Summary
A cilium is an extension of the cell that contains an axonemal complex of microtubules and associated proteins bounded by a membrane which is contiguous with the cell body membrane. Cilia may be nonmotile or motile, the latter having additional specific roles in cell or fluid movement. The term flagellum refers to the motile cilium of free-living single cells (e.g., bacteria, archaea, spermatozoa and protozoa). In eukaryotes, both nonmotile and motile cilia possess sensory functions. The ciliary interior (cilioplasm) is separated from the cytoplasm by a selective barrier that prevents passive diffusion of molecules between the two domains. The sensory functions of cilia reside largely in the membrane and signals generated in the cilium are transduced into a variety of cellular responses. In this review we discuss the structure and biogenesis of the cilium, with special attention to the trypanosome flagellar membrane, its lipid and protein composition and its proposed roles in sensing and signaling.
Introduction
Most eukaryotic cells possess one or more unique organelles known as cilia, membranated projections that regulate cell motility and environmental sensing, and serve important roles in human health and disease [1]. Cilia are commonly classified as nonmotile (primary) or motile. All cilia share many structural and functional properties. An expanding group of human disorders, collectively known as the ciliopathies, has been linked to defects in ciliary structure, function and associated signaling pathways, generating renewed interest in the biology of these organelles [2]. The ciliopathies are widely diverse, ranging from embryonic patterning defects to renal and ocular diseases [3]. The subset of motile cilia includes flagella that primarily function in the propulsion of the single celled organisms, including trypanosomes (Trypanosoma cruzi and brucei) and Leishmania, flagellated, parasitic protozoa that cause a range of debilitating human diseases. The focus of this review is on common principles of ciliary structure, with special attention to the trypanosome flagellar membrane, its lipid and protein composition, and its proposed roles in sensing and signaling.
Ciliary structure
Eukaryotic cilia contain three distinct domains: the cytoskeleton axoneme, the soluble ciliary compartment (cilioplasm), and the ciliary membrane [4]. The central axoneme consists of a microtubular array that provides the structural support and gives the cilium its recognizable form. The most common axonemal arrangements includes the 9 + 2 arrangement, in which a central pair of microtubules is surrounded by nine outer double microtubules, and the 9 + 0 arrangement, in which the central pair of microtubules is absent. Positioned at the base of the axoneme is a barrel-shaped structure called the basal body that consists of nine triplet microtubules and serves as a differentiated centriole [5]. Transition fibers, sheet-like projections radiate outwards from the mature basal body and serve as a permeability barrier that restricts the free diffusion of vesicles and macromolecules between the cell cytoplasm and the soluble ciliary compartment [6]. This property of transition fibers enables the cilium to maintain its unique composition of lipids and proteins that is distinct from the cytoplasm, thereby leading to a vision of the cilium as a separate cellular organelle. The ciliary membrane wraps around the ciliary axoneme and conforms to its shape. It is a highly specialized domain of the plasma membrane which, while contiguous with cell body and ciliary pocket membranes, nevertheless maintains a unique lipid and protein composition and distinct functions [7].
Lipid composition of trypanosome flagellar membrane
The flagella of several microorganisms have been isolated to study their lipid composition in comparison to the cell body. These studies demonstrate that the flagellar membrane is enriched in sterols [8–10], glycolipids [11] and sphingolipids [12,13], all which are components of canonical lipid raft microdomains. Numerous lipid raft associated proteins from the kinetoplastids are also dually acylated. These include T. brucei calflagins (Tb17, Tb24 and Tb44) [14], T. cruzi flagellar calcium-binding protein (FCaBP) (Maric, et. al., manuscript in preparation) and Leishmania major small myristoylated protein (SMP-1) [15]. Furthermore, lipid raft association is essential for the flagellar localization in the case of the T. brucei calflagins, where ablation of the palmitoylation site or inhibition of the enzyme that palmitoylates calflagin (TbPAT7) leads to protein mislocalization to the cell body membrane and disrupts its association with lipid rafts [16]. Hence it has been proposed that protein association with lipid rafts might serve to recruit and/or retain flagellar membrane proteins [14]. Despite these studies, it remains unclear how the organelle generates and maintains a lipid restrictive environment and the functional role of its membrane organization.
