (1↔2 and 3↔4); and rostrocaudal phase lag of ipsilateral burst activity (1→4 and 2→3). Cycle times (T), burst proportions (BP), intersegmental phase lags (ϕINT), and right–left phase values (ϕRT–LT) (Table 1; n=8) were similar to those previously described for larval (McClellan, 1990; Davis et al., 1993; McClellan and Hagevik, 1997) and adult lampreys (Wallén and Williams, 1984; McClellan, 1984).
In normal larval lamprey, application of HRP to the spinal cord at 25% BL (n=17) labeled an average of 1177 brain neurons in eleven cell groups (Figs. 1B, 4A2; Table 2). For each cell group, the numbers of labeled neurons on the left and right sides of the brain were not significantly different, indicative of relatively symmetrical labeling (Table 2; p>0.05, t-test). Reticulospinal (RS) neurons were labeled in the mesencephalic reticular nucleus (MRN), as well as the anterior (aARRN, lARRN, and mARRN), middle (aMRRN and pMRRN), and posterior (PRRN) rhombencephalic reticular nuclei (see Fig. 1B) (Davis and McClellan, 1994a,b). Neurons in the MRN, ARRN, MRRN, and PRRN accounted for ~4%, ~15%, ~9%, and ~49%, respectively, of the total numbers of labeled descending brain neurons (Table 2). Other descending brain neurons were labeled in the diencephalic (Di) and three rhombencephalic cell groups (ALV, DLV, PLV). In the present study, the numbers of labeled descending brain neurons projecting to 25% BL were not significantly different from those described in larval lamprey for HRP application to the spinal cord at 20% BL (p>0.05; ANOVA) (Davis and McClellan, 1994a; Zhang et al., 2002). Müller and Mauthner cells, which are large, identified RS neurons, were labeled in three reticular nuclei, as previously shown (Rovainen, 1978; Davis and McClellan, 1994b): M cells (M1–M3) in the MRN; I cells (I1–I4) in the ARRN; and B cells (B1–B5), Mauthner (Mau) cells, and auxillary Mauthner (AM) cells in the MRRN (Figs. 1B, 4A2).
Hemi-transections
Animals with hemi-transections of the rostral spinal cord at 15% BL (Fig. 4B1; n=9) were still able to produce flexure responses and locomotor movements in response to stimulation of the oral hood. However, during flexure responses, animals appeared to have difficulty turning toward the side of the hemi-transection, and during locomotion, animals sometimes spiraled in a direction away from the lesioned side of the cord. Presumably these deficits were due to weaker descending drive to locomotor networks on the lesioned side of the spinal cord that resulted in an imbalance of the force vectors generated on the two sides of the body (McClellan and Hagevik, 1997). Nonetheless, the pattern of locomotor muscle activity generated by these animals was relatively normal and displayed left–right alternation (1↔2 and 3↔4) and a rostrocaudal phase lag (1→4 and 2→ 3) (Fig. 4B1). The parameters of locomotor activity were not significantly different from those during swimming in normal whole animals (p>0.05, ANOVA) (Table 1).
Application of HRP to the spinal cord at 25% BL, ~10 mm caudal to hemi-transections (n=19), labeled descending brain neurons in both ipsilateral and contralateral cell groups (Fig. 4B2, Table 2; also see Fig. 3B). Brain neurons labeled ipsilateral to the spared half of the spinal cord and with non-decussating descending axons included all Müller cells. However, since descending axons of M cells in the MRN (M1–M3, see Fig. 1B) are very close to the midline (Rovainen et al., 1973), following hemi-transections these neurons were sometimes labeled on both sides of the brain or on neither side of the brain (Fig. 4B), presumably because of slight variations in the extent of the hemi-transections. In the MRN, mARRN, MRRN, and PRRN cell groups, labeling was significantly greater ipsilateral to the spared half of the spinal cord (p≤0.05, paired t-test), while in the Di, lARRN, ALV, DLV, and PLV cell groups, labeling was significantly greater contralateral to the spared half of the cord (p≤0.05, paired t-test). The aARRN had approximately equal numbers of labeled neurons with ipsilateral and contralateral projections (see Fig. 4B2 and Table 2). On average, ~33% of descending brain neurons had crossed descending axons, and neurons in the ARRN, PRRN, and PLV accounted for ~25%, ~22%, and ~34%, respectively, of the total numbers of neurons with contralateral descending projections.
Lateral spinal tracts spared
All animals with spared lateral tracts in the rostral spinal cord (Fig. 5A1; n=8) were able to initiate and maintain locomotion, spontaneously as well as in response to stimulation of the oral hood or tail. The locomotor pattern consisted of the typical left–right alternation and a rostrocaudal phase lag (Fig. 5A1), and the parameters of locomotor activity were not significantly different from those in normal animals (Table 1). These results suggest that the lateral spinal tracts are important for conveying descending drive from the brain to the spinal locomotor networks and that some of the descending brain neurons that project in these tracts are the output elements of the locomotor command system. Furthermore, the results confirm previous data (McClellan, 1988) that Müller cells, which are large, identified RS neurons with descending axons mostly in the medial spinal tracts (Fig. 1B) (Rovainen et al. 1973), are not necessary for the initiation of locomotion, at least under the present experimental conditions (see Discussion).
Fig. 5.
