Summary
Background
Tissue factor (TF) is an initiator of coagulation. The serine protease factor Xa (FXa) is the convergence point of the extrinsic and intrinsic components of the coagulation cascade. In addition to its hemostatic function, FXa elicits inflammatory responses in endothelial cells that may be important in surgical procedures in which inflammation is triggered. This study tested the hypothesis that FXa can upregulate TF on vascular endothelial cells by a MAPK- and NF-κB- dependent pathway.
Methods and results
Incubation of cultured human umbilical vein endothelial cells (HUVECs) with FXa increased TF protein expression and activity in a dose–dependent manner. Pre-incubation of HUVECs with the serine protease inhibitor antithrombin, which targets not only thrombin but also FXa and FIXa, inhibited FXa-induced TF expression, but the selective thrombin inhibitor hirudin did not inhibit FXa-induced TF expression, ruling out a thrombin-mediated pathway. After 10 min incubation with HUVECs, FXa rapidly induced P44/42 MAPK activation (immunoblotting of phosphorylated P44/42 MAPK) with a peak at 30 minutes. The MEK 1/2 inhibitor PD98059 partially reduced FXa-induced TF expression and activity (3.82±0.11 vs 6.54±0.08 fmol/min/cm2, P<0.05). NF-κB was activated by FXa, confirmed by cytoplasmic IkB α degradation and increased NF-κB P65 nuclear translocation. Interruption of the NF-κB pathway by the IkB α phosphorylation inhibitor Bay 11-7802 abrogated FXa-induced TF protein expression and activity (1.93± 0.02 vs 6.54±0.08 fmol/min/cm2, P<0.05). However, inhibition of PI3 kinase by LY 294002 did not attenuate FXa-induced TF protein expression and activity.
Conclusions
1) FXa upregulates TF protein expression and activity in HUVECs 2) FXa-induced upregulation of TF is independent of the thrombin-PAR1 pathway, and 3) the MAPK and NF-kB pathways, but not PI3 kinase pathway, are involved in FXa-induced TF expression on human umbilical endothelial cells. FXa may be a feed-forward alternative mechanism of activating TF expression and activity, thereby increasing a procoagulant state or inflammation. This mechanism may be important in the pro-inflammatory state initiated by cardiac surgical procedures.
Keywords: endothelial cell, Factor Xa, MAP kinase, NF-κB, tissue factor
Introduction
Tissue factor (TF) is a 47 kDa transmembrane glycoprotein that is involved in initiation of coagulation in vivo. Normally TF is absent from vascular cells that come in contact with blood. However, in pathological conditions and in cardiac surgery requiring ischemia and cardiopulmonary bypass, many factors such as cytokines, oxidants and reperfusion can induce the expression of TF on the surface of vascular endothelium [1–4]. Thrombin, the downstream product of TF activity, has been reported to enhance TF expression on human vascular endothelial cells [4]. During thrombosis, cells in the vascular wall (tunica media) are exposed to platelet activating factors as well as to procoagulant factors, including the serine proteases (serpins) thrombin and factor Xa (FXa). Both thrombin and FXa are known to induce cell signaling. FXa is the convergence point of the extrinsic and intrinsic components of the coagulation cascade. Factor X, the precursor of FXa, circulates as an inactive zymogen until activated to FXa by proteolytic cleavage between Arg52 and Ile53 by the Factor VIIa (FVIIa)–TF complex, which acts as a high-affinity receptor for FX [5]. Activation of FX to FXa occurs through multiple pathways. The FVIIa–TF complex (extrinsic pathway) and the FIXa–FVIIIa complex (intrinsic pathway) are the principle modes of activation in blood coagulation. In addition, FX bound to CD11b/CD18 (MAC-1) on monocytes can be activated to form FXa by cathepsin G.[6] During hemostasis, FXa associates with FVa and negatively charged phospholipids on platelets to form the prothrombinase complex, which in turn converts prothrombin to thrombin by proteolytic cleavage and formation of fibrin, thereby forming clots and potentially microemboli.
In addition to its essential roles in hemostasis FXa, like thrombin, functions as a signaling molecule. Recent studies have indicated that FXa upregulates TF expression [7;8], and induces expression of both pro-inflammatory cytokines, chemokines (MCP-1, IL-6, IL-8) and adhesion molecules (ICAM-1, VCAM-1) on human endothelial cells[9;10]. However, the signaling mechanisms involved in FXa–induced TF expression are not well-known. The present study tested the hypothesis that FXa upregulates TF protein expression and activity on human umbilical vein endothelial cells (HUVECs) through kinase (PI3-kinase and extracellular signal-regulated kinase (ERK, P44/42 MAPK) and NF-kB - dependent pathways. Inhibitors to each pathway were used to show a functional link between FXa-induction of TF expression and activity and the specific target pathway.
Methods and materials
Materials
Antibodies
Antibodies used were goat anti-human tissue factor (American Diagnostica, Inc., Stamford, CT); mouse anti-human NFκB p65, mouse anti-human IκBα (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); rabbit anti-phosphor P44/42 MAPK (ERK1/2 ) and phosphor Akt(Cell Signaling Technology, Beverly, MA); Mouse anti-Actin (Sigma). All secondary antibodies were obtained from Santa Cruz Biotechnology, Inc.(Santa Cruz, CA)
Protein and inhibitors
Anhibitors of MEK 1/2 - P44/42 MAPK, PI3 kinase and NF-κB pathways were used to demonstrate a functional link between FXa-induced TF xpression and activity to the related pathways. Factor VII/VIIa, Factor X and Xa, Antithrombin-III were purchased from Enzymatic Research Laboratories. Hirudin was urchased from Sigma.
Cell culture
HUVECs were isolated as previously described [11] and were cultured in 199 medium (GIBCO BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; GIBCO BRL, Grand Island, NY), 30 μg/mL of endothelial cell growth supplement (ECGS; Biomedical Technologies), 16 U/mL of heparin sodium (Elkins-Sinn Inc. Cherry Hill, NJ) and 1% penicillin/streptomycin (GIBCO-BRL). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2/95% air. For all experiments, confluent monolayers at passage 2–5 were grown in 35, 100mm culture dishes, or in 6, 24 well plates coated with 0.15% gelatin.