Protein composition of trypanosome flagellar membrane
In addition to unique lipid composition, flagellar membrane of trypanosomes is also characterized by the asymmetric distribution of certain proteins in comparison to other membrane domains. Proteins across the kinetoplastids that are heavily enriched or restricted to the flagellar membrane include: flagellar calcium-binding protein (FCaBP) (Figure 1 and [17]), calflagins (Tb 17, Tb24 and Tb44) [16], receptor adenylate cyclases (ESAG4) [18], low density lipoprotein receptor (LDL) [19], glucose transporter isoform 1 (Iso-1) [20] and the small myristoylated protein (SMP-1) [21]. A detailed listing of flagellar membrane proteins that have been confirmed in Trypanosomes, Leishmania, and Chlamydomonas is given in Table 1. The protein synthesis machinery is absent from the flagellum [22]; therefore, cells have evolved mechanisms to target newly synthesized flagellar proteins to their proper locations [1]. This is true for proteins of flagellar axoneme, flagellar matrix, and membrane, although the route of transport may differ among these classes of proteins. The mechanism by which proteins are selectively targeted to the flagellar membrane is somewhat controversial. The two prevailing models include: (i) a diffusion-retention model, in which proteins are delivered by a common pathway to the plasma membrane and can move laterally into the flagellar membrane and simply diffuse through the barrier imposed by the flagellar pocket and necklace, and (ii) a targeted delivery model, in which proteins destined for either flagellar or cell body membranes are sorted at an earlier point in biosynthesis and are then carried in vesicles to the base of the flagellum to be separately delivered to their final destinations [23]. The evidence for the first model is for the most part based on the studies of flagellar attachment molecules called agglutinins in Chlamydomonas [24–26]. Flagellar agglutinins from the two gametes adhere to each other, enabling fertilization in Chlamydomonas. Upon attachment, the flagellar pool of active agglutinins is lost and is subsequently replaced by a pool from the cell body that is delivered by the lateral transport of the agglutinins from the plasma membrane to the flagellar membrane and doesn’t involve vesicle transport to the base of the flagella or IFT [26,27]. More recently, Miletnkovic et. al. provided further evidence for lateral transport from the plasma membrane to the ciliary membrane in case of the ciliary protein Smoothened (Smo), an important protein in the Hedgehog signal transduction pathway. The binding of the ligand Sonic Hedgehog (Shh) to its receptor Patched 1 (Ptc1) serves as a trigger for lateral transport of Smo from the cell body membrane to the ciliary membrane and accumulation of Smo herein leads to the initiation of signaling [28]. Based on the work of others and recent findings from our own laboratory, it seems that the latter mechanism of protein delivery to the flagellar membrane is more likely in trypanosomes. Proteins that are part of the intraflagellar transport (IFT) machinery are synthesized in the cytoplasm on free polyribosomes and