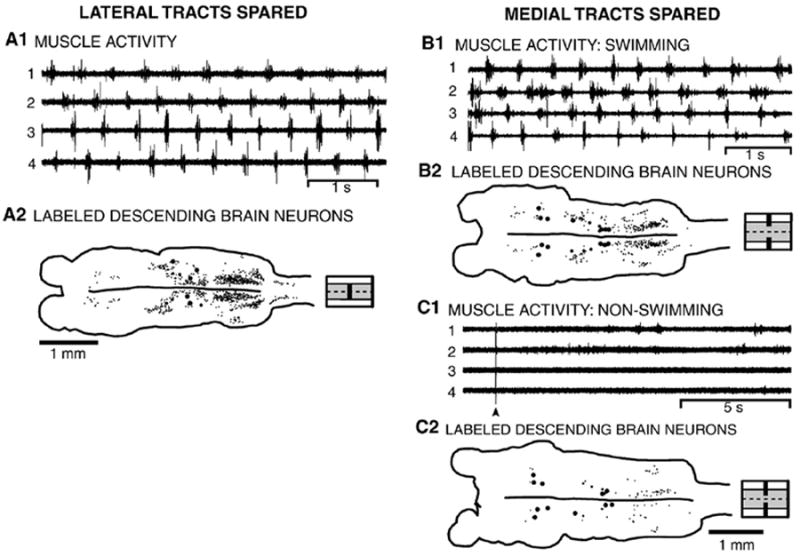
(A) Whole animal in which the lateral spinal tracts were spared (see Fig. 2A). (A1) Locomotor muscle burst activity consisted of left–right alternation and a rostrocaudal phase lag (see Table 1). (A2) Brain (left) and rostral spinal cord showing labeled descending brain neurons resulting from application of HRP to the spinal cord at 25% BL, caudal to a medial spinal lesion at 15% BL (diagram to right shows partial spinal lesion). (B, C) Whole animal in which the medial spinal tracts were spared (see Fig. 2A). (B1) For animals with the “smaller” lateral lesions (“1” in Fig. 2A), locomotor activity consisted of left–right alternation and a rostrocaudal phase lag (see Table 1). (B2) Brain (left) and rostral spinal cord showing labeled descending brain neurons resulting from application of HRP to the spinal cord at 25% BL, caudal to lateral lesions at 15% BL (diagram to right shows partial spinal lesion). (C1) For animals with the “larger” lateral lesions (“2” in Fig. 2A), brief electrical stimulation of the oral hood (arrowhead) did not initiate locomotion (channel gains were increased by 2x to show lack of locomotor burst activity). (C2) Mostly Müller cells and some scattered neurons in the PRRN and a few other cell groups were retrogradely labeled (diagram to right shows partial spinal lesion).
Application of HRP to the spinal cord caudal to lesions of the medial tracts in the rostral cord (n=14) resulted in labeling of ~44% of the numbers of descending brain neurons in normal animals (Fig. 5A2; also see Fig. 3C). In the ARRN, MRRN, PRRN, and PLV, ~40–70% of the normal numbers of neurons were labeled (Fig. 5A2; Table 2), indicating that many of the neurons in these cell groups have descending axons in the lateral spinal tracts. Also, ~85% of the total numbers of neurons projecting in the lateral tracts were located in the ARRN, MRRN, and PRRN. The numbers of left and right labeled descending brain neurons were not significantly different (p>0.05, t-test; not shown), indicative of symmetrical labeling.
Medial spinal tracts spared
The behavioral capabilities of animals with the medial spinal tracts spared in the rostral cord depended on the extent of the partial lesions. In animals with the “smaller” lateral lesions that extended only slightly into the “gray matter” (see “1” in Fig. 2A), locomotor behavior still occurred in all animals (Fig. 5B1; n=23), and the parameters of locomotor activity were not significantly different from those in normal animals (Table 1; n=5), except that cycle times were significantly longer than in normal animals (p≤0.05, ANOVA, Dunnett multiple comparison post-test). In contrast, for the “larger” lateral lesions that extended well into the “gray matter” (see “2” in Fig. 2A), locomotion was abolished in all animals (Fig. 5C1; n=12).
The numbers of descending brain neurons that were retrogradely labeled following application of HRP also depended on the extent of the lateral lesions. In animals with the “smaller” lesions of the lateral tracts (n=19), ~30% of the normal numbers of descending brain neurons were labeled (Fig. 5B2; also see Fig. 3D1). Usually, at least ~50% of Müller cells were labeled (M, I, and B cells; see Fig. 1B), and ~30–50% of the normal numbers of neurons in the Di, MRN, lARRN, PRRN, ALV, DLV, and PLV were labeled (Fig. 5B2; Table 2).
In contrast, application of HRP caudal to the “larger” lesions of the lateral tracts (n=11) resulted in labeling of ~18% of the normal number of descending brain neurons (Fig. 5C2; also see Fig. 3D2). Slightly fewer Müller cells were labeled than with the “smaller” lateral spinal lesions, ~30–50% of the normal numbers of neurons in the MRN, ALV, and DLV were labeled, and less than 20% of the neurons were labeled in the remaining cell groups (Fig. 5C2; Table 2). The numbers of labeled neurons in the MRN, ALV and DLV were only slightly less than for the “smaller” lateral lesions, suggesting that descending brain neurons in these cell groups project their axons near the midline in the medial spinal tracts. In contrast, many of the neurons in the Di, ARRN, MRRN, PRRN, and PLV appeared to have projections in the more lateral parts of the medial spinal tracts, which correspond to the intermediate tracts (see below). For either the “smaller” or “larger” lateral tract lesions, the numbers of left and right labeled descending brain neurons were not significantly different (p>0.05, paired t-test; not shown). It is important to note that the numbers of labeled descending brain neurons for lateral and medial lesions do not add up to the ~1200 neurons that project their axons to 25% BL (Table 2) because these lesions partially overlapped (Fig. 2A).
Intermediate spinal tracts spared
For lesions that spared the intermediate tracts of the spinal cord, the medial and lateral lesions were less extensive than those described above (see Fig. 2A). All animals with spared intermediate spinal cord tracts were able to initiate and generate locomotor behavior (n=16), and the parameters of locomotor muscle activity (Fig. 6A) were not significantly different from those in normal animals (Table 1; n=6), except that cycle times were significantly longer than in normal animals (p≤0.05, ANOVA, Dunnett multiple comparison post-test).
