Abstract
Objective
To compare the prevalence of left ventricular (LV) diastolic dysfunction in subjects with and without rheumatoid arthritis (RA), among those with no history of heart failure (HF), and to determine risk factors for diastolic dysfunction in RA.
Methods
We conducted a cross-sectional, community-based study comparing cohorts of adult RA and non-RA subjects without a history of HF. Standard 2D/Doppler echocardiography was performed in all participants. Diastolic dysfunction was defined as impaired relaxation (with or without increased filling pressures) or advanced reduction in compliance or reversible or fixed restrictive filling.
Results
The study included 244 RA subjects and 1448 non-RA subjects. Mean age was 60.5 years in the RA cohort (71% female) and 64.9 years (50% female) in the non-RA cohort. The vast majority (>98%) of both cohorts had preserved ejection fraction (EF≥50%). Diastolic dysfunction was more common in RA subjects at 31% compared to 26% (age and sex adjusted) in non-RA subjects (OR 1.6; 95% CI 1.2, 2.4). RA subjects had significantly lower LV mass, higher pulmonary arterial pressure, and higher left atrial volume index than non-RA subjects. RA duration and IL-6 level were independently associated with diastolic dysfunction in RA even after adjustment for cardiovascular risk factors.
Conclusion
Subjects with RA have a higher prevalence of diastolic dysfunction than those without RA. RA duration and IL-6 are independently associated with diastolic dysfunction suggesting the impact of chronic autoimmune inflammation on myocardial function in RA. Clinical implications of these findings require further investigation.
INTRODUCTION
We have previously shown that patients with rheumatoid arthritis (RA) have about a 2-fold increased risk of heart failure (HF) and mortality, which is not explained by traditional cardiovascular (CV) risk factors and/or clinical ischemic heart disease. (1-3) We have also recently shown that RA subjects with HF have fewer typical signs and symptoms of HF and are more likely to have preserved ejection fraction (EF≥50%) compared to non-RA subjects with HF. (4) The reasons for this are poorly understood, but these observations suggest there may be inherent differences in ventricular function, particularly diastolic, in persons with RA compared to persons without RA. The important questions are whether these differences in ventricular function may be evident in RA patients even before symptoms of HF develop clinically and whether there are RA-specific determinants for these changes.
Isolated diastolic dysfunction occurs frequently without clinically recognized HF (5), and may be one mechanism for the excess development of HF in RA patients. In previous population-based studies, isolated diastolic dysfunction was common and was associated with a marked increase in all-cause mortality in the general population, underscoring the importance of this disease entity. (5,6) Previous studies have shown that RA patients without clinically evident CV disease have a higher prevalence of left ventricular (LV) diastolic dysfunction compared to controls without RA. (7-16) However, these studies recruited highly selected patients from hospitals or academic centers rather than from community populations, and most included less than 60 consecutive patients with RA. Thus, information from population-based studies is lacking and the mechanisms underlying the development of diastolic dysfunction in RA remain unclear.
The purpose of this study was to compare the prevalence of left ventricular diastolic dysfunction in subjects with RA, without a history of HF, to non-RA subjects without HF in a community-based population and to determine risk factors associated with diastolic dysfunction in RA.
METHODS
Study Subjects and Design
Using the resources of the Rochester Epidemiology Project (REP) (17), a population-based medical records linkage system that allows access to complete medical records from all community medical providers, we conducted a population-based study of residents of Olmsted County, Minnesota aged ≥18 years who first fulfilled 1987 American College of Rheumatology (ACR) classification criteria for RA between 1/1/1980 and 12/31/2005. (18) From this RA incidence cohort (19), we identified eligible RA subjects, alive and living in Olmsted County without a history of HF (based on Framingham criteria (20)). We recruited 244 (61%) of the 397 eligible RA subjects.
We performed a cross-sectional study comparing these RA subjects to subjects drawn from the same underlying community without either RA or a history of HF. The latter subjects, referred to as the non-RA cohort, were part of a population-based study examining the burden of ventricular dysfunction in the community. (5) This previous study recruited patients from a random sample of Olmsted County residents age ≥ 45 years in 1997. Study participants returned for a second study visit in the years 2001-2004. These data were used for comparison to our RA subjects. All subjects had undergone echocardiography according to previously defined protocols. The institutional review boards of the Mayo Clinic and Olmsted Medical Center approved this study.