are imported post-translationally to the flagellum via the flagellar pore [4]. IFT protein complexes A and B move axonemal subunits to the tip of the flagellum (anterograde transport) and following cargo unloading at the flagellar tip, they return back to the base of the flagellum to be recycled (retrograde transport). Membrane-associated and transmembrane proteins are synthesized on the rough endoplasmatic reticulum (RER). Herein, proteins that need dual acylation, such as FCaBP/calflagins will get modified by the addition of a myristate to the N-terminal glycine residue by N-myristoyltransferase (NMT) and then will proceed to Golgi to get further modified via cysteine palmitoylation by one or more palmitoyltransferases (PAT). Golgi-derived vesicles differ in their lipid composition and this may in turn determine what kind of cargo they carry [29]. For example, vesicles that are enriched in sterols and sphingolipids may be preferentially loaded with lipid-modified proteins and proteins that contain flagellar targeting sequences. Additionally, since palmitoylation is reversible process, one or more of the PATs may also get loaded into these vesicles to confer association of their protein substrates to lipid raft-enriched vesicles. Vesicles of other composition may preferentially load other types of cargo, for example cargo destined for the cell body membrane. As the vesicles containing flagellum-bound cargo make their way towards the base of the flagellum, they interact with a multiprotein complex named BBSome [30,31] which facilitates transport of these vesicles to the base of the flagellum proximal to the flagellar pocket region [30,31]. Membrane vesicles cannot be targeted to the flagellar membrane directly and they accumulate at the base of the flagellum giving the appearance of a so called “flagellar necklace” structure [32]. At this point vesicles must fuse with the membrane, and in trypanosomes the fusion most likely occurs with the membrane of the flagellar pocket [26], a specialized invagination of the plasma membrane where all of the endo- and exocytosis takes place [33]. Leishmania glucose transporter isoforms 1 and 2 (Iso-1 and Iso-2) are both found in the flagellar pocket and are subsequently targeted to either the flagellar membrane (Iso-1) or to the plasma membrane (Iso-2) [20]. The differential localization of the two glucose transporter isoforms suggests that sorting takes place after the proteins reach the plasma membrane and that the flagellar pocket is the main sorting domain for membrane proteins in kinetoplastids [33]. Based on their intrinsic properties proteins get further sorted from the flagellar pocket to either flagellar or cell body membranes [33]. IFT proteins are likely also involved in the transport of the flagellar membrane proteins [1,34,35]. While the mode of protein entry into the flagella is controversial it is clear that flagellar membrane has a distinct protein composition in comparison to the flagellar pocket and cell body membranes. Presumably, the unique protein environment is what gives differential function to each membrane region.
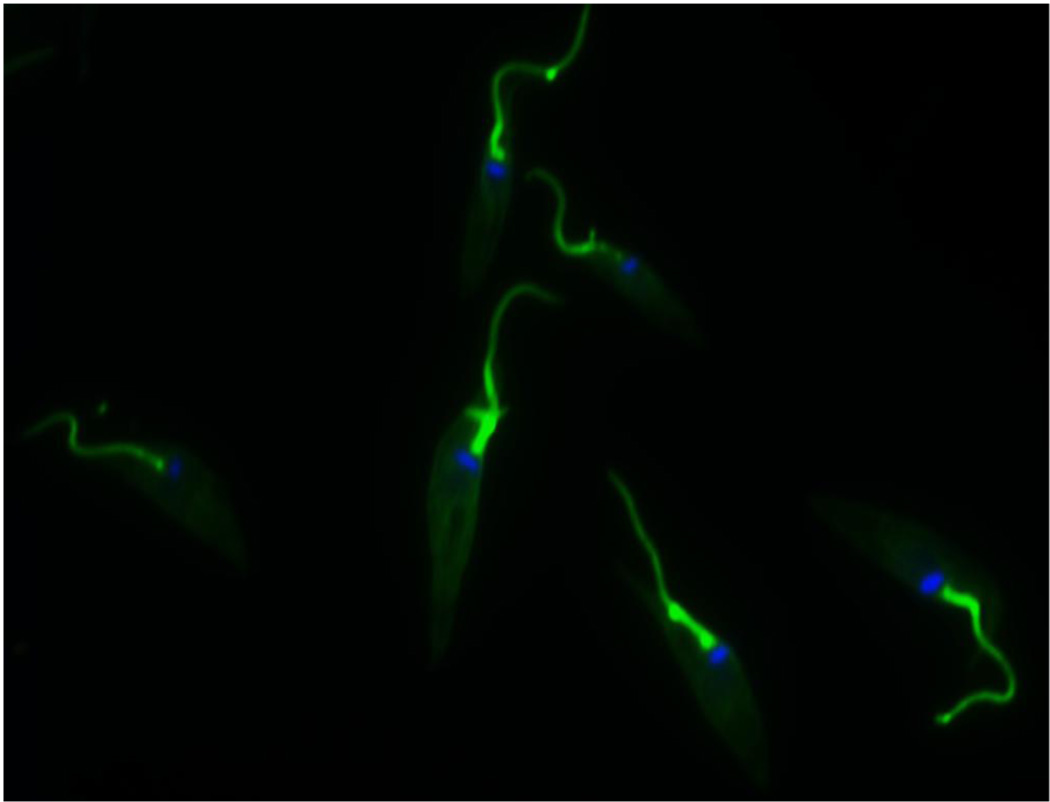
Figure 1.