Application of HRP caudal to lesions that spare the intermediate tracts in the rostral spinal cord (n=11; Fig. 3E) resulted in symmetrical labeling of ~27% of the number of descending brain neurons in normal animals (Table 2). Less than 10% of the normal numbers of M and B cells were labeled, while ~30% of I cells were labeled. In the Di, ARRN, PRRN, ALV, and PLV (Fig. 6B), ~25–45% of the normal numbers of neurons were labeled, while less than ~20% of the normal numbers were labeled in the remaining cell groups. Thus, many of the cell groups with brain neurons that project primarily in the lateral tracts (ARRN, PRRN, and PLV) or primarily in the medial tracts (Di and ALV) also contain substantial numbers of neurons with projections in intermediate parts of the spinal cord.
In vitro brain/spinal cord preparations
In whole animals with partial lesions of the rostral spinal cord, mechanosensory inputs were operational and might have contributed to the locomotor capabilities of these animals. Therefore, in in vitro brain/spinal cord preparations, in which mechanosensory inputs were eliminated (see Methods), locomotor activity was analyzed before and after making partial lesions in the rostral spinal cord at 15% BL.
Control recordings
Prior to making partial spinal lesions for in vitro brain/spinal cord preparations (n=17), pharmacological microstimulation in brain locomotor areas initiated well-coordinated spinal locomotor activity (Figs. 7A, 8A1, B1; Table 3) with features similar to those for locomotor muscle burst activity observed in whole animals (Table 1): left–right alternation of burst activity at the same segmental level (1↔2 and 3↔4); and a rostrocaudal phase lag of ipsilateral burst activity (1→4 and 2→3). However, for in vitro locomotor activity, intersegmental phase lags were significantly smaller (p=0.0023; unpaired t-test) and cycle times were significantly longer (p=0.0001; unpaired t-test with Welsh correction) than in whole animals (McClellan, 1994; Hagevik and McClellan, 1994), suggesting that mechanosensory inputs normally contribute to coordination of locomotor activity and the overall excitability of the locomotor networks, respectively.
Hemi-transections
Brain-initiated in vitro locomotor activity was similar before (Fig. 7A) and after (Fig. 7B) hemi-transections of the rostral spinal cord at 15% BL (n=5). The parameters of in vitro locomotor activity after spinal cord hemi-transections were not significantly different from those for control activity (Table 3; ANOVA).
Lesions that spared the lateral spinal tracts
In in vitro brain/spinal preparations (n=7), locomotor activity was similar before (Fig. 8A1) and after (Fig. 8A2) lesions of the medial tracts in the rostral spinal cord. In particular, the parameters of in vitro locomotor activity after the lesions were not significantly different from those for control activity (Table 3; ANOVA).
Lesions that spared the medial spinal tracts
Brain-initiated control locomotor activity prior to performing spinal lesions (Fig. 8B1) usually was abolished after lesions of the lateral spinal tracts (Fig. 8B2; n=5). In one preparation, locomotor activity was present but attenuated after making lesions of the lateral tracts that only encompassed the “white” matter, but this activity was abolished after the lesions were extended slightly into the “gray” matter (see “1” in Fig. 2A).
Specific features of descending brain–spinal cord axonal projections
Test for descending brain neurons with axons that branch
Double labeling was performed to determine if individual descending brain neurons in certain cell groups project their axons in only one region of the spinal cord or if they might have two or more branches that project in multiple spinal tracts. First, TRDA was applied to the right half of the spinal cord at 20% BL and Alexa or FDA was applied to the left half of the cord at 10% BL to determine if some individual descending brain neurons project their axons in both ipsilateral and contralateral spinal tracts (n=27). Under these conditions, double-labeled descending brain neurons were not observed in any of the cell groups (Fig. 9A), suggesting that, as a rule, individual neurons project either ipsilaterally or contralaterally, at least in the rostral spinal cord.
Second, TRDA was applied to the lateral spinal tracts at 20% BL and Alexa or FDA was applied to the medial tracts at 10% BL to determine if individual descending brain neurons had branched axons that projected in multiple areas of the cord (n=11). Again, no double-labeled neurons were observed (Fig. 9B), suggesting that the axons of individual descending brain neurons project either medially or laterally, at least in the rostral spinal cord.
Test for descending axons that switch tracts
Results from the HRP retrograde labeling experiments did not indicate whether axons of descending brain neurons remain in the same spinal cord tracts or might switch tracts during their descending trajectories. To investigate this aspect of descending projections, several types of lesions and HRP application protocols were used. Specifically, HRP was applied to the spinal cord caudal to a pair of “complementary partial lesions” of the spinal cord at 15% BL and 20% BL (see Methods; Fig. 10, right): rostral right plus caudal left hemi-transections (n=6); rostral medial plus caudal lateral lesions (n=6); or rostral lateral plus caudal medial lesions (n=9). None of the animals with these types of complementary lesions were able to initiate locomotor movements (data not shown), and an average of 2% or less of the total numbers of descending brain neurons were retrogradely labeled (Fig. 10, left). Thus, most descending brain neurons have axons that appear to be relatively straight and do not switch tracts in their descending trajectories, at least in the rostral spinal cord.
Test for descending brain neurons with axons that cross the midline
Several experimental manipulations were performed to determine if axons of descending brain neurons cross the midline in the rostral spinal cord (Fig. 11, right side; see Methods; n=34). A partial transverse lesion was made at 10% BL, and 7 days later, HRP was applied longitudinally from 15→20% BL in the spinal cord, either along the midline or more laterally. The partial transverse lesions prevented labeling of descending brain neurons that projected directly to the tracer application site. Thus, any labeled descending brain neurons presumably had axons or branches that crossed the midline. For the various manipulations performed, an average of 3% or less of the total numbers of descending brain neurons were labeled (Fig. 11, left). Thus, most descending brain neurons have relatively straight axons that do not cross the midline, at least in the rostral spinal cord.