Data Collection
Data collection for subjects in both the RA and non-RA cohorts was identical except RA subjects were asked additional questions pertaining to their RA disease. Subjects in both cohorts completed a questionnaire, provided a blood sample, and underwent an echocardiogram. The questionnaire included identical questions pertaining to HF symptoms, CV risk factors and medication usage. Demographic characteristics were recorded. Presence of the following traditional CV risk factors was ascertained: smoking (current or former); diabetes mellitus (based on physician diagnosis and/or documented use of insulin and/or oral hypoglycemic agents); hyperlipidemia (based on clinically obtained elevated fasting lipid values of total cholesterol ≥240 mg/dL, low-density cholesterol ≥160 mg/dL, triglycerides ≥200 mg/dL or high-density cholesterol <40 mg/dL and/or documented use of lipid lowering agents); body mass index (BMI, kg/m2) and hypertension (based on physician diagnosis and/or documented use of antihypertensive drugs). Data on history of ischemic heart disease (IHD) (presence of angina pectoris, coronary artery disease, myocardial infarction [MI; including silent events], and coronary revascularization procedures [i.e. coronary artery bypass graft, percutaneous angioplasty, insertion of stents and atherectomy]) were also gathered.
For individuals in the RA cohort, rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-alpha) and interleukin 6 (IL-6) levels were measured in the provided blood sample. RF testing was performed by nephelometry (latex enhanced assay; Behring Nephelometer II, Dade Behring, Inc., Newark, DE). Anti-CCP testing was performed by enzyme immunoassay from INOVA Diagnostics (San Diego, CA). IL-6 and TNF-alpha tests were performed by enzyme immunoassay from R & D Systems (Minneapolis, MN). CRP testing was performed by immunoturbidimetric assay (Roche CRPLX reagent, Indianapolis, IN). Medical records were reviewed to collect data on RA disease characteristics. The questionnaire was augmented to obtain the health assessment questionnaire (HAQ) disability score and RA medication usage at the time of the echocardiography visit, including systemic corticosteroids, disease-modifying antirheumatic drugs (DMARDs), biologic agents, and nonsteroidal anti-inflammatory drugs (NSAIDs). Systemic corticosteroid use included either oral or intravenous forms (e.g., prednisone, methylprednisolone, hydrocortisone, and/or dexamethasone); DMARDs included methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, and/or azathioprine; and biologic agents included tumor necrosis factor alpha (TNF-alpha) blockers, anakinra, abatacept, and/or rituximab. RA medication usage at the echocardiography visit was verified with patients’ pill bottles and by reviewing the most recent medication list in the medical record for discrepancies.
Two-dimensional and Doppler echocardiograms were performed on all subjects in the RA cohort following identical protocols as those used in the non-RA cohort, as previously described (5). All echocardiograms were performed by registered diagnostic cardiac sonographers and interpreted in the Mayo Clinic Echocardiographic Laboratory (by BLK and DDB). The following echocardiographic parameters were measured and/or estimated in each subject: pulmonary arterial pressure, left atrial volume index, LV mass, LV mass index, tricuspid regurgitant jet velocity, pulsed wave Doppler examination of mitral inflow peak early filling velocity (E) and velocity at atrial contraction (A) (before and during Valsalva maneuver) and of pulmonary venous inflow; as well as Doppler tissue imaging of the mitral annulus, E/A ratio, E/E’ ratio and deceleration time.
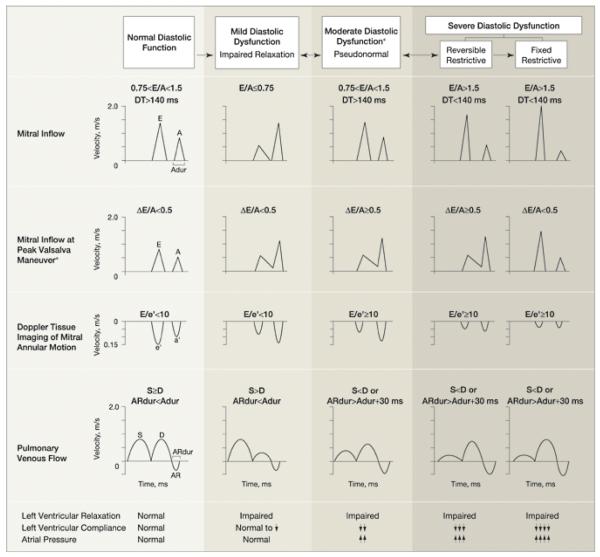
Diastolic dysfunction was categorized as none (normal diastolic function); mild, defined as impaired relaxation without evidence of increased filling pressures; moderate, defined as impaired relaxation associated with moderate elevation of filling pressures or pseudonormal filling; and severe, defined as advanced reduction in compliance or reversible or fixed restrictive filling (Figure 1). (5) Diastolic function was classified as indeterminate when these criteria could not be fully assessed, such as in cases of dysrhythmias. Subjects were classified into 4 mutually exclusive categories of normal diastolic function; mild diastolic dysfunction; moderate to severe diastolic dysfunction; or indeterminate diastolic function.