The flagellar calcium-binding protein of Trypanosoma cruzi. FCaBP is a calcium sensor that localizes to the inner aspect of the flagellar membrane in a manner dependent on (1) dual acylation at the N-terminus by myristate and palmitate and (2) a high calcium concentration. Green: FCaBP immunofluorescence using a polyclonal FCaBP antibody. Blue: DAPI staining of parasite DNA (only the kinetoplast (mitochondrial) DNA) is clearly visible in this image.
Table 1.
Inventory of confirmed flagellar membrane proteins in the trypanosomatids (T. brucei, T. cruzi and Leishmania spp.) and Chlamydomonas reinhardtii.
| Protein | Organism | Reference |
|---|---|---|
| Calflagins Tb17, Tb24, Tb44 | Trypanosoma brucei | [60,64] |
| Small kinetoplastid calpain-related proteins TbSKCRP7.2 and TbSKCRP1.5 |
Trypanosoma brucei | [65] |
| Glycosylphosphatidylinositol-specific phospholipase C, GPI-PLC |
Trypanosoma brucei | [66] |
| Expression site associated gene 4, ESAG4 | Trypanosoma brucei | [18] |
| Low density lipoprotein receptor, LDL | Trypanosoma brucei | [19] |
| Vacuolar type Ca2+ ATPase 1, TbA1 and TbA2 | Trypanosoma brucei | [67] |
| Membrane-bound histidine acid phosphatase, TbMBAP1 |
Trypanosoma brucei | [68] |
| Flagellar calcium-binding protein, FCaBP | Trypanosoma cruzi | [69] |
| Small myristoylated protein-1, SMP-1 | Leishmania major | [21] |
| Glucose transporter isoform-1, ISO-1 | Leishmania enriettii | [20] |
| Glucose transporter 1, LmGT1 | Leishmania mexicana | [70] |
| SST-2 | Leishmania braziliensis | [71] |
| Aquaglyceroporin 1, AQP1 | Leishmania major | [72] |
| Agg2p | Chlamydomonas reinhardtii | [54] |
| Kinesin-like protein FLA10 | Chlamydomonas reinhardtii | [73] |
| Polycystin-2, TRPP2 | Chlamydomonas reinhardtii | [74] |
| Polycystic kidney disease protein 1, PKD1 | Chlamydomonas reinhardtii | [75] |
| Polycystic kidney disease protein 2, PKD2 | Chlamydomonas reinhardtii | [76] |
| Agglutinins | Chlamydomonas reinhardtii | [77] |
| Flagella Membrane Glycoprotein 1B, FMG-1B | Chlamydomonas reinhardtii | [78,79] |
| Mastigoneme | Chlamydomonas reinhardtii | [79,80] |
Sensory and signaling functions of cilia in vertebrates
Both primary and motile cilia engage in signaling across diverse cells and tissues of multicellular eurkaryotes. In vertebrates, cilia transduce a variety of signals, including mechanical stressors from deformation or fluid flux (airway and kidney epithelia) [36,37], bending of otic hair cells in response to sound [38], others detect gradients for chemosensation [39], responsiveness to sex steroids [40] and light sensitivity by retinal photoreceptors [41]. The impact of ciliary function is equally diverse, ranging from tissue development via sensing of Hedgehog gradients [42] to the bulk movement of mucus during airway clearance. These processes generally result in signaling through alterations in cytoplasmic calcium mediated by voltage-sensitive channels, notably the transient receptor potential channels (TRP family), G-protein coupled receptor signaling, and/or activation of phospholipase C [43–45].
Sensory and signaling functions of cilia/flagella in invertebrates and protists
The evidence that both motile and nonmotile cilia have sensory and integrative signaling is not limited to vertebrates. Other eukaryotic organisms, notably Caenorhabditis elegans expresses the G-protein coupled receptor ODR-10 linked to ODR-3, which regulates a cation channel at the cilia of olfactory neurons [46–49]. Also in C. elegans, the TRP polycystin complex of proteins localizes to the distal ciliary tip and signals through phosphoinositides [50]. The protozoan Paramecium and green alga Chlamydomonas have several described integrative signaling pathways mediated by the flagellum. In Paramecium, a contact-sensitive process triggers calcium flux enabling a change in swimming trajectory [51], and sexual mating is initiated through cilium-cilium adhesion [52]. Examination of the detergent-resistant membranes of the Chlamydomonas flagellum revealed that light and oxidation state are sensed through a rhodopsin protein linking through flagellar AGG2/3 [53,54]. In the mating of Chlamydomonas, bidirectional flagellar contact initiates a signaling cascade through transient receptor potential (TRP) channels, involving a rise in both calcium and cyclic adenosine monophosphate (camp) [55]. These examples demonstrate that the varied and robust signaling role of cilia in vertebrate tissues is likely operative in many eukaryotic cells.