Discussion
Experimental considerations
In whole animals and in vitro preparations from larval lamprey, partial lesions were made in the rostral spinal cord, and the locomotor capabilities were determined. In addition, in whole animals descending brain neurons that projected in spared parts of the spinal cord were retrogradely labeled. There are several potential considerations regarding these approaches. First, because of possible surgical trauma in the vicinity of the lesion site, descending axons in addition to those directly lesioned might have been compromised. This probably is not a significant factor, because for a particular partial spinal lesion, the behavioral capabilities (Figs. 4-6) and labeling of spared axons above the partial lesions (Fig. 3) were relatively consistent between animals. In addition, in a previous study, the extent of the lesions under the dissecting microscope was similar to that in histological sections (McClellan, 1988). Second, ischemia near the lesion site potentially might have compromised unlesioned axons, but this probably was not a significant problem, because the lamprey spinal cord receives oxygen and nutrients via diffusion from the cerebrospinal fluid (Rovainen, 1979). Third, there are very few descending propriospinal neurons in the very rostral spinal cord that might have contributed to the initiation of locomotor activity caudal to the lesion (Rouse and McClellan, 1997). Fourth, although part of the PLV extends into the very rostral spinal cord (Fig. 1B), stimulation lateral to the PRRN, in the region of the PLV, does not initiate spinal locomotor activity (Paggett et al., 2004; Jackson et al., 2007). Fifth, activation of trigeminal afferents can initiate locomotor behavior (McClellan, 1984), and some of these afferents project their axons to the very rostral spinal cord (Calton et al., 1998) and potentially might activate spinal CPGs. However, the behavioral capabilities of animals with partial spinal cord lesions were very similar for locomotion that occurred spontaneously or was elicited by tactile stimulation of the head or tail. In conclusion, the locomotor capabilities of animals with partial lesions of the rostral spinal cord very likely were due to axons from descending brain neurons that projected in spared parts of the spinal cord.
Descending brain–spinal cord projections in larval lamprey
Spinal cord tracts involved in the initiation of locomotion
In the present study, all larval lampreys with spared lateral tracts or spared intermediate spinal tracts (Figs. 3C, E) were able to initiate and generate relatively normal locomotor movements and locomotor activity, suggesting that descending axons in these spinal tracts are important for the initiation of locomotor behavior. Similar results were obtained in adult lamprey when partial spinal lesions were made slightly more rostrally than in the present study (McClellan, 1988; Ullen et al., 1997). First, the present results confirm and extend previous studies suggesting that the medial spinal tracts are not critical for the initiation of locomotion (McClellan, 1988) (see below). Second, ~85% of the neurons that projected in the lateral/intermediate spinal tracts were RS neurons in the ARRN, MRRN, and PRRN (Table 2; Fig. 12). In addition, in larval lamprey bilaterally symmetrical pharmacological microstimulation in these reticular nuclei can initiate locomotor patterns in in vitro brain/spinal cord preparations (Hagevik et al. 1996; Hinton and McClellan, 1997) and semi-intact preparations (Jackson et al., 2007). Thus, output neurons of the locomotor command system are distributed in several reticular nuclei. Third, RS neurons in the above nuclei project in relatively wide areas of the spinal cord, including both dorsal and ventral spinal tracts (McClellan, 1988). In contrast, in mammals, RS neurons for locomotion are located in medullary reticular nuclei and project primarily in the ventral or ventrolateral spinal tracts (Jordan, 1998; Jordan et al., 2008). Unfortunately, in the lamprey, descending spinal tracts are not well-defined (Nieuwenhuys and Nicholson, 1998). In studies with adult lamprey, stimulation of reticular nuclei or individual RS neurons can sometimes speed up an on-going spinal locomotor rhythm that is activated by pharmacological agents applied to the spinal cord (Wannier et al. 1998; Zelenin et al., 2001), but it is unclear how relevant this result is with regard to initiation of swimming from the brain.
Fig. 12.
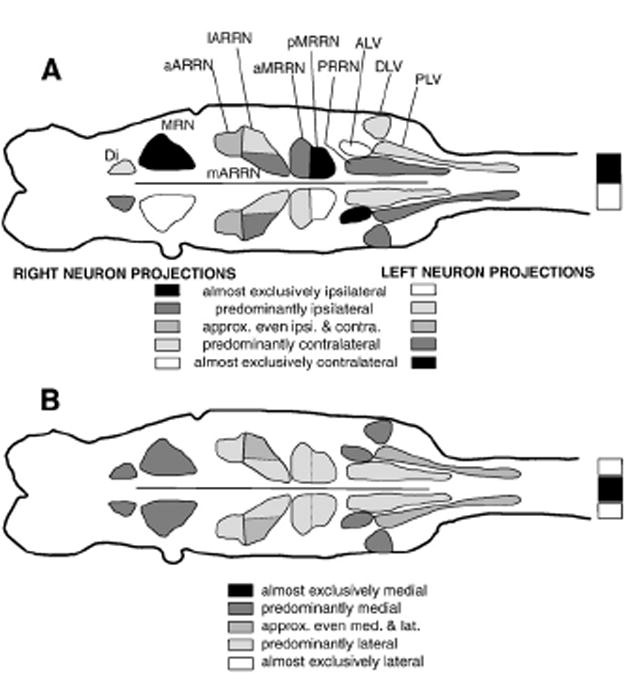
Descending brain–spinal cord projections in larval lamprey. (A) Summary of cell groups of descending brain neurons with ipsilateral and contralateral spinal projections (see below the brain diagram for code for shading of cell groups). (B) Summary of cell groups of descending brain neurons with medial (darker shades) and lateral (lighter shades) spinal projections (see below the brain diagram for code for shading of cell groups).
In general, the results from in vitro preparations supported those from whole animals, with one exception. For in vitro preparations, lesions of the lateral spinal tracts that abolished locomotor activity (“smaller” lesion “1” in Fig. 2A) were less extensive than those that were necessary to reliably abolish locomotor activity in whole animals (“larger” lesion “2” in Fig. 2A). In whole animals that displayed locomotor behavior following the “smaller” lesions of the lateral tracts, mechanosensory inputs might have contributed to the locomotor capabilities. In addition, in whole animals the average frequencies of locomotor burst activity, and presumably the CNS excitability, are higher than for in vitro brain/spinal cord preparations.