Figure 1. Doppler Criteria for Classification of Diastolic Function*.
*Adapted with permission from Redfield MM, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289(2):194-202. Copyright © (2003) American Medical Association. All Rights reserved.
Statistical Methods
Descriptive statistics were used to summarize the demographics, CV risk factors, and echocardiographic features for both cohorts, as well as the RA disease characteristics for the RA cohort. Descriptive statistics in the non-RA cohort were adjusted to the age and sex distribution of the RA cohort to allow for visual comparison. This adjustment was performed by weighting the non-RA data according to the proportions of RA patients in each sex and decade of age. This technique is often used to perform direct standardization to allow comparison of rates in epidemiology studies. Differences between the two cohorts were tested using linear and logistic regression models adjusted for age and sex. Logistic regression models were also used to examine factors associated with the presence of any diastolic dysfunction. Secondary analyses were performed using matched cohorts. In these analyses, each RA subject was matched to a non-RA subject with similar age (±1 year), sex, and CV risk factors (smoking status, presence of hypertension (defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg), BMI (±2 kg/m2) and presence of diabetes mellitus). Conditional logistic regression was used to examine the association between diastolic dysfunction and RA/non-RA status in the matched cohorts.
RESULTS
The study population included 244 subjects with RA and 1448 subjects without RA. Subjects with clinically diagnosed heart failure were excluded from both cohorts. Baseline characteristics for both cohorts are reported in Table 1. RA patients were somewhat younger than non-RA subjects. A higher proportion of RA subjects were female. The proportion of ever smokers, but not current smokers, was higher in RA vs non-RA subjects. RA subjects were more likely to have a history of hypertension. Blood pressure at the time of the echocardiographic study in both RA patients and non-RA subjects was within the normal range, although RA patients had somewhat higher measures of systolic and diastolic blood pressure compared to non-RA subjects (Table 1). There were no significant differences in diabetes mellitus, hyperlipidemia, BMI or history of IHD in the 2 cohorts.
Table 1.
Cardiovascular risk factors in RA and non-RA patients
| Cardiovascular risk factor | RA (n=244) |
Non-RA* (n=1448) |
p-value |
|---|---|---|---|
| Age, mean ± SD (years) | 60.5 ± 13.2 | 64.9 ± 9.5 | <0.001 |
| Female, number (%) | 174 (71) | 730 (50) | <0.001 |
| Smoking | |||
| - Ever | 113 (56) | 661 (36) | 0.005 |
| - Current | 15 (6) | 86 (4) | 0.98 |
| Diabetes mellitus | 28 (11) | 128 (6) | 0.19 |
| Hyperlipidemia | 97 (40) | 695 (38) | 0.46 |
| BMI, mean ± SD (kg/m2) | 28.4 ± 5.8 | 28.4 ± 5.4 | 0.39 |
| Blood pressure at the time of echo examination: |
|||
| – Systolic Blood pressure, mm Hg | 129.6 ± 17.8 | 122.4 ± 18.8 | <0.001 |
| – Diastolic blood pressure, mm Hg | 71.1 ± 8.9 | 68.6 ± 10.3 | <0.001 |
| Diagnosis of hypertension/use of antihypertensive drugs |
157 (64) | 738 (42) | <0.001 |
| History of ischemic heart disease | 29 (12) | 214 (10) | 0.48 |
All reported percentages are adjusted to the age and sex distribution of the RA patients.
RA = rheumatoid arthritis; BMI = body mass index; SD = standard deviation
The vast majority (98%) of both cohorts had preserved ejection fraction (EF≥50%), and the proportions with preserved EF in the two cohorts were similar (Table 2). However, diastolic dysfunction of any severity was significantly more common in the RA subjects at 31% compared to 26% in non-RA subjects (odds ratio [OR] 1.6; 95% confidence interval [CI] 1.2, 2.4; p=0.006).