Sensory and signaling functions of trypanosome flagella
Although less well established, sensory and signaling roles for the trypanosome flagellum are highly predictable, given the structural similarities shared with other cilia. As discussed previously, the trypanosomal flagellar membrane is enriched in lipid rafts, platforms known to organize transmembrane signaling events [14]. In addition, many proteins, including several acylated proteins, are either enriched or are completely restricted to the flagellar membrane and many associate with lipid rafts [14,15 and Maric, et. al., manuscript in preparation]. Restricted localization of these molecules to the flagellar membrane suggests, but does not prove, specialization of their function. Several of these restricted proteins are predicted to be involved in sensing and signaling, extending the role of the trypanosome flagella beyond motility and host cell invasion. In motile cilia of protists and other invertabrates, such as Chalmydomonas and Paramecium, a rise in intracellular calcium and production of cAMPs in the cilia are known triggers that are important for sensory reception and initiation of downstream signaling events (reviewed in [56]). Similar themes are just beginning to emerge for the trypanosomatids. In T. brucei, the protein encoded by expression site associated gene 4 (ESAG4), an adenylyl cyclase, is restricted to the flagellar membrane and may mediate environmental sensing by regulating cAMP [18]. A flagellar cAMP signaling pathway in T. brucei through phosphodiesterases TbrPDEB1/B2 has been implicated in parasite virulence [57], and a calcium handling protein calmodulin links to the paraflagellar rod [58]. The family of dually acylated flagellar calcium-binding proteins, T. cruzi FCaBP [59] and the T. brucei calflagins Tb17, Tb24, Tb44 [60] are all calcium sensors that associate with flagellar membrane in calcium-dependent and palmitoylation-dependent manner [17]. These proteins resemble the neuronal calcium sensors, which undergo a calcium-dependent conformational change that modulates the interaction between the acyl groups and the membrane [61]. This switch mechanism permits the association with two different binding partners, permitting an on/off switch to regulate their signaling functions [61]. Although the specific partner proteins of FCaBP/ calflagins are just now being identified, calcium-sensitive conformational changes have been confirmed [62]. Interestingly, a role for the calflagins in host survival and immune evasion has also recently been described [63]. Given the resurgence of interest in the cilia as a signaling platform linked to disease, that new proteins and signaling networks will be identified in the trypanosomal flagellum. We speculate that the calflagins will emerge as regulators of environmental sensing signaling through calcium and a regulated membrane association dependent upon their acylation state.
Conclusion
Cilia and flagella are fascinating organelles with a rich impact on tissue development, cell motility and environmental sensing. An improved understanding of ciliarly biology and has revealed several human disorders linked to defects in motility, IFT, and the BBsome complex. Beyond the common structural and motile elements that underlie most cilia, a new understanding of integrated environmental sensing is evident, and these recurring themes are likely extend to other protozoan organisms. Still, major questions remain about cilia and flagella: How is the specialized composition of the ciliary membrane established and then maintained? Where and how are cilium-bound vesicles formed and what are the precise molecular mechanisms of their transport and entry into the cilium? What is the molecular mechanism for each type of ciliopathy? What are the sensory functions of each type of cilium and the mechanisms by which the ciliary sensors transduce their signals into specific cellular responses? Finally, for digenetic organisms like trypanosomes, which spend part of their lives in invertebrate hosts and part in mammalian hosts (either intracellularly in T. cruzi and Leishmania species or extracellularly in T. brucei), what are the specific functions of the flagellum for adhesion to epithelial (insect) or endothelial (mammal) surfaces, cell invasion, differentiation and environmental sensing?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:111–145. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 2.Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- 3.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 4. Bloodgood RA. Protein targeting to flagella of trypanosomatid protozoa. Cell Biol Int. 2000;24:857–862. doi: 10.1006/cbir.2000.0598. •• A review that nicely summarizes known proteins with flagellar targeting sequences and delineates molecular determinants for flagellar protein targeting.