Why are some whole animals with lesions of the lateral spinal tracts able to locomote? Both lateral and intermediate spinal tracts support brain-initiated locomotor behavior. Therefore, “smaller” lesions of the lateral spinal tracts that mostly encompass just the “white” matter (“1” in Fig. 2A) still spare substantial numbers of axons from RS neurons that project in intermediate regions of the spinal cord and that can activate spinal locomotor networks.
Role of Müller and Mauthner cells
First, although Müller cells (M, I, and B cells; Fig. 1B) are sometimes active during swimming (Deliagina et al. 2000; Zelenin 2005), these cells probably are not critical for the initiation of locomotor behavior (McClellan, 1988, and present study). For example, these neurons have descending axons mostly in the ventromedial parts of the rostral spinal tracts (Rovainen et al., 1973), and following medial tract lesions, animals could still initiate relatively normal locomotion, even when the axon of only one Müller cell was spared. However, in the present study, the full range of locomotor capabilities of animals with partial spinal cord lesions was not explored. Thus, it is possible that these large RS neurons might contribute to some aspects of locomotion, such as rapid changes in speed, fast turns, or quick postural adjustments (e.g. Deliagina et al., 2000; Zelenin 2005). Second, the functions of the Mauthner cells, whose axons descend in the intermediate spinal tracts (Rovainen et al., 1973), are unclear because stimulation of this cell in semi-intact preparations does not elicit a clear behavioral response of the body (Rovainen, 1967; however, see Currie and Carlsen 1987).
Comparison to other vertebrates
In a large variety of vertebrates, locomotion can be elicited by stimulation of similar higher-order locomotor regions: lateral hypothalamus; SLR; MLR; PLS; and CLR (see Introduction). These higher-order locomotor areas do not appear to directly activate spinal CPGs, since lesions or blocking activity “downstream” in medial reticular nuclei can abolish activity initiated by certain higher locomotor centers (Shefchyk et al., 1984; Garcia-Rill and Skinner, 1987a; Bernau et al., 1991; Noga et al., 1991). In addition, direct stimulation in reticular nuclei elicits spinal locomotor activity (Noga et al., 1988; Bernau et al., 1991), suggesting that RS neurons are the main output neural elements of the command system.
In most vertebrates, relatively discrete areas of the spinal cord, including the ventral and/or ventrolateral spinal tracts, are important for initiation of locomotion. For example, lesions of these spinal tracts in monkey (Eidelberg et al. 1981b), cat (Windle et al., 1958; Afelt 1974; Steeves and Jordan, 1980; Eidelberg et al. 1981a; Noga et al. 1991), rat (Schucht et al., 2002), bird (Sholomenko and Steeves, 1987), and stingray (Williams et al., 1984) abolish the initiation of locomotion, or raise the threshold for initiating locomotion (Marlinskii and Voitenko, 1992) (reviewed in Eidelberg, 1981; McClellan, 1986; Rossignol et al., 1996, 1999). In addition, stimulation of these tracts can initiate spinal locomotor activity (Jacobson and Holliday, 1982; Yamaguchi, 1986). However, more recent evidence suggests that the DLF may also contribute to over-ground locomotion (Yamaguchi, 1986; Bem et al., 1995; Loy et al., 2002b; also see Stein, 1978; Loy et al., 2002a). In addition, descending pathways via the gray matter may also be important (Shik, 1983; Magnuson et al., 1999; also see Cowley et al., 2008). Finally, following lesions of the ventrolateral or lateral tracts in the caudal thoracic cord in mammals, the locomotor system can adapt after relatively long recovery times and lead to some return of voluntary locomotor function (reviewed in Rossignol et al., 1996, 1999).
Conclusions
Relatively wide areas of the lamprey spinal cord, including lateral and intermediate spinal cord tracts, are important for the initiation of locomotor behavior. The RS neurons with descending axons in these tracts are located in multiple reticular nuclei, including the ARRN, MRRN and PRRN (Fig. 12). The medial spinal tracts, which contain most of the descending axons of large, identified Müller cells, are neither necessary nor generally sufficient for initiation of locomotion. Following spinal cord injury in larval lamprey, injured descending axons regenerate and behavioral functions recover within a few weeks (reviewed in McClellan, 1998; Selzer, 2003). The present results form the basis for examining whether restoration of specific descending brain–spinal cord projections is important for behavioral recovery in spinal cord-transected lamprey.
Acknowledgments
Thanks are extended to Janelle Johns, Sarah McBee, Amy Oxner, and Paul Hayter for technical assistance and/or providing preliminary data.
Grant support: Supported by NIH grant NS29043, NSF grant IBN 9817605, and American Paralysis Association grant MA1-9605 awarded to A.D.M. T.H., and S.T. were supported by the Howard Hughes Internship Program at the University of Missouri-Columbia.