Table 2.
Echocardiographic measurements in RA and non-RA patients
| Measurement | RA n |
RA (n=244) |
Non-RA n |
Non-RA* (n=1448) |
p- value |
|---|---|---|---|---|---|
| Diastolic dysfunction (any) | 76 (31) | 449 (26) | 0.006 | ||
| - None | 118 (48) | 709 (57) | |||
| - Mild | 56 (23) | 261 (15) | |||
| - Moderate/severe | 20 (8) | 188 (11) | |||
| - Indeterminate | 50 (20) | 290 (17) | |||
| Left atrial volume index (cc/m2) >28 cc/m2 |
199 | 26.0 ± 6.4 60 (30) |
1393 | 22.9 ± 7.2 344 (19) |
<0.001 <0.001 |
| Tricuspid regurgitant jet velocity (m/sec) |
171 | 2.43 ± 0.27 | 932 | 2.33 ± 0.33 | <0.001 |
| E/A ratio | 233 | 1.76 ± 0.59 | 1420 | 1.09 ± 0.37 | <0.001 |
| E/E’ ratio | 237 | 9.5 ± 4.2 | 1426 | 10.2 ± 4.3 | 0.010 |
| Pulmonary artery pressure (mm Hg) |
146 | 29.7 ± 6.3 | 932 | 22.1 ± 5.4 | <0.001 |
| LV mass index (g/m2) | 200 | 83.1 ± 15.6 | 1071 | 88.3 ± 20.4 | 0.001 |
| Ejection fraction (%) <50% |
244 | 61.8 ± 6.4 4 (2) |
1435 | 64.8 ± 6.6 35 (2) |
<0.001 0.76 |
All reported percentages are adjusted to the age and sex distribution of the RA patients.
RA = rheumatoid arthritis; LV = left ventricular
Age, sex, blood pressure and BMI were significantly associated with diastolic dysfunction overall, and a history of IHD approached statistical significance (p=0.06) (data not shown). There were no significant interactions between these variables and RA/non-RA status. After adjustment for age, sex, blood pressure, BMI and history of IHD, RA subjects were still more likely to have diastolic dysfunction compared to non-RA subjects (OR 1.4; 95% CI 0.9, 2.0). However, adjusting for these risk factors somewhat attenuated the risk of diastolic dysfunction in RA and the association was not statistically significant. This association remained unchanged when blood pressure was removed from the list of adjustors.
A secondary analysis was performed to address the concern of higher prevalence of some traditional CV risk factors in RA versus non-RA subjects, in which RA and non-RA subjects were matched for age, sex, smoking status, BMI, presence of hypertension, and presence of diabetes mellitus. Diastolic dysfunction was still more common in RA subjects compared to non-RA subjects (OR 1.6, 95% CI 0.9, 2.7). However, this association did not reach statistical significance.
Our finding of increased prevalence of diastolic dysfunction in RA subjects was further supported by other echo findings (Table 2). RA subjects had a higher pulmonary arterial pressure and higher left atrial volume index compared to non-RA subjects (Table 2). The proportion of subjects with high left atrium volume index (>28 cc/m2) was also significantly higher in the RA as compared to the non-RA cohort. The tricuspid regurgitant jet velocity and E/A ratio were both significantly higher in the RA than non-RA subjects, consistent with increased filling pressures seen in diastolic dysfunction (Table 2). The mean LV mass index was lower in RA subjects as compared to non-RA subjects (p=0.001).
We further investigated the impact of RA characteristics on myocardial function. Disease characteristics of RA subjects are summarized in Table 3. Median disease duration was 8.2 years, with 71% seropositive for RF and 45% seropositive for CCP. About one half of RA subjects had erosive disease on radiography. At the time of the echocardiography visit 54% of RA subjects used methotrexate, 27% used hydroxychloroquine and 10% of patients used other DMARDs. About one third of RA subjects were treated with corticosteroids, 63% used NSAIDs and 16% were on biologic therapy.
Table 3.