- 5.Ralston KS, Kabututu ZP, Melehani JH, Oberholzer M, Hill KL. The Trypanosoma brucei flagellum: moving parasites in new directions. Annu Rev Microbiol. 2009;63:335–362. doi: 10.1146/annurev.micro.091208.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci. 2010;123:511–518. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balber AE. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit Rev Immunol. 1990;10:177–201. [PubMed] [Google Scholar]
- 8.Chailley B, Boisvieux-Ulrich E. Detection of plasma membrane cholesterol by filipin during microvillogenesis and ciliogenesis in quail oviduct. J Histochem Cytochem. 1985;33:1–10. doi: 10.1177/33.1.3965567. [DOI] [PubMed] [Google Scholar]
- 9. Kaneshiro ES. Lipids of ciliary and flagellar membranes. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. Plenum Press; 1990. pp. 241–265. •• This book chapter systematically details lipid enrichment in ciliary and flagellar membranes across eukaryotic species.
- 10.Tetley L. Freeze-fracture studies on the surface membranes of pleomorphic bloodstream and in vitro transformed procyclic Trypanosoma brucei. Acta Trop. 1986;43:307–317. [PubMed] [Google Scholar]
- 11.Bloodgood RA, Woodward MP, Young WW. Unusual distribution of a glycolipid antigen in the flagella of Chamydomonas. Protoplasma. 1995;185:123–130. [Google Scholar]
- 12.Kaneshiro ES, Matesic DF, Jayasimhulu K. Characterizations of six ethanolamine sphingophospholipids from Paramecium cells and cilia. J Lipid Res. 1984;25:369–377. [PubMed] [Google Scholar]
- 13.Kaya K, Ramesha CS, Thompson GA., Jr On the formation of alpha-hydroxy fatty acids. Evidence for a direct hydroxylation of nonhydroxy fatty acid-containing sphingolipids. J. Biol. Chem. 1984;259:3548–3553. [PubMed] [Google Scholar]
- 14. Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. doi: 10.1242/jcs.037721. • Lipid rafts are enriched in the trypanosome flagellar membrane, providing a unique mechanism for flagellar protein localization.
- 15.Tull D, Naderer T, Spurck T, Mertens HD, Heng J, McFadden GI, Gooley PR, McConville MJ. Membrane protein SMP-1 is required for normal flagellum function in Leishmania. J Cell Sci. 2010;123:544–554. doi: 10.1242/jcs.059097. [DOI] [PubMed] [Google Scholar]
- 16.Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci. 2009;122:867–874. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18:2057–2065. doi: 10.1093/emboj/18.8.2057. • Detailed analysis of determinants for flagellar membrane localization suggesting a potentially conserved evolutionary mechanism.
- 18.Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux JC, Dinsart C, Huet G, Pays E. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol. Cell. Biol. 1992;12:1218–1225. doi: 10.1128/mcb.12.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppens I, Baudhuin P, Opperdoes FR, Courtoy PJ. Receptors for the host low density lipoproteins on the hemoflagellate Trypanosoma brucei: purification and involvement in the growth of the parasite. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6753–6757. doi: 10.1073/pnas.85.18.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snapp EL, Landfear SM. Cytoskeletal association is important for differential targeting of glucose transporter isoforms in Leishmania. J Cell Biol. 1997;139:1775–1783. doi: 10.1083/jcb.139.7.1775. • This paper describes two distinct trafficking routes for glucose transporter isoforms 1 and 2.
- 21. Tull D, Vince JE, Callaghan JM, Naderer T, Spurck T, McFadden GI, Currie G, Ferguson K, Bacic A, McConville MJ. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol. Biol. Cell. 2004;15:4775–4786. doi: 10.1091/mbc.E04-06-0457. • Well executed study of molecular determinants for flagellar protein localization.