Abbreviations
- Alexa
Alexa 488 dextran amine
- ALV
anterolateral vagal group
- AM
auxillary Mauthner cells
- ARRN
anterior rhombencephalic reticular nucleus
- aARRN
anterior division of ARRN
- aMRRN
anterior division of MRRN
- BL
body length
- Di
diencephalic group
- DLF
dorsolateral funiculus
- DLM
dorsolateral mesencephalon (brain locomotor area)
- DLV
dorsolateral vagal group
- FDA
fluorescein dextran amine
- HRP
horseradish peroxidase
- lARRN
lateral division of ARRN
- mARRN
medial division of ARRN
- Mau
Mauthner cells
- MLR
mesencephalic locomotor region (brain locomotor area)
- MRN
mesencephalic reticular nucleus
- MRRN
middle rhombencephalic reticular nucleus
- PLV
posterolateral vagal group
- pMRRN
posterior division of MRRN
- PLS
pontomedullary locomotor strip
- PON
posterior octavomotor nucleus
- PRRN
posterior rhombencephalic reticular nucleus
- RLR
rostrolateral rhombencephalon (brain locomotor area)
- RS
reticulospinal
- SLR
subthalamic locomotor region
- TRDA
Texas red dextran amine
- VLF
ventrolateral funiculus
- VMD
ventromedial diencephalon (brain locomotor area)
References
- Afelt Z. Functional significance of ventral descending tracts of the spinal cord of the cat. Acta Neurobiol Exp. 1974;34:393–407. [PubMed] [Google Scholar]
- Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem T, Gorska T, Majczynski H, Zmyslowski W. Different patterns of fore– hindlimb coordination during overground locomotion in cats with ventral and lateral spinal lesions. Exp Brain Res. 1995;104:70–80. doi: 10.1007/BF00229856. [DOI] [PubMed] [Google Scholar]
- Bernau NA, Puzdrowski RL, Leonard RB. Identification of the midbrain locomotor region and its relation to descending locomotor pathways in the Atlantic stingray, Dasyatis sabina. Brain Res. 1991;557:83–94. doi: 10.1016/0006-8993(91)90119-g. [DOI] [PubMed] [Google Scholar]
- Boyd M, McClellan AD. Variations in locomotor activity parameters versus cycle time in larval lamprey. J Exp Biol. 2002;23:3707–3716. doi: 10.1242/jeb.205.23.3707. [DOI] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in cat. I. Deficits and adaptive mechanisms. J Neurophysiol. 1998;80:1245–1267. doi: 10.1152/jn.1998.80.3.1245. [DOI] [PubMed] [Google Scholar]
- Calton JL, Philbrick K, McClellan AD. Anatomical regeneration and behavioral recovery following crush injury of the trigeminal root in lamprey. J Comp Neurol. 1998;396:322–337. [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, Schmidt BJ. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol. 2008;586:1623–1635. doi: 10.1113/jphysiol.2007.148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SN, Carlsen RC. Functional significance and neural basis of larval lamprey startle behaviour. J Exp Biol. 1987;133:121–135. doi: 10.1242/jeb.133.1.121. [DOI] [PubMed] [Google Scholar]
- Davis GR, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal-transected lamprey. J Comp Neurol. 1994a;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- Davis GR, McClellan AD. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp Neurol. 1994b;127:94–105. doi: 10.1006/exnr.1994.1083. [DOI] [PubMed] [Google Scholar]
- Davis GR, Troxel MT, Kohler VJ, Grossmann EM, McClellan AD. Time course of locomotor recovery and functional regeneration in spinal-transected lamprey: kinematics and electromyography. Exp Brain Res. 1993;97:83–95. doi: 10.1007/BF00228819. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Fagerstedt P, Grillner S, Orlovsky GN. Activity of reticulospinal neurons during locomotion in the freely behaving lamprey. J Neurophysiol. 2000;83:853–863. doi: 10.1152/jn.2000.83.2.853. [DOI] [PubMed] [Google Scholar]
- Eidelberg E. Consequences of spinal cord lesions upon motor function, with special reference to locomotor activity. Prog Neurobiol. 1981;17:185–202. doi: 10.1016/0301-0082(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Story JL, Walden JG, Meyer BL. Anatomical correlates of return of locomotor function after partial spinal cord lesions in cats. Exp Brain Res. 1981a;42:81–88. doi: 10.1007/BF00235732. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Walden JG, Nguyen H. Locomotor control in macaque monkeys. Brain. 1981b;104:647–663. doi: 10.1093/brain/104.4.647-a. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987a;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987b;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Conrad C, Mosley D, Campbell C. Projections of the mesencephalic locomotor region in the rat. Brain Res Bull. 1986;17:33–40. doi: 10.1016/0361-9230(86)90158-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Gilmore RD, Owings R. Connections of the mesencephalic locomotor region (MLR): II. Afferents and efferents. Brain Res Dev. 1983;10:63–71. doi: 10.1016/0361-9230(83)90076-x. [DOI] [PubMed] [Google Scholar]
- Gorska T, Bem T, Majczynski H. Locomotion in cats with ventral spinal lesions: support patterns and duration of support phases during unrestrained walking. Acta Neurobiol Exp. 1990;50:191–200. [PubMed] [Google Scholar]
- Gorska T, Bem T, Majczynski H, Zmyslowski W. Unrestrained walking in cats with partial spinal lesions. Brain Res Bull. 1993a;32:241–249. doi: 10.1016/0361-9230(93)90183-c. [DOI] [PubMed] [Google Scholar]
- Gorska T, Majczynski H, Bem T, Zmyslowski W. Hindlimb swing, stance and step relationships during unrestrained walking in cats with lateral funicular lesion. Acta Neurobiol Exp. 1993b;53:133–142. [PubMed] [Google Scholar]
- Gorska T, Ioffe M, Zmyslowski W, Bem T, Majczynski H, Mats VN. Unrestrained walking in cats with medial pontine reticular lesions. Brain Res Bull. 1995;38:297–304. doi: 10.1016/0361-9230(95)00102-k. [DOI] [PubMed] [Google Scholar]
- Gorska T, Bem T, Majczynski H, Zmyslowski W. Different forms of impairment of the fore–hindlimb coordination after partial spinal lesions in cats. Acta Neurobiol Exp. 1996;56:177–188. doi: 10.55782/ane-1996-1119. [DOI] [PubMed] [Google Scholar]