Disease characteristics of RA patients and association with diastolic dysfunction
| Disease characteristic | RA (n=194) † |
Odds ratio (95% CI)** Adjusted for age, sex and RA duration |
Odds ratio** (95% CI) Adjusted for age, sex, RA duration and CV risk factors†† |
|---|---|---|---|
| RA duration, years, median (IQR) |
8.2 (4.7, 13.6) | 3.2 (1.8, 5.4) | 3.3 (1.8, 5.9) |
| RF positive, number (%) | 138 (71) | 1.0 (0.5, 2.1) | 1.0 (0.4, 2.1) |
| CCP positive, number (%) | 80 (45) | 0.8 (0.4, 1.7) | 0.8 (0.4, 1.8) |
| C-reactive protein, mg/L, median (IQR) |
2.3 (0.9, 4.8) | 1.1 (0.8, 1.5) | 1.1 (0.8, 1.5) |
| IL-6, pg/mL, median (IQR) | 2.4 (1.4, 4.2) | 1.2 (1.02, 1.4) | 1.2 (1.01, 1.4) |
| TNF-alpha, pg/mL, median (IQR) |
1.6 (1.1, 3.3) | 1.0 (0.98,1.01) | 1.0 (0.99, 1.00) |
| Erosions / destructive changes on radiographs, number (%) |
125 (51) | 0.8 (0.4, 1.6) | 0.8 (0.4, 1.6) |
| HAQ score, median (IQR) | 0.3 (0.0, 0.8) | 1.2 (0.7, 2.1) | 1.1 (0.6, 1.9) |
| Medication usage * | |||
| Methotrexate | 105 (54) | 2.1 (1.01, 4.2) | 1.8 (0.9, 3.8) |
| Hydroxychloroquine | 53 (27) | 0.7 (0.3, 1.6) | 0.8 (0.3, 1.7) |
| Other DMARDs | 19 (10) | 1.9 (0.6, 5.6) | 2.0 (0.6, 6.5) |
| Biologics | 32 (16) | 1.0 (0.4, 2.6) | 1.1 (0.4, 2.7) |
| Corticosteroids (systemic) | 53 (27) | 1.2 (0.6, 2.6) | 1.1 (0.5, 2.4) |
| NSAIDs | 122 (63) | 0.9 (0.4, 1.8) | 0.9 (0.4, 2.0) |
RA = rheumatoid arthritis; RF = rheumatoid factor; CCP = cyclic citrullinated peptide antibody; IL-6 = interleukin 6; TNF-alpha = tumor necrosis factor alpha; IQR = interquartile range; HAQ = Health Assessment Questionnaire; DMARDs = disease-modifying antirheumatic drugs, NSAIDs = nonsteroidal anti-inflammatory drugs
At time of echocardiographic visit. Systemic corticosteroids included either oral or intravenous forms (e.g., prednisone, methylprednisolone, hydrocortisone, dexamethasone). Other DMARDs included sulfasalazine, leflunomide, azathioprine. Biologics included TNF-alpha blockers, anakinra, abatacept, rituximab
Odds ratios reported per IQR where indicated.
Patients with indeterminate diastolic dysfunction excluded
CV risk factors include those listed in Table 1
RA duration and IL-6 level were significantly associated with diastolic dysfunction (OR 3.2, 95%CI 1.8, 5.4 and OR 1.2 per 2.8 pg/mL, 95%CI 1.02, 1.4, respectively), even after adjustment for CV risk factors (Table 3). There was no apparent association between TNF-alpha level and LV diastolic dysfunction (OR 1.0 per 2.2 pg/mL, 95%CI 0.98, 1.01). Methotrexate use was significantly associated with diastolic dysfunction (OR 2.1, 95%CI 1.01, 4.2). Use of other DMARDs was associated with 1.9-fold increased risk of LV diastolic dysfunction; however, this association did not reach statistical significance (OR 1.9, 95%CI 0.6, 5.6). After adjustment for CV risk factors patients treated with methotrexate and other DMARDs were still more likely to have diastolic dysfunction (OR 1.8, 95%CI 0.9, 3.8 and OR 2.0, 95% 0.6, 6.5, respectively), although these associations did not reach statistical significance (Table 3).
To examine the possibility of confounding by indication in methotrexate users, we performed additional analyses adjusting for RA characteristics (RF positivity, CRP, and IL-6). Following this additional adjustment, the association between methotrexate and diastolic dysfunction was somewhat attenuated and was no longer statistically significant (OR 1.7, 95%CI 0.7, 3.8, p=0.21). Furthermore, there was no association between duration of methotrexate use and diastolic dysfunction. We did not find statistically significant associations of diastolic dysfunction with other RA characteristics (i.e. RF and CCP positivity, CRP level, erosive changes and HAQ score) or with medications (including biologics, corticosteroids and NSAIDs) (Table 3).