Cell treatment
Confluent HUVECs were pre-incubated for 1 hour in heparin-free and serum-free medium with 10 μM PD98059 (MEK1/2 inhibitor which inhibits downstream P42/44 - ERK1/2 phosphorylation, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 10μM Bay11-7802 (Calbiochem) and 10μM Ly294002 (PI3 kinase inhibitor, Calbiochem). The doses of PD 98059 (IC50 = 2 μM), Ly294002 (IC50 = 1.4 μM) and Bay 11-7802 (IC50 = 10 μM) were chosen according to previous studies. We performed preliminary studies which showed that HUVECs became detached when the doses of the three inhibitors were increased to 30 uM,. PD98059 and Ly294002 are well documented to exert non-specific effects only when they are used in large doses (>50 μM). Ly294002 does not affect the activities of EGF receptor kinase, MAP kinase, PKC, Pl 4-kinase, S6 kinase, and c-Src even at 50 μM. In our study, the dose of PD98059 was 10 μM, which may not exert non-specific effects.[23, 27, 33] For protease inhibitory experiments, 100 nmol/L Hirudin or 150 nmol/L Antithrombin III were preincubated with HUVECs for 1 hour before FXa was added to the medium. Cultures were incubated with (range 10nM to 100nM) or without (controls) increasing concentrations of FXa.
Functional TF activity assay
TF activity on the surface of HUVECs was quantified by measuring the enzymatic conversion of factor X to FXa by the TF/FVIIa complex[10] [12]. In brief, monolayers of HUVECs were washed with Hank’s Balanced Salt Solution (HBSS), followed by addition of a reaction buffer containing10mM HEPES, 140mM NaCl, 5 mM Ca2+, 10 nM FVIIa, and 100 nM FX (Enzymatic Research Laboratories, South Bend, IN). After 60 minutes incubation at 37°C, the reaction was stopped using a buffer containing 7.5 mM EDTA. After the addition of the chromogenic substrate Spectrazyme Xa (America Diagnostica), aliquots of samples were assayed for absorbance at 405 nm with the reference absorbance set at 492 nm. The rate of formation of FXa in various samples was calculated, based on a standard curve, which had been established by using known amounts of FXa (Enzymatic Research Laboratories). To confirm the specificity for TF, HUVECs were pre-incubated for 30 minutes with anti-human monoclonal TF antibody (10 μg/mL) or goat anti-human polyclonal TF antibody (15 μg/mL; American Diagnostica Inc, Greenwich, CT).
Whole cell lysates, nuclear, and cytosolic fraction extraction protocol
HUVEC monolayers were washed twice with ice cold PBS prior to scraping in extraction buffer containing 50 mM Tris, 150 mM NaCl, 1mM EGTA, 1% Triton X-100, 1% sodium deoxycholate, 1 mM sodium vanadate, 50 mM sodium fluoride, 2 mM EDTA (pH 8.0), 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml of leupeptin/pepstatin A/aprotinin. After solubilization on ice for 15 min with intermittent vortexing, the whole cell extracts were centrifuged at 16,000 × G for 15 minutes, and the supernatants were collected. Nuclear and cytosolic fractions were prepared by NE-PERR nuclear and cytoplasmic protein extraction kit (PIERCE Biotechnology, Rockford, IL) according to the manufacturer’s instructions. All steps were carried out at 4°C. The final protein concentration of the samples was determined by the Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules CA).
Western Blot Analysis
Samples containing equal amounts of protein (30ug) were reduced with 100 mM DTT, and denatured at 95°C for 5 minutes. The samples were separated by SDS-polyacrylamide gel electrophoresis at 200 V for 30 minutes, and proteins were transferred to nitrocellulose (Hybond ECL, Amersham ) at 100 V for 1 hour. The blot was blocked overnight at 4°C with 5% non fat milk in Tris-buffered saline (TBS, pH 7.6), containing 0.1% Tween-20 (TBS-T), followed by three washes with TBS-T. The blot was incubated overnight at 4°C with primary antibody, followed by washing in TBS-T, and incubation with a goat anti-rabbit or a goat anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz CA) at room temperature for 1 hour. After washing, the immunoblots were incubated with ECL detection system to enhance visualization of the immunoreactive bands, and then exposed to ECL chemiluminescent film for 2–5 minutes.