- Grillner S. Some aspects on the descending control of the spinal circuits generating locomotor movements. In: Herman M, Grillner S, Stein P, Stuart D, editors. Neural Control of Locomotion. Plenum; 1976. pp. 351–375. [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Hagevik A, McClellan AD. Coupling of spinal locomotor networks in larval lamprey revealed by receptor blockers for inhibitory amino acids: Neurophysiology and computer modeling. J Neurophysiol. 1994;72:1810–1829. doi: 10.1152/jn.1994.72.4.1810. [DOI] [PubMed] [Google Scholar]
- Hagevik A, Oxner-McGaha A, McClellan AD. Chemical microstimulation in brain locomotor regions in larval lamprey. Soc Neurosci Abst. 1996;22:1372. [Google Scholar]
- Harris RM, Little JW, Goldstein B. Spared descending pathways mediate locomotor recovery after subtotal spinal cord injury. Neurosci Lett. 1994;180:37–40. doi: 10.1016/0304-3940(94)90908-3. [DOI] [PubMed] [Google Scholar]
- Hinton P, McClellan AD. Chemical microstimulation in brain locomotor regions in larval lamprey: non-excitatory amino acids. Soc Neurosci Abst. 1997;23:205. [Google Scholar]
- Jackson AW, Horinek D, Boyd M, McClellan AD. Disruption of left–right reciprocal coupling in the spinal cord of larval lamprey abolishes brain-initiated locomotor activity. J Neurophysiol. 2005;94:2031–2044. doi: 10.1152/jn.00039.2005. [DOI] [PubMed] [Google Scholar]
- Jackson AW, Pino FA, Wiebe ED, McClellan AD. Movements and muscle activity initiated by brain locomotor areas in semi-intact preparations from larval lamprey. J Neurophysiol. 2007;97:3229–3241. doi: 10.1152/jn.00967.2006. [DOI] [PubMed] [Google Scholar]
- Jacobson RD, Holliday M. Electrically evoked walking and fictive locomotion in the chick. J Neurophysiol. 1982;48:238–256. doi: 10.1152/jn.1982.48.1.257. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Kalla R, Hüfner K, Wagner J, Strupp M, Brandt T. Supraspinal locomotor control in quadrupeds and humans. Prog Brain Res. 2008;171:353–362. doi: 10.1016/S0079-6123(08)00652-3. [DOI] [PubMed] [Google Scholar]
- Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol. 1996;76:849–866. doi: 10.1152/jn.1996.76.2.849. [DOI] [PubMed] [Google Scholar]
- Jordan LM. Initiation of locomotion in mammals. Ann N Y Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res Bull. 1990;24:509–516. doi: 10.1016/0361-9230(90)90104-8. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Leonard RB. Locomotion evoked by stimulation of the brain stem in the Atlantic stingray, Dasyatis sabina. J Neurosci. 1990;10:194–204. doi: 10.1523/JNEUROSCI.10-01-00194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002a;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002b;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC. Locomotor rhythm evoked by ventrolateral funiculus stimulation in the neonatal rat spinal cord in vitro. J Neurophysiol. 1997;77:200–206. doi: 10.1152/jn.1997.77.1.200. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Marlinskii VV, Voitenko LP. Participation of the medial reticular formation of the medulla oblongata in the supraspinal control of locomotor and postural activities in the guinea pig. Neurosci Behav Physiol. 1992;22:336–342. doi: 10.1007/BF01182876. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Descending control and sensory gating of “fictive” swimming and turning responses elicited in an in vitro preparation of the lamprey brainstem/ spinal cord. Brain Res. 1984;302:151–162. doi: 10.1016/0006-8993(84)91294-0. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Command systems for initiating locomotor responses in fish and amphibians — parallels to initiation of locomotion in mammals. In: Grillner S, Herman R, Stein P, Stuart D, editors. Neurobiology of Vertebrate Locomotion. Wenner-Gren Symposium Series. MacMillan Press; 1986. pp. 1–20. [Google Scholar]
- McClellan AD. Brainstem command systems for locomotion in the lamprey: localization of descending pathways in the spinal cord. Brain Res. 1988;457:338–349. doi: 10.1016/0006-8993(88)90704-4. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Locomotor recovery in spinal-transected lamprey: role of functional regeneration of descending axons from brainstem locomotor command neurons. Neuroscience. 1990;37:781–798. doi: 10.1016/0306-4522(90)90108-g. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Time course of locomotor recovery and functional regeneration in spinal-transected lamprey. In vitro brain/spinal cord preparations. J Neurophysiol. 1994;72:847–860. doi: 10.1152/jn.1994.72.2.847. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Organization of spinal locomotor networks: contributions from model systems. Comments Theor Biol. 1996;4:63–91. [Google Scholar]
- McClellan AD. Spinal cord injury: lessons from locomotor recovery and axonal regeneration in lower vertebrates. Neuroscientist. 1998;4:250–263. [Google Scholar]
- McClellan AD, Davis GR. Reticulospinal neurons and descending initiation pathways for locomotion in the lamprey. Soc Neurosci Abst. 1992;8:314. [Google Scholar]
- McClellan AD, Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J Neurophysiol. 1997;78:214–228. doi: 10.1152/jn.1997.78.1.214. [DOI] [PubMed] [Google Scholar]
- Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain. 1994;117:337–346. doi: 10.1093/brain/117.2.337. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Nicholson C. Lampreys, Petromyzontoidea. In: Nieuwenhuys R, Ten Donkelaar HJ, Nicholson C, editors. The Central Nervous System of Vertebrates. Springer; Heidelberg: 1998. pp. 397–495. [Google Scholar]
- Noga BR, Kettler J, Jordan LM. Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip. J Neurosci. 1988;8:2074–2086. doi: 10.1523/JNEUROSCI.08-06-02074.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Jordan LM. The effect of selective brainstem or spinal cord lesions on treadmill locomotion evoked by stimulation of the mesencephalic or pontomedullary locomotor regions. J Neurosci. 1991;11:1691–1700. doi: 10.1523/JNEUROSCI.11-06-01691.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Brownstone RM, Jordan LM. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol. 2003;90:1464–1478. doi: 10.1152/jn.00034.2003. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Connections of the reticulo-spinal neurons with the ‘locomotor regions’ of the brain stem. Biophysics. 1970;15:171–177. [Google Scholar]