DISCUSSION
Herein, we report the first large, population-based echocardiographic investigation of myocardial function among RA subjects compared to non-RA subjects without a history of heart failure from the same community. We have shown that patients with RA have a higher prevalence of diastolic dysfunction (31%) than non-RA subjects (26%) based on currently accepted diagnostic criteria. Diastolic dysfunction was more common in RA than in the non-RA subjects even after adjustment for or matching for CV risk factors, although the associations were marginally significant. We have also found that diastolic dysfunction in RA is associated with RA duration and IL-6 level, even after adjustment for CV risk factors. Notably, the vast majority of subjects had preserved EF. The presence of diastolic dysfunction with preserved EF, or isolated diastolic dysfunction, has been previously associated with a marked increase in mortality in the general population (5,6). Hence, the increased prevalence of isolated diastolic dysfunction in RA may have implications on excess mortality in RA patients.
Other echocardiographic findings supportive of the increased prevalence of diastolic dysfunction in RA subjects included higher pulmonary arterial pressure and left atrial volume index in the RA cohort compared to the non-RA cohort. Pulmonary arterial hypertension has been reported to be more prevalent among RA patients than controls. (21, 22) While this finding resonates with the higher prevalence of any diastolic dysfunction in RA compared to non-RA subjects, we cannot exclude the possibility that higher pulmonary arterial pressures were due to pulmonary abnormalities such as interstitial lung disease in the RA cohort. However, a larger left atrial volume index would not be expected from interstitial lung disease alone.
Previous studies have reported an association between RA and features of impaired diastolic ventricular function, as reviewed by Giles et al. (23) Using Doppler determination of transmitral flow velocity, alterations of the E/A ratio have been demonstrated for RA patients compared to controls in several studies (14,16,17,24-27). Left atrial size and left atrial volume have been noted to be increased among RA patients. Increased LV diameter, increased LV mass and LV hypertrophy (16) have been reported and were independently associated with RA (28). However, similar to our findings, some other authors found lower LV mass index in RA compared to non-RA subjects, as measured by cardiac magnetic resonance imaging (cMRI) (29). Despite the differences in the direction of changes of LV mass in different studies these findings are not necessarily contradictory. There is a possibility of sequential changes in LV mass in RA from myocardial hypertrophy to myocardial wasting (i.e. myocardial remodeling). It is notable that RA subjects in the study reporting high LV mass were somewhat younger (mean age 46.7 years) than RA subjects in either our study (mean age 60.5 years) or the Giles study (mean age 59 years) both reporting low LV mass. (28,29)
Concurrent with several earlier studies (24-26), the duration of RA disease in our study was strongly associated with diastolic dysfunction, even after adjustment for traditional CV risk factors. This may reflect a chronic subclinical myocardial process leading to impairment of myocardial function as previously hypothesized (12). Concordant with this hypothesis, a number of studies suggested a strong relationship between RA characteristics, including extra- articular RA, and diastolic dysfunction (8,9,24,31). The association of diastolic dysfunction with IL-6 in our study further emphasizes the impact of immune dysregulation on myocardial abnormalities in RA. IL-6 was previously found to closely correlate with LV systolic and diastolic dysfunction in the general population (32). Our data suggest that this cytokine may also be implicated in diastolic dysfunction in RA.
Antirheumatic medications did not appear to have a major impact on diastolic dysfunction in our study. Confounding by indication or contraindication might account for the increased likelihood of LV dysfunction in methotrexate users. For example, given concerns about heart failure with the use of TNF-alpha blockers, patients at high risk of heart failure may be preferentially placed on methotrexate, rather than TNF-alpha blockers. While some evidence from recent studies suggests that TNF-alpha blockers may confer beneficial effect with regard to the risk of HF (33), studies on the changes of diastolic function with TNF-alpha blockade are lacking. In our study there was no apparent association of biologics with the risk of LV diastolic dysfunction. The reasons for this are uncertain, and the impact of antirheumatic medications on diastolic dysfunction requires further investigation.