Statistical analysis
All experiments were repeated at least four separate times. In each of the at least four experiments, either duplicate or triplicate plates were analyzed for each parameter observed. All values are expressed as means ± standard error of the mean (SEM). Comparisons between groups were analyzed using one way analysis of variance (ANOVA) followed by Student-Newman-Kuels post hoc analysis for multiple comparisons. A p value <0.05 was considered significant.
Results
Factor Xa increases TF protein expression and activity in HUVECs
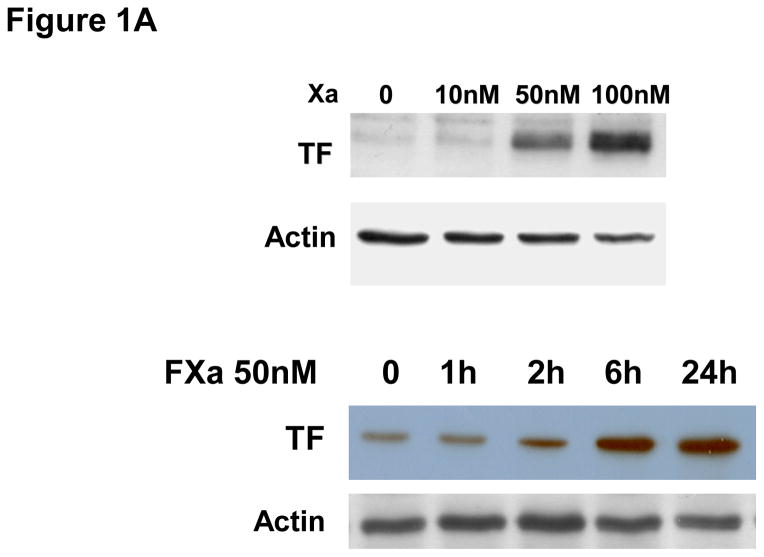
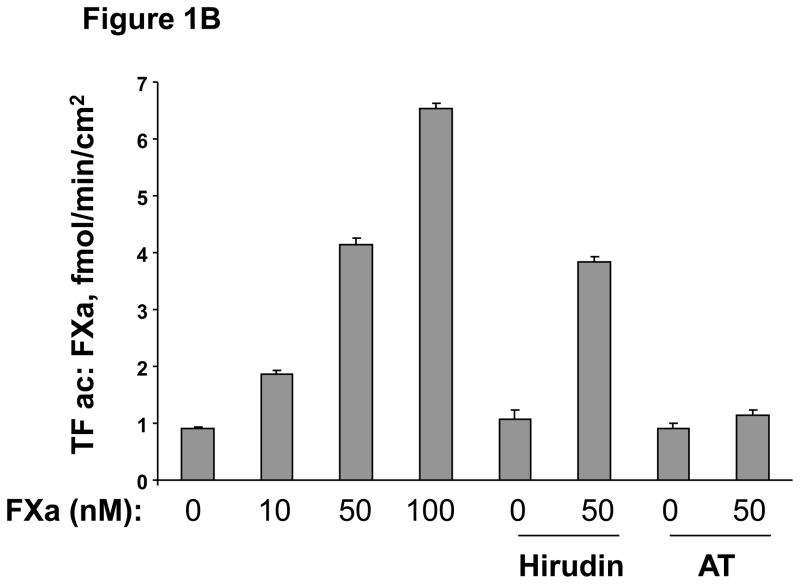
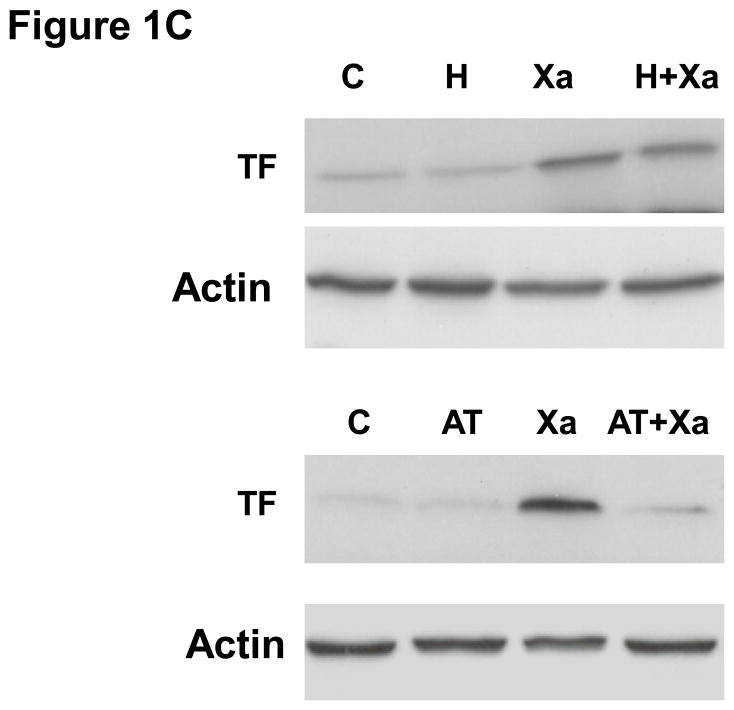
To determine if Factor Xa increases the expression of TF in HUVECs, cells were treated with increasing concentrations of Factor Xa (10nM to 100nM) for 6 hours, after which FXa was washed out. TF protein was almost undetectable in cells in the absence of FXa (Figure 1A). Western blot results show that incubation of HUVECs with FXa for 6 hours induced TF protein expression in a concentration-dependent manner (Fig 1A). Increased TF protein levels may suggest an increased level of activity. Accordingly, we measured TF activity on the surface of HUVECs (Fig 1B) after incubation with incremental concentrations of FXa. FXa increased TF activity in a concentration-dependent manner, consistent with the increases in protein level. Therefore, incubation of HUVECs with FXa increased both TF protein levels and activity. Next we investigated the expression of TF on HUVECs incubated with FXa (50 nM) for incremental periods of time. Western blots showed that FXa - induced TF expression appeared after 1 hour treatment, had peaked by 6 hours and persisted for 24 hours (Figure 1A). In the presence of Ca2+ and phospholipids, FXa converts prothrombin to thrombin (accelerated by FVa)[13]. To verify that increased TF expression was not stimulated by thrombin generated by the TF/FVIIa/FXa complex, the HUVECs were incubated in medium containing FXa and either antithrombin-III (AT-III), which inhibits both FXa and thrombin, or the thrombin-specific inhibitor hirudin for 30 minutes. The cells were then washed and co-incubated with 50 nM FXa for 6 hour, and TF protein and activity were again quantified. AT-III completely inhibited FXa-induced TF expression and activity (Fig 1B, C). However, hirudin had no effect on Factor Xa-induced TF protein expression and activity. These data suggest that FXa induces TF expression via a thrombin-independent mechanism.
Figure 1.
A. Factor Xa-induced TF expression in HUVECs in a concentration-dependent manner. HUVECs were incubated with increasing concentrations of Factor Xa for 6 hours. Lower panel: Time course of FXa–induced TF expression. HUVECs were incubated with 50 nM FXa for indicated time periods. Whole cell lysates were extracted and immunoblotted for TF. Actin was used as a loading control. The immunoblot is representative of 4 independent experiments.
B. FXa increased tissue factor activity on the surface of HUVECs in a concentration-dependent manner. HUVECs were cultured and incubated with or without increasing concentration of FXa for 6 hours. Tissue factor activity was assayed by a chromogenic assay as described in material and methods. TF activity was not affected by Hirudin pretreatment, but was inhibited by AT-III. C. TF protein level was not affected by hirudin, but was suppressed by antithrombin-III.