- Paggett K, Jackson AW, McClellan AD. Organization of higher-order brain areas that initiate locomotor activity in larval lamprey. Neuroscience. 2004;125:25–33. doi: 10.1016/j.neuroscience.2004.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp. 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res. 1999;123:349–365. doi: 10.1016/s0079-6123(08)62870-8. [DOI] [PubMed] [Google Scholar]
- Rouse DT, McClellan AD. Descending propriospinal neurons in normal and spinal cord-transected lamprey. Exp Neurol. 1997;146:113–124. doi: 10.1006/exnr.1997.6511. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I Muller and Mauthner cells. J Neurophysiol. 1967;30:1000–1023. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Müller cells, Mauthner cells, and other reticulospinal neurons in the lamprey. In: Faber D, Korn H, editors. Neurobiology of the Mauthner Cell. Raven Press; New York: 1978. pp. 245–269. [Google Scholar]
- Rovainen CM. The neurobiology of lampreys. Physiol Rev. 1979;59:1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- Rovainen CM, Johnson PA, Roach EA, Mankowsky JA. Projections of individual axons in lamprey spinal cord determined by tracing through serial sections. J Comp Neurol. 1973;149:193–201. doi: 10.1002/cne.901490205. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Selzer ME. The sea lamprey. What this primitive animal can teach us about thepotential for repair of the injured spinal cord. Top Spinal Cord Inj Rehabil. 2003;8:14–36. [Google Scholar]
- Shaw AC, Holmes T, Johns JL, Thurman S, Jackson AW, Davis GR, McClellan AD. Pathfinding of regenerating descending axons in spinal cord-transected larval lamprey: functional anatomy. Soc Neurosci Abst. 2001;27:960. [Google Scholar]
- Shefchyk SJ, Jell RM, Jordan LM. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp Brain Res. 1984;56:257–262. doi: 10.1007/BF00236281. [DOI] [PubMed] [Google Scholar]
- Shik ML. Action of the brainstem locomotor region on spinal stepping generators via propriospinal pathways. In: Koa CC, Bunge RP, Reier RJ, editors. Spinal Cord Reconstruction. Raven Press; New York: 1983. pp. 421–434. [Google Scholar]
- Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976;56:465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Sholomenko GN, Steeves JD. Effects of selective spinal cord lesions on hind limb locomotion in birds. Exp Neurol. 1987;95:403–418. doi: 10.1016/0014-4886(87)90148-8. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Kinjo N, Ishikawa Y, Biedermann JA, Garcia-Rill E. Locomotor projections from the pedunculopontine nucleus to the medioventral medulla. Neuroreport. 1990;1:207–210. doi: 10.1097/00001756-199011000-00008. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Localization of a descending pathway in the spinal cord which is necessary for controlled treadmill locomotion. Neurosci Lett. 1980;20:283–288. doi: 10.1016/0304-3940(80)90161-5. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res. 1984;307:263–276. doi: 10.1016/0006-8993(84)90480-3. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Sholomenko GN, Webster DMS. Stimulation of the pontomedullary reticular formation initiates locomotion in decerebrate birds. Brain Res. 1987;401:205–212. doi: 10.1016/0006-8993(87)91406-5. [DOI] [PubMed] [Google Scholar]
- Stein PSG. Swimming movements elicited by electrical stimulation of the turtle spinal cord: the high spinal preparation. J Comp Physiol A. 1978;124:203–210. [Google Scholar]
- Ullen F, Deliagina TG, Orlovsky GN, Grillner S. Visual pathways for postural control and negative phototaxis in lamprey. J Neurophysiol. 1997;78:960–976. doi: 10.1152/jn.1997.78.2.960. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Moore AM, Eidelberg E, Walden JG. Recovery of locomotion in monkeys with spinal cord lesions. J Mot Behav. 1992;24:288–296. doi: 10.1080/00222895.1992.9941624. [DOI] [PubMed] [Google Scholar]
- Wallén P, Williams T. J Physiol. Vol. 347. London: 1984. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal; pp. 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannier T, Deliagina TG, Orlovsky GN, Grillner S. Differential effects of reticulospinal system on locomotion in lamprey. J Neurophysiol. 1998;80:103–112. doi: 10.1152/jn.1998.80.1.103. [DOI] [PubMed] [Google Scholar]
- Williams BJ, Livingston CA, Leonard RB. Spinal cord pathways involved in initiation of swimming in the stingray, Dasyatis sabina: spinal cord stimulation and lesions. J Neurophysiol. 1984;51:578–591. doi: 10.1152/jn.1984.51.3.578. [DOI] [PubMed] [Google Scholar]
- Windle WF, Smart JO, Beers JJ. Residual function after subtotal spinal cord transection in adult cats. Neurology. 1958;8:518–521. doi: 10.1212/wnl.8.7.518. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. Descending pathways eliciting forelimb stepping in the lateral funiculus: experimental studies with stimulation and lesion of the cervical cord in decerebrate cats. Brain Res. 1986;379:125–136. doi: 10.1016/0006-8993(86)90264-7. [DOI] [PubMed] [Google Scholar]
- You SW, Chen BY, Liu HL, Lang B, Xia JL, Jiao XY, Ju G. Spontaneous recovery of locomotion induced by remaining fibers after spinal cord transection in adult rats. Restor Neurol Neurosci. 2003;21:39–45. [PubMed] [Google Scholar]
- Zelenin PV. Activity of individual reticulospinal neurons during different forms of locomotion in the lamprey. Eur J Neurosci. 2005;22:2271–2282. doi: 10.1111/j.1460-9568.2005.04395.x. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina TG. Heterogeneity of the population of command neurons in the lamprey. J Neurosci. 2001;21:7793–7803. doi: 10.1523/JNEUROSCI.21-19-07793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McClellan AD. Axonal regeneration of descending brain neurons in larval lamprey demonstrated by retrograde double labeling. J Comp Neurol. 1999;410:612–626. doi: 10.1002/(sici)1096-9861(19990809)410:4<612::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Zhang L, Palmer R, McClellan AD. Increase in descending brain–spinal cord projections with age in larval lamprey: implications for spinal cord injury. J Comp Neurol. 2002;447:128–137. doi: 10.1002/cne.10208. [DOI] [PubMed] [Google Scholar]


