Abstract
Many antipsychotic medications carry a substantial liability for weight gain, and one mechanism common to all antipsychotics is binding to the dopamine D2 receptor. We therefore examined the relationship between −141C Ins/Del (rs1799732), a functional promoter region polymorphism in DRD2, and antipsychotic-induced weight gain in 58 first episode schizophrenia patients enrolled in a randomized trial of risperidone (RIS) vs. olanzapine (OLZ). Carriers of the deletion allele (n=29) were compared to Ins/Ins homozygotes (non-carriers, n=29) in a mixed model encompassing 10 weight measurements over 16 weeks. Deletion allele carriers demonstrated significantly more weight gain after 6 weeks of treatment regardless of assigned medication. While deletion carriers were prescribed higher doses of OLZ (but not RIS), dose did not appear to account for the genotype effects on weight gain. Given previous evidence that deletion carriers demonstrate reduced symptom response to medication, additional study of appropriate treatment options for these patients appears warranted.
Keywords: DRD2, dopamine, antipsychotic, weight gain, pharmacogenetics
Clinically significant weight gain is a commonly reported adverse event associated with antipsychotic use, and represents a major public health concern in the clinical management of schizophrenia and other serious mental illnesses [1]. Antipsychotic-induced weight gain is particularly pronounced in the first episode of schizophrenia; effects of prior treatment lead to an under-estimation of the extent of this potentially serious outcome in studies of chronic patients [2]. Weight gain has been noted across most currently available antipsychotic agents, including first-generation “typical” antipsychotics, but large inter-individual differences are observed and mechanisms remain poorly understood [3].
Pharmacogenetic studies of antipsychotic-induced weight gain have primarily focused on serotonergic mechanisms, with a modest but replicable effect detected for the −759T/C variant of the 5-HT2C receptor gene [4]. However, dopamine D2 receptor blockade is a property of all known antipsychotics [5], and substantial weight gain has been observed in patients taking antipsychotic agents with minimal or no serotonergic action [2,3]. It is therefore surprising that there have been no prospective, controlled trials examining dopamine D2 receptor genetic variation in antipsychotic-induced weight gain. Two naturalistic studies have reported no significant relationship between the DRD2 Taq1A polymorphism and treatment-associated weight gain in Asian cohorts [6,7].
However, Taq1A is located downstream from DRD2, and its significance to gene expression is unclear. On the other hand, a polymorphism in the 5′ promoter region (−141C Ins/Del, rs1799732), which has been demonstrated to alter gene expression in vitro and striatal receptor density in vivo [8,9], has demonstrated a replicable effect on antipsychotic treatment response [10-12]. We therefore selected this polymorphism as our top candidate for investigation. Notably, prior reports have generally demonstrated a dominant or additive effect of the deletion allele, permitting combination of relatively rare deletion homozygotes with heterozygotes into a single deletion carrier group; this methodology was adopted for the present study as well.
Because long-term treatment with antipsychotics causes substantial changes (specifically, upregulation) in dopamine receptor physiology [5], pharmacogenetic studies of patients in the first episode (FE) of schizophrenia may be methodologically advantageous in examining effects of this gene. We therefore examined FE subjects participating in a prospective, randomized, clinical trial supported by the National Institute for Mental Health (clinicaltrials.gov identifier: NCT00000374). Details of the trial have been previously reported [13]. Briefly, this trial enrolled inpatients at two facilities in the New York area spanning the socioeconomic spectrum, with research assessments made by a single team of reliable, blinded raters trained at the Zucker Hillside Hospital. All patients were in the first episode of a diagnosis with schizophrenia, schizoaffective, or schizophreniform disorder and had no more than 12 weeks prior exposure to antipsychotic medications. The majority (79%) of subjects in the overall trial were antipsychotic-naïve, and a similar proportion (76%) of subjects in the pharmacogenetic study were antipsychotic-naïve. In this study, only five subjects had more than seven days of prior treatment with antipsychotics (haloperidol and/or risperidone). Nine additional subjects had 1-7 days of prior antipsychotic treatment (median=3 days). Exclusion criteria were 1) meeting DSM-IV criteria for a current substance-induced psychotic disorder, psychotic disorder due to a general medical condition, or mental retardation; 2) medical condition/treatment known to affect the brain; 3) any medical condition requiring treatment with a medication with psychotropic effects; 4) medical contraindications to treatment with olanzapine or risperidone; 5) significant risk of suicidal or homicidal behavior.