- 22.Rosenbaum JL, W GB. Intraflagellar transport. Nature Reviews Molecular Cell Biology. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 23.Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111:1605–1616. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodenough UW. Cyclic AMP enhances the sexual agglutinability of Chlamydomonas flagella. J Cell Biol. 1989;109:247–252. doi: 10.1083/jcb.109.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan J, Snell WJ. Kinesin II and regulated intraflagellar transport of Chlamydomonas aurora protein kinase. J Cell Sci. 2003;116:2179–2186. doi: 10.1242/jcs.00438. [DOI] [PubMed] [Google Scholar]
- 28.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao L, Scholey JM. Intraflagellar transport at a glance. J Cell Sci. 2009;122:889–892. doi: 10.1242/jcs.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin H, Nachury MV. The BBSome. Curr Biol. 2009;19:R472–R473. doi: 10.1016/j.cub.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 35.Sloboda RD. Intraflagellar transport and the flagellar tip complex. J Cell Biochem. 2005;94:266–272. doi: 10.1002/jcb.20323. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara H, Asano T, Kato K, Kameshima T, Semba R. Localization of a G protein Gi2 in the cilia of rat ependyma, oviduct and trachea. Eur J Neurosci. 1998;10:699–707. doi: 10.1046/j.1460-9568.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- 37.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmood T, Saridogan E, Smutna S, Habib AM, Djahanbakhch O. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum Reprod. 1998;13:2991–2994. doi: 10.1093/humrep/13.11.2991. [DOI] [PubMed] [Google Scholar]
- 41.Ramamurthy V, Cayouette M. Development and disease of the photoreceptor cilium. Clin Genet. 2009;76:137–145. doi: 10.1111/j.1399-0004.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- 42.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 43.Delmas P. Polycystins: polymodal receptor/ion-channel cellular sensors. Pflugers Archiv. European Journal of Physiology. 2005;451:264–276. doi: 10.1007/s00424-005-1431-5. [DOI] [PubMed] [Google Scholar]
- 44.Oro AE. The primary cilia, a 'Rab-id' transit system for hedgehog signaling. Curr Opin Cell Biol. 2007;19:691–696. doi: 10.1016/j.ceb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 46.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/s0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 48.Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 49.Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–466. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 50.Bae YK, Kim E, L'Hernault SW, Barr MM. The CIL-1 PI 5-phosphatase localizes TRP Polycystins to cilia and activates sperm in C. elegans. Curr Biol. 2009;19:1599–1607. doi: 10.1016/j.cub.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Ondarza J, Symington SB, Van Houten JL, Clark JM. G-protein modulators alter the swimming behavior and calcium influx of Paramecium tetraurelia. J Eukaryot Microbiol. 2003;50:349–355. doi: 10.1111/j.1550-7408.2003.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 52.Kitamura A, Hiwatashi K. Mating-reactive membrane vesicles from cilia of Paramecium caudatum. J Cell Sci. 1976;69:736–740. doi: 10.1083/jcb.69.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster KW, Saranak J, Patel N, Zarilli G, Okabe M, Kline T, Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984;311:756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- 54.Iomini C, Li L, Mo W, Dutcher SK, Piperno G. Two flagellar genes, AGG2 and AGG3, mediate orientation to light in Chlamydomonas. Curr Biol. 2006;16:1147–1153. doi: 10.1016/j.cub.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Snell WJ. Flagellar adhesion-dependent regulation of Chlamydomonas adenylyl cyclase in vitro: a possible role for protein kinases in sexual signaling. J Cell Biol. 1994;125:617–624. doi: 10.1083/jcb.125.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 57.Oberholzer M, Marti G, Baresic M, Kunz S, Hemphill A, Seebeck T. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J. 2007;21:720–731. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- 58.Ridgley E, Webster P, Patton C, Ruben L. Calmodulin-binding properties of the paraflagellar rod complex from Trypanosoma brucei. Mol Biochem Parasitol. 