Our study had several potential limitations. First, the nature of a cross-sectional study limits the types of conclusions that can be drawn, as these observations are associations and not necessarily causal. Second, the population of Olmsted County is over 90% white, and the generalizability of our findings to populations with different race/ethnicity may be limited. In addition, we could not fully exclude the possibility of participation bias, as subjects in both cohorts had to agree to filling out questionnaires and receiving echocardiograms. Finally, the higher proportion of subjects with history of hypertension in the RA versus non-RA cohorts could be expected to affect the diastolic dysfunction measurements unfavorably. However, this seems unlikely as blood pressures at the time of the study in both cohorts were within the normal range suggesting that hypertension was well-controlled. Further, adjustment for blood pressure did not affect the likelihood of LV diastolic dysfunction in RA suggesting that other factors including RA-related factors may affect myocardial function.
Our study also had several strengths. It is the first large community-population based study of subjects without a history of HF, comparing the prevalence of diastolic dysfunction in RA subjects to non-RA subjects and determining risk factors for diastolic dysfunction in RA. Because it was a large population based study, the RA cohort was representative of those patients meeting ACR classification criteria for RA in a defined community population. By utilizing the comprehensive medical record linkage system of the REP, a large number of patients in each cohort could be identified, integrated within two large population-based NIH-funded cohorts. Furthermore, our study utilized a standardized echocardiogram protocol with rigorous definitions for mild, moderate, and severe diastolic dysfunction.
In conclusion, among subjects without a history of HF, subjects with RA have a higher prevalence of diastolic dysfunction than those without RA. Diastolic dysfunction in RA was associated with RA duration and IL-6 level, even after adjustment for CV risk factors. Our findings suggest the role of chronic autoimmune inflammation in development of diastolic dysfunction in RA. The clinical implications of these findings, particularly the impact of diastolic dysfunction on the excess risk of HF and mortality in RA, require further investigation.
Acknowledgments
Funding Source: This work was funded by grants from the National Institutes of Health, NIAMS (R01 AR46849) and NHLBI (R01 HL 55502) and made possible by a grant from the National Institutes of Health, NIAMS (AR-30582)
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicenses such use and exploit all subsidiary rights, as set out in our license (http://ARD.bmjjournals.com/ifora/licence.pdf)
REFERENCES
- 1.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Crowson CS, Maradit-Kremers H, Ballman KV, Roger VL, Jacobsen SJ, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006;54(1):60–7. doi: 10.1002/art.21560. [DOI] [PubMed] [Google Scholar]
- 3.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–20. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 4.Davis JM, 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58(9):2603–11. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 7.Yazici D, Tokay S, Aydin S, Toprak A, Inanc N, Khan SR, et al. Echocardiographic evaluation of cardiac diastolic function in patients with rheumatoid arthritis: 5 years of follow-up. Clin Rheumatol. 2008;27(5):647–50. doi: 10.1007/s10067-007-0820-x. [DOI] [PubMed] [Google Scholar]
- 8.Wislowska M, Jaszczyk B, Kochmanski M, Sypula S, Sztechman M. Diastolic heart function in RA patients. Rheumatol Int. 2008;28(6):513–9. doi: 10.1007/s00296-007-0473-8. [DOI] [PubMed] [Google Scholar]
- 9.Udayakumar N, Venkatesan S, Rajendiran C. Diastolic function abnormalities in rheumatoid arthritis: relation with duration of disease. Singapore Med J. 2007;48(6):537–42. [PubMed] [Google Scholar]
- 10.Meune C, Wahbi K, Assous N, Weber S, Kahan A, Allanore Y. Myocardial dysfunction in rheumatoid arthritis: a controlled tissue-Doppler echocardiography study. J Rheumatol. 2007;34(10):2005–9. [PubMed] [Google Scholar]