Randomization to olanzapine or risperidone was stratified by sex, current DSM-IV-defined substance abuse or dependence (excluding nicotine and caffeine), and site. Our strategy was to find the lowest effective dose, starting at 2.5 mg for olanzapine and 1 mg for risperidone. A slowly increasing titration schedule was used: after week 1, dose increases occurred at intervals of 1–3 weeks until the subject improved or reached a maximum daily dose of 20 mg of olanzapine or 6 mg of risperidone. Compliance was checked by report of both the patient and a caregiver, supplemented by pill counts. Dose was computed as a function of total medication ingested each week. Subjects with persistent mood symptoms unresponsive to antipsychotic treatment were prescribed sertraline for depression (n=2) or divalproex sodium for manic symptoms (n=8). The acute treatment phase lasted 16 weeks.
Our sample comprised 58 subjects (76% male; mean age 23.5±4.9 years, range 16-38) who provided written informed consent to IRB-approved protocols for both the clinical trial and genetic analysis. (Three subjects from a prior pharmacogenetic study of treatment response [8] did not undergo weight assessment after baseline and were excluded from the present study.) This subset did not significantly differ from the total clinical trial cohort [13] on basic demographics or cumulative rates of response. Subjects were treated with randomly-assigned olanzapine (2.5-20 mg/day, n=26) or risperidone (1-6 mg, n=32). Because of ethnic heterogeneity within the sample (40% African-American; 28% Caucasian (European); 19% Hispanic; 5% Asian; 8% Other), subject ethnicity was entered as a factor into all statistical analyses.
Patients were genotyped for −14lC Ins/Del by 5′-exonuclease fluorescence assay. Genotype frequencies were in Hardy-Weinberg equilibrium (p=0.39). Because of the relatively low frequency of the deletion allele, most prior association studies have combined the small number of deletion homozygotes with heterozygous carriers [12]. Moreover, both postmortem and in vivo functional assays have demonstrated substantial effects of a single deletion allele on D2 receptors [8,9], suggesting that the deletion allele may exert a dominant effect. Because only nine subjects were homozygous for the deletion allele, patients were dichotomized for primary analyses as homozygous for the common allele or as carriers of the rare allele (n=29 Ins/Ins, n=29 Del carriers). Genotype groups (Ins/Ins vs. Del carriers) did not significantly differ on sex, diagnosis, medication assignment, or prior treatment (all p's >.30).
Weight data was collected at baseline and weekly through week 6, and biweekly thereafter. (Results were not substantively different for either raw weight or BMI, so the former measure was used for this brief report.) A mixed models approach to repeated measures analysis of variance was utilized (PROC MIXED in SAS v9.1), with time as the primary within-subject variable and genotype as the primary between-subject variable. In the mixed model, weight was expressed as the ratio of weight at a given time point relative to baseline weight, and the log was taken of this ratio; this log-ratio is comparable to percent change from baseline, while removing effects of any covariates entered in the model, including drug assignment, ethnicity, sex, and treatment site.
The potentially mediating effect of dose on the relationship between DRD2 genotype and weight gain was tested in secondary analyses, also using mixed models. Relationships between medication dose, genotype, and weight gain were examined in separate mixed models for each medication group due to the different dosing ranges for the two medications. Dose was computed as the average actual dose taken each week based on a synthesis of all sources of information about medication adherence. Because we have previously observed a significant increase in weight gain for patients in the olanzapine group who were also prescribed adjunctive divalproex sodium [12], use of concomitant divalproex was coded as a time-dependent covariate in the olanzapine analysis.