Activation of the MEK 1/2 - P44/42 MAPK (ERK-1/2) pathway is required for FXa-induced TF expression
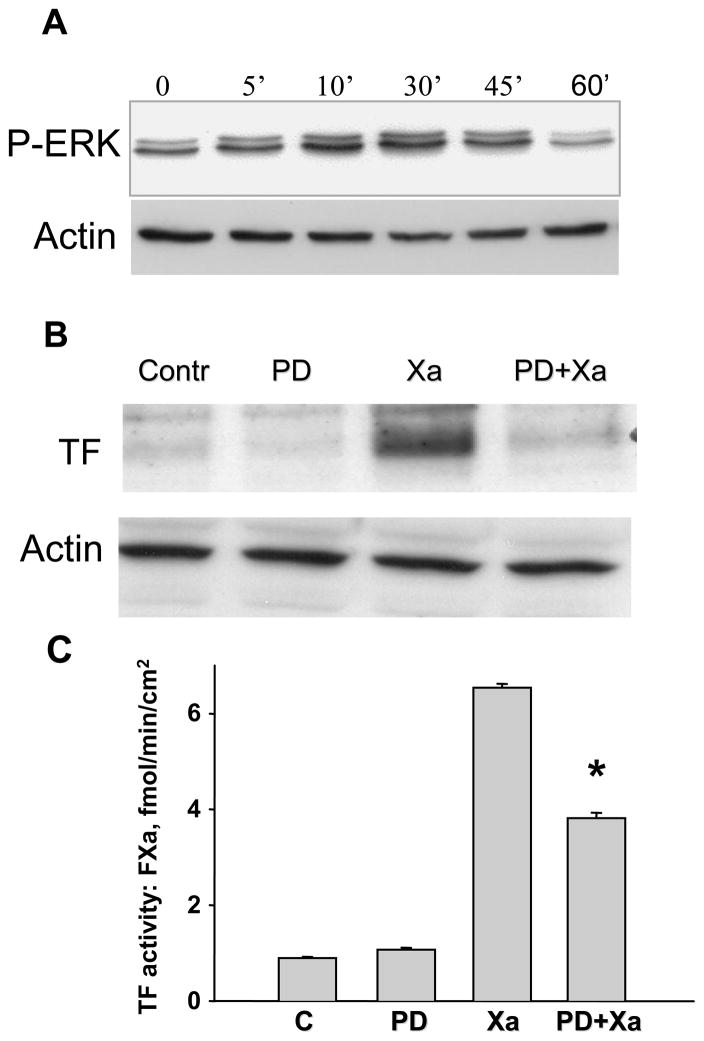
To define the signal transduction pathways that link FXa-induced TF expression, we investigated whether FXa induction of TF expression and activity could be inhibited by antagonists to MEK 1/2/P44/42 MAPK (ERK1/2), PI3 kinase, or NF-κB. P44/42 MAPK (ERK-1/2) phosphorylation (Fig 2 Panel A) increased as early as 5 minutes after treatment with FXa, peaking 30 minutes after exposure to FXa. This entire time course was inhibited by the MEK1/2 inhibitor PD 98059 (data not shown). To determine a functional link between the P44/42 MAPK - ERK1/2 pathway and FXa-induced TF protein expression, HUVECs were pre-incubated for 1 hour with the selective MEK1/2 inhibitor PD 98059 (10 μM), after which HUVECs were incubated with FXa (50 nM) for an additional 6 hours; parallel controls without inhibitor and FXa were incubated for a similar time course. As shown in Figure 2 (Panel B), PD 98059 alone in the absence of FXa (Control) had no effect on TF protein expression. In contrast, 10 μm PD 98059 inhibited FXa-induced TF protein expression. TF activity, quantified by generation of FXa from FX substrate, was unaffected by PD 98059 alone; the activity stimulated by transient incubation with FXa was partially inhibited by 10 μm PD 98059 by approximately 40% (Figure 2C). These data suggest that the stimulation of TF protein expression and activity by FXa involves, at least in part, the P44/42 MAPK - ERK-1/2 pathway.
Figure 2.
A. Time course of P44/42 MAPK ERK1/2 activation by FXa. HUVECs were incubated with or without 50 nM FXa for 0, 5, 10, 30, or 60 minutes. Whole lysates were prepared and immunoblotted for active ERK using phosphospecific antibody. B. TF protein expression was significantly attenuated by a selective inhibitor of MEK 1/2 (PD 98059). HUVECs were pre-incubated for 1 hour with or without 10 μM PD 98059. The cultures were then incubated for 6 hour with or without Factor Xa. Immunoblots are representative of 3 independent experiments. C. Inhibition of MEK 1/2 - ERK1/2 attenuated FXa-induced TF activity. HUVECs were cultured in a 24 well plates and pretreated for 1hour with or without 10 μM of the MEK 1/2 inhibitor PD 98059. The cultures were then incubated for 6 hours with or without Factor Xa. Tissue factor activity was assayed by a chromogenic assay as described in material and methods. * P<0.05, compared to FXa treatment.
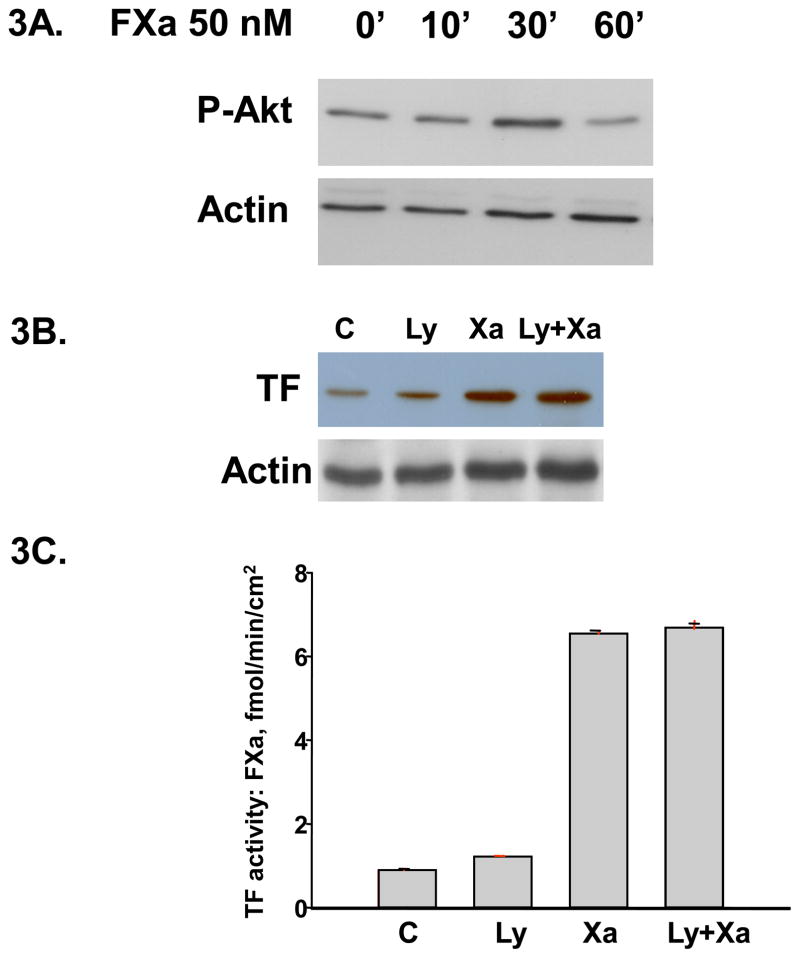
Inhibition of PI-3 Kinase did not attenuate Factor Xa - induced TF expression
To investigate whether the PI3 kinase pathway is involved in TF expression by Factor Xa, we first determined whether FXa can induce the activation of PI3 Kinase/Akt pathway. HUVEcs were incubated with 50nM FXa for different periods of 0, 10, 30 and 60 minutes and whole lysates were extracted. Western blot analysis showed that phosphor-Akt significantly increased after 30 minutes incubation of FXa (Figure 3A). To further investigate the functional link of PI3 kinase/Akt activation in FXa-induced TF expression, HUVECs were pre-incubated with or without (controls) 10μM LY294002, a specific inhibitor of PI-3 kinase, for 1 hour, followed by 6 hours incubation with 50 nM Factor Xa. LY294002 alone did not affect TF protein expression (Figure 3B) or TF activity assessed by generation of FXa (Figure 3C). The FXa-induced increase in TF activity was not attenuated by inhibition of PI-3 kinase by Ly294002 (Figure 3C). These results suggest that FXa-induced increases in TF expression are not mediated via the PI-3 kinase pathway.