2000;109:195–201. doi: 10.1016/s0166-6851(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 59.Godsel LM, Olson CL, Lacava ZG, Engman DM. Comparison of the 24 kDa flagellar calcium-binding protein cDNA of two strains of Trypanosoma cruzi. J Eukaryot Microbiol. 1995;42:320–322. doi: 10.1111/j.1550-7408.1995.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y, Deford J, Benjamin R, Lee MG, Ruben L. The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Biochem J. 1994;304:833–841. doi: 10.1042/bj3040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araujo AP, Engman DM. A flagellum-specific calcium sensor. J Biol Chem. 2005;280:40104–40111. doi: 10.1074/jbc.M505777200. [DOI] [PubMed] [Google Scholar]
- 62.Wingard JN, Ladner J, Vanarotti M, Fisher AJ, Robinson H, Buchanan KT, Engman DM, Ames JB. Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J Biol Chem. 2008;283:23388–23396. doi: 10.1074/jbc.M803178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emmer BT, Daniels MD, Taylor JM, Epting CL, Engman DM. Calflagin Inhibition Prolongs Host Survival and Suppresses Parasitemia in Trypanosoma brucei Infection. Eukaryot Cell. 2010 doi: 10.1128/EC.00086-10. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Haghighat NG, Ruben L. The predominant calcimedins from Trypanosoma brucei comprise a family of flagellar EF-hand calcium-binding proteins. Biochem. J. 1992;287:187–193. doi: 10.1042/bj2870187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, Apagyi K, McLeavy L, Ersfeld K. Expression and cellular localisation of calpain-like proteins in Trypanosoma brucei. Mol Biochem Parasitol. 2010;169:20–26. doi: 10.1016/j.molbiopara.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Hanrahan O, Webb H, O'Byrne R, Brabazon E, Treumann A, Sunter JD, Carrington M, Voorheis HP. The glycosylphosphatidylinositol-PLC in Trypanosoma brucei forms a linear array on the exterior of the flagellar membrane before and after activation. PLoS Pathog. 2009;5:e1000468. doi: 10.1371/journal.ppat.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo S, Rohloff P, Cox J, Uyemura SA, Docampo R. Trypanosoma brucei plasma membrane-type Ca2+-ATPase 1 (TbPMC1) and 2 (TbPMC2) genes encode functional Ca2+-ATPases localized to the acidocalcisomes and plasma membrane, and essential for Ca2+ homeostasis and growth. J Biol Chem. 2004;279:14427–14439. doi: 10.1074/jbc.M309978200. [DOI] [PubMed] [Google Scholar]
- 68.Engstler M, Weise F, Bopp K, Grunfelder CG, Gunzel M, Heddergott N, Overath P. The membrane-bound histidine acid phosphatase TbMBAP1 is essential for endocytosis and membrane recycling in Trypanosoma brucei. J Cell Sci. 2005;118:2105–2118. doi: 10.1242/jcs.02327. [DOI] [PubMed] [Google Scholar]
- 69.Engman DM, Krause K-H, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca2+ -binding protein in trypanosomes. J Biol Chem. 1989;264:18627–18631. [PubMed] [Google Scholar]
- 70.Burchmore RJ, Rodriguez-Contreras D, McBride K, Merkel P, Barrett MP, Modi G, Sacks D, Landfear SM. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc Natl Acad Sci USA. 2003;100:3901–3906. doi: 10.1073/pnas.0630165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silveira TG, Takahashi HK, Straus AH. Immunolocalization of Leishmania (Viannia) braziliensis membrane antigens recognized by mAbs SST-2, SST-3, and SST-4. Parasitology. 2003;127:449–456. doi: 10.1017/s0031182003003986. [DOI] [PubMed] [Google Scholar]
- 72.Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 73.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179:501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 76.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 77.Solter KM, Gibor A. Evidence for role of flagella as sensory transducers in mating of Chlamydomonas reinhardtii. Nature. 1977;265:444–445. doi: 10.1038/265444a0. [DOI] [PubMed] [Google Scholar]
- 78.Bloodgood RA, Salomonsky NL. Calcium influx regulates antibody-induced glycoprotein movements within the Chlamydomonas flagellar membrane. J Cell Sci. 1990;96:27–33. doi: 10.1242/jcs.96.1.27. [DOI] [PubMed] [Google Scholar]
- 79.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura S, Tanaka G, Maeda T, Kamiya R, Matsunaga T, Nikaido O. Assembly and function of Chlamydomonas flagellar mastigonemes as probed with a monoclonal antibody. J Cell Sci. 1996;109:57–62. doi: 10.1242/jcs.109.1.57. [DOI] [PubMed] [Google Scholar]