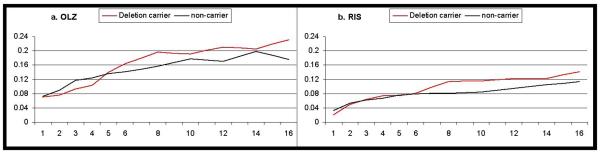
The primary mixed models analysis demonstrated that deletion carriers gained significantly more weight over time (time-by-genotype interaction F10,242=2.11, p=0.024). As demonstrated in Figure 1, deletion carriers began to separate from Ins/Ins homozygotes after about 6 weeks of treatment on either medication. The figure also clearly demonstrates the expected main effect of drug assignment (F1,44=6.10, p=0.0175), with greater weight gain on olanzapine (Fig. 1a) as compared to risperidone (Fig. 1b); however, the time-by-genotype effect was not specific to either drug, as indicated by a non-significant 3-way interaction (time-by-genotype-by-medication F10,242=0.84, p=0.60). Ethnicity was also significant in the model (F4,44=3.47, p=0.015), but this effect entered the model independently of the other effects described. There was no significant effect of sex or treatment site, and there was no main effect of genotype (genotype groups were well-matched on baseline weight). Results did not substantially change when the 5 subjects with >1 week prior exposure were excluded (time-by-genotype interaction F10,219=1.85, p=0.053). As described above, the mixed models analysis provides results in the form of log-ratios; untransformed absolute measures 16-week weight change (completers analysis) and 6-week weight change (interim analysis) are provided in the supplementary data file.
Figure 1.
Weight change from baseline over the course of the 16-week trial for patients receiving (a) olanzapine or (b) risperidone. Weight change (y-axis) is expressed as the log of the ratio of weight at a given time point relative to baseline weight. Mean values for carriers of the DRD2 −141C deletion allele are depicted in red; non-carriers are depicted in black.
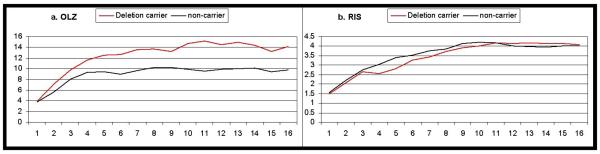
Because we previously demonstrated poorer clinical response in deletion carriers [10], we tested whether the pharmacogenetic effect on weight gain might be mediated by dose. Specifically, we wanted to rule out the possibility that the increased weight gain in deletion carriers was merely a function of higher doses administered to non-responsive patients. Mixed models analysis revealed that average dose of olanzapine was higher in deletion carriers (p=0.0258, Figure 2a, no time-by-genotype interaction, p=0.1378). However, average dose risperidone was not significantly influenced by genotype (p=0.7694; no time-by-genotype interaction, p=0.3492, Figure 2b).
Figure 2.
Mean daily medication dose (mg) received for patients receiving (a) olanzapine or (b) risperidone. Mean values for carriers of the DRD2 −141C deletion allele are depicted in red; non-carriers are depicted in black.
Consequently, we re-examined the genetic effect on olanzapine-induced weight gain adding dose to the model as a time-dependent covariate. As noted, we also added divalproex use (yes/no) as a time-dependent covariate. Ethnicity was maintained in the model, but sex and site were not entered as they were nonsignificant in all prior analyses. The time-by-genotype interaction remained marginally significant, despite the reduction in sample size (F10,115=1.82, p=0.065), and presence of adjuvant divalproex also entered the model as marginally significant (F1,3=8.47, p=0.062).
To our knowledge, this is the first study to detect a significant relationship between genetic variation in DRD2 and antipsychotic-induced weight gain in schizophrenia; our findings suggest that liability to antipsychotic-induced weight gain may be related to variation in density of D2 receptors. It should be noted that our results, which were observed in risperidone- and olanzapine-treated patients, would likely only explain some portion of the variance in weight gain that is common to most antipsychotic medications [3]. While all antipsychotics demonstrate significant D2 binding, there is no evidence of a direct relationship between D2 affinity and propensity for weight gain [3,5]. Clearly, our results do not account for the differential effects of the various medications within the class of antipsychotics, which encompass a range of additional pharmacologic effects beyond D2 binding [5].