Figure 3.
A. Time course of PI3 kinase/Akt activation by FXa. HUVECs were incubated with 50 nM FXa for indicated time periods. Whole cell lysates were extracted and immunoblotted for phosphor-Akt. Actin was used as a loading control. The immunoblot is representative of 3 independent experiments. B. and C. Inhibition of PI3 Kinase had no effect on Xa-induced TF protein and activity. HUVECs were cultured and pretreated for 1h with or without 10 μM LY 294002 (an PI3K inhibitor). The cultures were then incubated for 6 hours with or without 50 nM FXa. TF protein was shown by Western blot and activity was assayed by a chromogenic assay as described in material and methods.
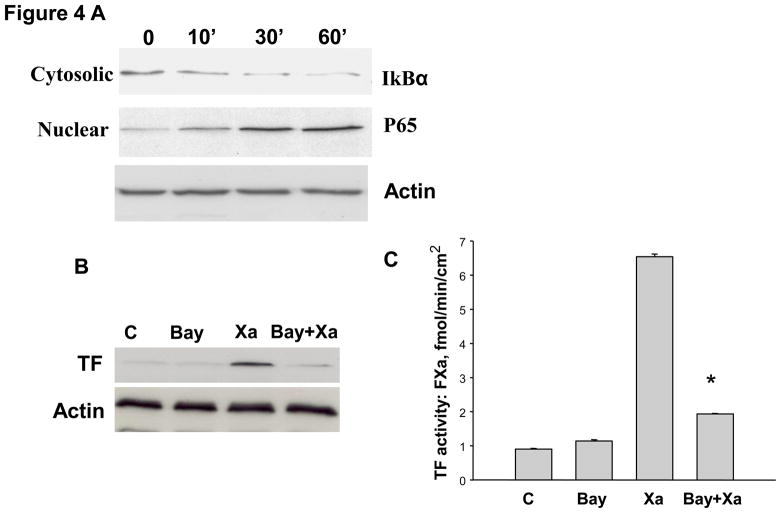
FXa - induced TF expression involves activation of NF-κB
Stimulation of TF expression by FXa involves activation of the transcription factor NF-kB. In unstimulated HUVECs, NF-kB is sequestered in the cytoplasm by IkB. Upon stimulation, IkB is phosphorylated and subsequently degraded, allowing NF-kB to translocate to the nucleus to initiate transcription.[14] To test the hypothesis that the stimulation of TF expression by FXa involves activation of NF-kB, the levels of IkBα in the cytoplasm of HUVECs and nuclear translocation of P65 fraction of NF-κB were determined with and without stimulation by FXa. HUVECs were incubated with 50nM FXa for incrementally increasing periods of time. At each time point, HUVECs were harvested and the cytoplasmic and nuclear protein fractions were extracted, and Western blot analysis was performed. FXa rapidly induced IκBα degradation starting at 10 minutes of incubation, which continued up to 60 minutes of incubation with FXa (Figure 4A). Accordingly, FXa increased the appearance of NF-κB P65 in the nuclear fraction in a similar time course to IκBa, suggesting nuclear translocation of P65 (Figure 4A). This finding suggests that FXa activates NF-kB through the classic pathway via activating IκB Kinase (IKK). To determine whether the NF-kB pathway is functionally involved in FXa-induced TF expression in HUVECs, Bay 11-7802 was used to selectively inhibit IkBα phosphorylation by IKK, and thereby inhibit IkBα degradation and subsequent NF-kB (P65) translocation to the nucleus during incubation of HUVECs with FXa. Bay 11-7802 alone had no effect on TF expression compared to controls (Figure 4B). However, TF expression was inhibited by Bay11-7802. In addition, Bay11-7802 alone did not alter TF activity, but significantly inhibited FXa-induced TF activity (Figure 4C). These data suggest that FXa upregulates TF protein and activity in a NF-kB–dependent manner.
Figure 4.
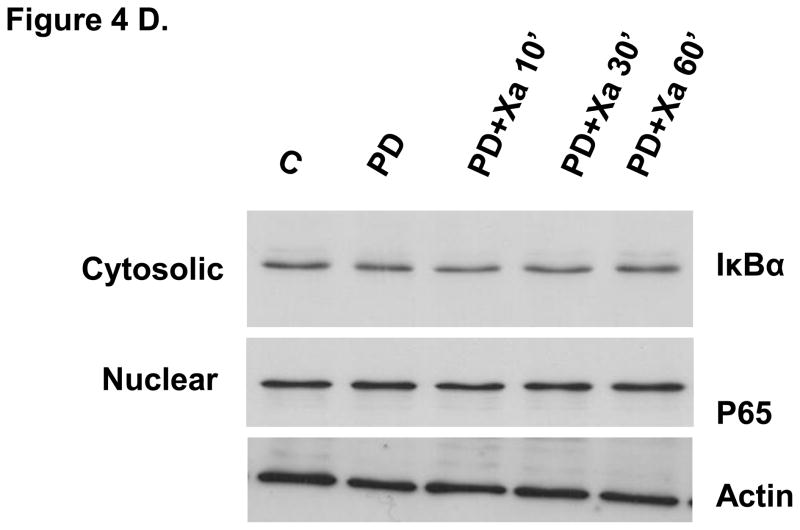
A. Factor Xa stimulated mobilization of NF-κB (P65) to the nucleus. HUVECs were incubated with 50 nM FXa for the indicated times. Nuclear and cytosolic extracts were prepared and immunoblotted for IκBα and NF-κB P65. Immunoblots are representative of 4 independent experiments. B. Tissue factor expression was greatly attenuated by NF-κB inhibitor. HUVECs were preincubated for 1 hour with or without 10 μM Bay 11-7802, an IKK inhibitor. The cultures were then incubated for 6 hour with or without Factor Xa. Immunoblots are representative of 3 independent experiments. C. Inhibition of NF-kB attenuated Xa-induced TF activity. HUVECs were cultured in 24 well plate and pretreated for 1h without or with 10 μM Bay 11-7802 (an IKK inhibitor). The cultures were then incubated for 6 h without or with Factor Xa. Tissue factor activity was assayed by a chromogenic assay as described in material and methods. * P<0.05, compared to FXa treatment. D. The effect of inhibition of MEK1/2–P44/42 MAPK on NF- κB. HUVECs were incubated with 50 nM FXa in the presence or absence of PD 98059 for the indicated times. Nuclear and cytosolic extracts were prepared and immunoblotted for IκBα and NF-κB P65. Immunoblots are representative of 4 independent experiments.
Regulation of NF-kB by the MEK 1/2–P44/42 MAPK pathway
We have demonstrated that both the MEK 1/2–P44/42 MAPK and NF-kB pathways are involved in the induction of TF by FXa. To determine whether FXa–induced MEK 1/2–P44/42 MAPK activation is involved in the activation of NF-κB, we analyzed cytoplasmic and nuclear extracts from HUVECs incubated with FXa in the presence or absence of PD98059 for NF-kB activation. Western blot analysis was performed to determine whether MEK 1/2 inhibitor PD98059 inhibits FXa induced IκBα degradation and P65 nuclear translocation (Figure 4A). PD 98059 completely inhibited IκBα degradation and attenuated P65 nuclear translocation (Figure 4D). This finding suggests that the activation of NF-κB is at least partially regulated by MEK 1/2–P44/42 MAPK pathway.
Discussion
The present study demonstrates that stimulation of HUVECs with FXa increases TF protein expression and activity, consistent with previous studies.[7;8] This stimulation of TF expression and activity was independent of thrombin since the substrate for thrombin formation was absent from the medium, and further confirmed by the failure of the direct thrombin inhibitor hirudin to block FXa-induced TF expression. However, these previous studies did not investigate the molecular signaling mechanisms by which transient exposure to FXa stimulates increased TF protein expression and the activity in HUVECs. In the present study, we demonstrated that the stimulation of TF expression by FXa involved MEK 1/2/P44/42 MAPK (ERK1/2), since blockade with PD98059 partially inhibited TF stimulation by FXa. However, the specific PI-3 kinase inhibitor LY 294002 did not attenuate FXa-induced TF expression or activity, suggesting that the PI-3 kinase pathway is not involved. In addition, stimulation of TF by FXa did involve the transcription factor NF-κB and translocation of P65 to the nucleus, since inhibition of IκBα phosphorylation by IKK inhibitor (Bay 11-7802) blocked FXa-induced increased expression of TF. Therefore, the plasma protease FXa may stimulate TF expression and activity in addition to other stimulating factors such as reactive oxygen species, cytokines, and exposure of subendothelial surfaces. This mechanism of TF upregulation may be important in the pro-inflammatory and procoagulant response of the vascular endothelium to oxidant generation, ischemia-reperfusion, shock, cardiac surgery involving cardiopulmonary bypass [15], and septicemia, in which activation of the vascular endothelium is a critical factor leading to myocardial and endothelial injury.
Recently, it has become evident that in addition to their critical role in hemostasis, plasma serine proteases trigger a diversity of cellular responses, including pro-inflammatory responses to cardiopulmonary bypass, reperfusion [16–18], and septic shock. For example, it is well known that the serine protease thrombin acts via its specific protease activated receptor type 1 (PAR1) as a potent pro-inflammatory mediator to endothelial cells by selectively upregulating the surface expression of P-selectin. This event is key in the recruitment of neutrophils to the endothelial surface and initiation of the process of “rolling” during reperfusion [19;20]. In addition, thrombin directly increases vascular permeability and induces cytokine release [8;21]. Factor Xa induces the release of the cytokines IL-6, IL-8 and TNFα in human endothelial cells.[9;10]. Factor Xa has also been shown to mediate a variety of biological effects in addition to its hemostatic functions. There is evidence that factor Xa itself can directly trigger inflammation both in vitro and in vivo[5] [10]. This pro-inflammatory effect would be consistent with the amplification of TF expression with subsequent increases in thrombin generation which may contribute to activation of the vascular endothelium. Herault et al[7] showed that factor Xa-induced TF expression in HUVECs was dependent on its serine protease catalytic activity. Data from the present study support the concept that FXa also directly stimulates both TF expression and activity independent of thrombin since the increased TF expression and activity were observed in the absence of prothrombin, and were not inhibited by the direct thrombin inhibitor hirudin.
Relatively few studies have focused on the signaling pathways involved in factor Xa-induced expression of TF in HUVECs. Recent studies provide compelling evidence that ERK1/2 and p38 MAPK are important in the upregulation of TF expression by cytokines and thrombin in vascular endothelial cells.[8;22–24] Although inhibition of P44/42 MAPK - ERK1/2 by PD 98059 attenuated TF expression, Western blot analysis and TF activity assay showed that this was only a partial (~40%) attenuation. These data suggest that other pathways may be involved in the stimulation of TF by FXa.
The present study demonstrates that NF-κB is a major pathway in the stimulation of TF expression and activity by factor Xa. NF-κB is a heterodimer composed of a 50 kDa DNA binding subunit and a 65 kDa transactivator unit (p65). The cytoplasmic protein IκB is phosphorylated by IκB kinases upon stimulation by proinflammatory mediators such as TNFα and LPS, leaving the P65 subunit free to translocate to the nucleus where it binds to the promoter region of many genes [14]. Indeed, the TF gene is regulated in part by NF-κB; the TF promoter contains binding sites not only for NF-κB,[25] but also for other transcription factors such as Egr-1 and AP-1. For instance, the induction of TF in endothelial cells and monocytes by agents such as TNFα and the bacterial toxin lipopolysaccharide (LPS) appears to be regulated primarily by the AP-1, NF-κB and Sp1 transcription factors,[26–28] whereas the transcriptional factor Egr1 has been shown to be important in promoting TF activation in endothelial cells under shear stress.[29] The present study demonstrates that the NF-κB pathway is a key pathway in the induction of TF protein expression by factor FXa in HUVECs under normoxic conditions since inhibition with Bay 11-7802 attenuated factor Xa-induced TF protein expression and activity. Factor Xa rapidly induced IκBα degradation necessary for translocation of P65 to the nucleus. Blockade of this step with Bay 11-7802 prevented translocation and attenuated the increased expression of TF by FXa.