It is possible that DRD2 promoter region variation may render D2 receptors differentially sensitive to the effects of antipsychotic medications on reward signals associated with food intake and satiety. While a reward-based mechanism is speculative at this time, such a model is consistent with multiple lines of evidence studies demonstrating a robust relationship between D2 activity and feeding behavior. For example, D2 agonists have long been shown to inhibit food intake in rodents [14]; by contrast, risperidone and other antipsychotics increase food intake and core body temperature, while reducing locomotor activity in mice [15]. Moreover, food restriction increases D2 receptor levels in rodents [16], while obesity has been associated with lower D2 levels in humans [17].
Prior studies of antipsychotics have tended to report a positive correlation between weight gain and clinical response in chronic patients [7]. However, in the present study of first episode patients, DRD2 −141C deletion carriers demonstrate increased liability for antipsychotic-induced weight gain, even though this same subgroup demonstrated reduced symptom response to medication [10]. Importantly, secondary analyses indicated that increased weight gain in deletion carriers was not primarily an effect of dose. Thus, our combined data suggests that −141C deletion carriers in their first episode of illness may be particularly vulnerable to a poor risk-benefit profile when treated with olanzapine or risperidone. Additional studies of antipsychotics with differing modes of action at the D2 receptor are warranted, as well as replication of these data in olanzapine and risperidone treated subjects. Such studies would benefit from inclusion of patients in the first episode of illness, for whom potentially confounding effects of prior treatment are minimized.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health: K01 MH65580 (Dr. Lencz), R01 MH60004 (Dr. Robinson), P30 MH60575 (Dr. Kane), K23 MH01760 and P50 MH080173 (Dr. Malhotra), and K23 DA15541 (Dr. Sevy), and by a General Clinical Research Center from the National Center for Research Resources (M01 RR18535; PI: Kevin Tracey). This work was also supported by NARSAD (Dr. Malhotra).
References
- 1.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–72. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Jiménez M, González-Blanch C, Crespo-Facorro B, et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs. 2008;22:547–62. doi: 10.2165/00023210-200822070-00002. [DOI] [PubMed] [Google Scholar]
- 3.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 4.Lencz T, Malhotra AK. Pharmacogenetics of antipsychotic-induced side effects. Dialogues in Clinical Neuroscience. 2009;11:405–15. doi: 10.31887/DCNS.2009.11.4/tlencz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZJ, Yao ZJ, Zhang XB, et al. No association of antipsychotic agent-induced weight gain with a DA receptor gene polymorphism and therapeutic response. Acta Pharmacol Sin. 2003;24:235–40. [PubMed] [Google Scholar]
- 7.Ujike H, Nomura A, Morita Y, et al. Multiple genetic factors in olanzapine-induced weight gain in schizophrenia patients: a cohort study. J Clin Psychiatry. 2008;69:1416–22. doi: 10.4088/jcp.v69n0909. [DOI] [PubMed] [Google Scholar]
- 8.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6:577–82. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–6. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 10.Lencz T, Robinson DG, Xu K, et al. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006;163:529–531. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Lencz T, Malhotra AK. Dopamine D2 receptor genetic variation and clinical response to antipsychotic drug treatment: A meta-analysis. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2009.09040598. Epub: March 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arranz MJ, de Leon J. Pharmacogenetics and pharmacogenomics of schizophrenia: a review of last decade of research. Mol Psychiatry. 2007;12(8):707–47. doi: 10.1038/sj.mp.4002009. [DOI] [PubMed] [Google Scholar]
- 13.Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163:2096–102. doi: 10.1176/ajp.2006.163.12.2096. [DOI] [PubMed] [Google Scholar]
- 14.Terry P, Gilbert DB, Cooper SJ. Dopamine receptor subtype agonists and feeding behavior. Obes Res. 1995;3:515S–523S. doi: 10.1002/j.1550-8528.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 15.Cope MB, Li X, Jumbo-Lucioni P, et al. Risperidone alters food intake, core body temperature, and locomotor activity in mice. Physiol Behav. 2009;96:457–63. doi: 10.1016/j.physbeh.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanos PK, Michaelides M, Piyis YK, et al. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.