In this study, we demonstrated that both MEK1/2- P44/42 MAPK and NF-κB pathways are involved in FXa–induced TF expression in human endothelial cells. The finding that inhibition of MEK1/2- P44/42 MAPK suppressed FXa–induced IκBα degradation and attenuated P65 nuclear translocation suggests that MEK 1/2–P44/42 MAPK may play a role in modulating NF-κB activity associate with FXa.
The PI-3 kinase–Akt pathway is involved in a number of responses, notably in the survival response to ischemia-reperfusion[30] and potentially postconditioning,[31] although the latter situation is controversial.[32] This pathway was shown in one study to inhibit TF expression stimulated by vascular endothelial growth factor (VEGF) in endothelial cells, and therefore may be a negative regulator of TF expression.[33] In the present study, the PI-3 kinase–Akt pathway is activated by FXa, however, it had no apparent functional involvement in factor Xa-induced TF expression.
Limitations
The present study used HUVECs, which may phenotypically differ from other endothelial cells such as coronary arterial and venous cells that undergo inflammatory responses in clinically relevant pathological situations such as ischemia-reperfusion and cardioplegic arrest. Factor X is produced in the liver and carried in plasma at concentrations normally approximating 140 nM. Therefore, the 50 nM concentration of FXa used to stimulate TF expression may be relevant to local concentrations achieved proximate to intravascular thrombi. However, in vivo, FXa generation is tightly regulated by AT and tissue factor pathway inhibitor (TFPI). Under certain conditions (e.g. cardiopulmonary bypass), AT and TFPI may be decreased, and FXa levels may increase (e.g. after rFVIIa administration). Another limitation is that cellular components of coagulation, e.g. platelets/monocytes, are not included in our model. Finally, we did not study the role of protease activated receptors in the increased protein expression and activity of TF stimulated by factor Xa. The serine protease activity of factor Xa cleaves and activates PAR1 or PAR2 receptors on endothelial cells, which involves many biological responses induced by FXa [8;37–39].
In conclusion, the present data demonstrate that Factor Xa induces the expression of TF via NF-kB – dependent and MEK 1/2 - ERK1/2-dependent pathways. These data provide support for a positive feedback mechanism between the coagulation protease Factor Xa and TF expression on cultured endothelial cells. This pathway may further amplify the TF-related inflammatory response to ischemia-reperfusion, cardiopulmonary bypass, in addition to other stimuli such as oxygen radical species and cytokines. Indeed, TF[34] and its downstream product thrombin [35;36] have been shown to increase in patients presenting with acute myocardial infarction.
Acknowledgments
The authors would like to thank L. Susan Schmarkey and Sara Katzmark for technical assistance. The study was supported in part by a grant from NIH (HL069487 to JV-J) and a postdoctoral fellowship award from the American Heart Association (to RJ). We appreciate the continued support from the Carlyle Fraser Heart Center Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shimizu T, Nishihira J, Watanabe H, Abe R, Honda A, Ishibashi T, Shimizu H. Macrophage migration inhibitory factor is induced by thrombin and factor Xa in endothelial cells. J Biol Chem. 2004;279:13729–13737. doi: 10.1074/jbc.M400150200. [DOI] [PubMed] [Google Scholar]
- 2.Bachli EB, Pech CM, Johnson KM, Johnson DJ, Tuddenham EGD, McVey JH. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J Thromb Haemost. 2003;1:1935–1944. doi: 10.1046/j.1538-7836.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 3.Hollenstein UM, Pernerstorfer T, Homoncik M, Hansen JB, Finzen H, Handler S, Jilma B. Effect of factor X inhibition on coagulation activation and cytokine induction in human systemic inflammation. J Infect Disease. 2002;186:1270–1276. doi: 10.1086/344646. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Pelekanakis K, Woolkalis MJ. Thrombin and tumor necrosis factor α synergistically stimulate tissue factor expression in human endothelial cells. J Biol Chem. 2004;279:36142–36147. doi: 10.1074/jbc.M405039200. [DOI] [PubMed] [Google Scholar]
- 5.Iba T, Kidokoro A, Fukunaga M, Fuse S, Suda M, Kunitada S, Hara T. Factor Xa-inhibitor (DX-9065a) modulates the leukocyte-endothelial cell interaction in endotoxemic rat. Shock. 2002;17:159–162. doi: 10.1097/00024382-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Akahane K, Okamoto K, Kikuchi M, Todoroki F, Higure A, Ohuchida T, Kitahara K, Takeda S, Itoh H, Ohsato K. Inhibition of factor Xa suppresses the expression of tissue factor in human monocytes and lipopolysaccharide-induced endotoxemia in rats. Surgery. 2001;130:809–818. doi: 10.1067/msy.2001.116452. [DOI] [PubMed] [Google Scholar]
- 7.Herault JP, Bono F, Avril C, Schaeffer P, Herbert JM. Activation of human vascular endothelial cells by factor Xa: effect of specific inhibitors. Biochem Pharmacol. 1999;57:603–610. doi: 10.1016/s0006-2952(98)00348-7. [DOI] [PubMed] [Google Scholar]
- 8.McLean K, Schirm S, Johns A, Morser J, Light DR. FXa-induced responses in vascular wall cells are PAR-mediated and inhibited by ZK-807834. Thromb Res. 2001;103:281–297. doi: 10.1016/s0049-3848(01)00330-9. [DOI] [PubMed] [Google Scholar]
- 9.Senden NHM, Jeunhomme TMAA, Heemskerk JWM, Wagenvoord R, van’t Veer C, Hemker HC, Buurman WA. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161:4318–4324. [PubMed] [Google Scholar]
- 10.Buschi G, Seitz I, Steppich B, Hess S, Eckl R, Schomig A, Ott I. Coagulation factor Xa stimulates interleukin-8 release in endothelial cells and mononuclear leukocytes implications in acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1–6. doi: 10.1161/01.ATV.0000151279.35780.2d. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe EA, Nechman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto Y, Kawai Y, Watanabe K, Murata M, Handa M, Nakamura S, Ikeda Y. Fluid shear stress attenuates tumor necrosis factor-α-induced tissue factor expression in cultured human endothelial cells. Blood. 1998;91:4164–4172. [PubMed] [Google Scholar]
- 13.Krishnaswamy S, Walker RK. Contribution of the prothrombin fragment 2 domain to the function of factor Va in the prothrombinase complex. Biochemistry. 1997;36:3319–3330. doi: 10.1021/bi9623993. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 15.Boyle EM, Verrier ED, Spiess BD. Endothelial cell injury in cardiovascular surgery: The procoagulant response. Ann Thorac Surg. 1996;62:1549–1557. doi: 10.1016/0003-4975(96)00836-3. [DOI] [PubMed] [Google Scholar]
- 16.Pruefer D, Makowski J, Dahm M, Guth S, Oelert H, Darius H, Buerke M. Aprotinin inhibits leukocyte-endothelial cell interactions after hemorrhage and reperfusion. Ann Thorac Surg. 2003;75:210–215. doi: 10.1016/s0003-4975(02)03932-2. [DOI] [PubMed] [Google Scholar]
- 17.Pruefer D, Buerke U, Khalili M, Dahm M, Darius H, Oelert H, Buerke M. Cardioprotective effects of the serine protease inhibitor aprotinin after regional ischemia and reperfusion on the beating heart. J Thorac Cardiovasc Surg. 2002;124:942–949. doi: 10.1067/mtc.2002.123703. [DOI] [PubMed] [Google Scholar]
- 18.Reeves JG, Jiang R, Mykytenko J, Schmarkey LS, Wang NP, Kin H, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Inhibition of serine protease activity at reperfusion reduces postischemic injury in a closed-chest model of acute myocardial infarction. JACC. 2006;47:170A. [Google Scholar]
- 19.Kubes P, Jutila M, Payne D. Therapeutic potential of inhibiting leukocyte rolling in ischemia/reperfusion. J Clin Invest. 1995;95:2510–2519. doi: 10.1172/JCI117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 21.Kranzhöfer R, Clinton SK, Ishii K, Coughlin SR, Fenton JW, II, Libby P. Thrombin potently stimulates cytokine production in human vascular smooth muscle cells but not in mononuclear phagocytes. Circ Res. 1996;79:286–294. doi: 10.1161/01.res.79.2.286. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson AC, Nachman RL, Altieri DC, Summers BD, Ruf W, Edgington TS, Hajjar DP. Effector cell protease receptor-1 is a vascular receptor for coagulation factor Xa. J Biol Chem. 1996;271:28407–28413. doi: 10.1074/jbc.271.45.28407. [DOI] [PubMed] [Google Scholar]
- 23.Nishibe T, Parry G, Ishida A, Aziz S, Murray J, Patel Y, Rahman S, Strand K, Saito K, Saito Y, Hammond WP, Savidge GF, Mackman N, Wijelath ES. Oncostatin M promotes biphasic tissue factor expression in smooth muscle cells: evidence for Erk-1/2 activation. Blood. 2001;97:692–699. doi: 10.1182/blood.v97.3.692. [DOI] [PubMed] [Google Scholar]
- 24.Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105:1756–1759. doi: 10.1161/01.cir.0000015465.73933.3b. [DOI] [PubMed] [Google Scholar]
- 25.Mackman N. Regulation of the tissue factor gene. FASEB Journal. 1995;9(10):883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- 26.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:612–621. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 27.Oeth P, Parry GC, Mackman N. Regulation of the tissue factor gene in human monocytic cells: role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arterioscler Thromb Vasc Biol. 1997;17:365–374. doi: 10.1161/01.atv.17.2.365. [DOI] [PubMed] [Google Scholar]
- 28.Parry GC, Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain kappa B-like sites that specifically bind c-Rel-p65 heterodimers. J Biol Chem. 1994;269:20823–20825. [PubMed] [Google Scholar]
- 29.Houston P, Dickson MC, Ludbrook V, White B, Schwachtgen JL, McVey JH, Mackman N, Reese JM, Gorman DG, Campbell C, Braddock M. Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. Arteriosclerosis Thrombosis and Vascular Biology. 1999;19:281–289. doi: 10.1161/01.atv.19.2.281. [DOI] [PubMed] [Google Scholar]
- 30.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. TCM. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 32.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol (Heart Circ Physiol) 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 33.Blum S, Issbruker K, Willuweit A, Hehlgans S, Lucerna M, Mechtcheriakova D, Walsh K, von der Ahe D, Hofer E, Clauss M. An Inhibitory Role of the Phosphatidylinositol 3-Kinase-signaling Pathway in Vascular Endothelial Growth Factor-induced Tissue Factor Expression. The Journal of Biological Chemistry. 2001;276:33428–33434. doi: 10.1074/jbc.M105474200. [DOI] [PubMed] [Google Scholar]
- 34.Moons AHM, Levi M, Peters RJG. Tissue factor and coronary artery disease. Cardiovasc Res. 2002;53:313–325. doi: 10.1016/s0008-6363(01)00452-7. [DOI] [PubMed] [Google Scholar]
- 35.Reganon E, Vila V, Martinez-Sales V, Vaya A, Aznar J. Inflammation, fibrinogen and thrombin generation in patients with previous myocardial infarction. Haematologica. 2002;87:740–745. [PubMed] [Google Scholar]
- 36.Roldan V, Marin F, Fernandez P, Lujan J, Martinez JG, Pineda J, Marco P, Sogorb F. Tissue factor/tissue factor pathway inhibitor system and long-term prognosis after acute myocardial infarction. Int J Cardiol. 2001;78:115–119. doi: 10.1016/s0167-5273(00)00471-x. [DOI] [PubMed] [Google Scholar]
- 37.Riewald M, Kravchenko VV, Petrovan RJ, O’Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood. 2001;97:3109–3116. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- 38.Bono F, Schaeffer P, Herault JP, Michaux C, Nestor AL, Guillemot JC, Herbert JM. Factor Xa activates endothelial cells by a receptor cascade between EPR-1 and PAR-2. Arteriosclerosis Thrombosis and Vascular Biology. 2000;20 :E107–E112. doi: 10.1161/01.atv.20.11.e107. [DOI] [PubMed] [Google Scholar]
- 39.Daubie V, Cauwenberghs S, Senden NH, Pochet R, Lindhout T, Buurman WA, Heemskerk JW. Factor Xa and thrombin evoke additive calcium and proinflammatory responses in endothelial cells subjected to coagulation. Biochim Biophys Acta. 2006;1763:860–869. doi: 10.1016/j.bbamcr.2006.04.010. [DOI] [PubMed] [Google Scholar]