- 11.Rexhepaj N, Bajraktari G, Berisha I, Beqiri A, Shatri F, Hima F, et al. Left and right ventricular diastolic functions in patients with rheumatoid arthritis without clinically evident cardiovascular disease. Int J Clin Pract. 2006;60(6):683–8. doi: 10.1111/j.1368-5031.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 12.Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional Doppler and tissue Doppler: relation with duration of disease. Clin Rheumatol. 2006;25(3):294–9. doi: 10.1007/s10067-005-0014-3. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. The increased risk of ventricular diastolic dysfunction and congestive heart failure in patients with rheumatoid arthritis is independent of the duration of the disease. Semin Arthritis Rheum. 2005;35(2):132–3. doi: 10.1016/j.semarthrit.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Alpaslan M, Onrat E, Evcik D. Doppler echocardiographic evaluation of ventricular function in patients with rheumatoid arthritis. Clin Rheumatol. 2003;22(2):84–8. doi: 10.1007/s10067-002-0677-y. [DOI] [PubMed] [Google Scholar]
- 15.Di Franco M, Paradiso M, Mammarella A, Paoletti V, Labbadia G, Coppotelli L, et al. Diastolic function abnormalities in rheumatoid arthritis. Evaluation By echo Doppler transmitral flow and pulmonary venous flow: relation with duration of disease. Ann Rheum Dis. 2000;59(3):227–9. doi: 10.1136/ard.59.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrao S, Salli L, Arnone S, Scaglione R, Pinto A, Licata G. Echo-Doppler left ventricular filling abnormalities in patients with rheumatoid arthritis without clinically evident cardiovascular disease. Eur J Clin Invest. 1996;26(4):293–7. doi: 10.1046/j.1365-2362.1996.133284.x. [DOI] [PubMed] [Google Scholar]
- 17.Kremers H Maradit, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30(4):819–34. vii. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel SECC, Kremers H Maradit, Therneau TM. The Rising Incidence of Rheumatoid Arthritis [abstract] Arthritis Rheum. 2008;58(suppl):S453. [Google Scholar]
- 20.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 21.Keser G, Capar I, Aksu K, Inal V, Danaoglu Z, Savas R, et al. Pulmonary hypertension in rheumatoid arthritis. Scand J Rheumatol. 2004;33(4):244–5. doi: 10.1080/03009740410005809. [DOI] [PubMed] [Google Scholar]
- 22.Dawson JK, Goodson NG, Graham DR, Lynch MP. Raised pulmonary artery pressures measured with Doppler echocardiography in rheumatoid arthritis patients. Rheumatology (Oxford) 2000;39(12):1320–5. doi: 10.1093/rheumatology/39.12.1320. [DOI] [PubMed] [Google Scholar]
- 23.Giles JT, Fernandes V, Lima JA, Bathon JM. Myocardial dysfunction in rheumatoid arthritis: epidemiology and pathogenesis. Arthritis Res Ther. 2005;7(5):195–207. doi: 10.1186/ar1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montecucco C, Gobbi G, Perlini S, Rossi S, Grandi AM, Caporali R, et al. Impaired diastolic function in active rheumatoid arthritis. Relationship with disease duration. Clin Exp Rheumatol. 1999;17(4):407–12. [PubMed] [Google Scholar]
- 25.Levendoglu F, Temizhan A, Ugurlu H, Ozdemir A, Yazici M. Ventricular function abnormalities in active rheumatoid arthritis: a Doppler echocardiographic study. Rheumatol Int. 2004;24(3):141–6. doi: 10.1007/s00296-003-0342-z. [DOI] [PubMed] [Google Scholar]
- 26.Yavasoglu I, Senturk T, Onbasili A. Diastolic dysfunction in rheumatoid arthritis and duration of disease. Rheumatol Int. 2008;29(1):113–4. doi: 10.1007/s00296-008-0625-5. [DOI] [PubMed] [Google Scholar]
- 27.Wislowska M, Sypula S, Kowalik I. Echocardiographic findings, 24-hour electrocardiographic Holter monitoring in patients with rheumatoid arthritis according to Steinbrocker’s criteria, functional index, value of Waaler-Rose titre and duration of disease. Clin Rheumatol. 1998;17(5):369–77. doi: 10.1007/BF01450894. [DOI] [PubMed] [Google Scholar]
- 28.Rudominer RL, Roman MJ, Devereux RB, Paget SA, Schwartz JE, Lockshin MD, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60(1):22–9. doi: 10.1002/art.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giles JMA, Fernandes V, Post W, Szklo M, Bluemke D, Petri M, Gelber A, Lima J, Bathon J. Rheumatoid Arthritis is Associated with a Reduction in Left Ventricular Mass Assessed by Cardiac Magnetic Resonance Imaging (cMRI). [abstract] Arthritis Rheum. 2008;58(suppl):S419. [Google Scholar]
- 30.Wutzen J. Ultrastructural examination of rat myocardium on low-magnesium diet during administration of methotrexate. Mater Med Pol. 1991;23:92–6. [PubMed] [Google Scholar]
- 31.Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, Ollier WE, et al. Echocardiographic and Doppler findings in long-term treated rheumatoid arthritis patients without clinically evident cardiovascular disease. Semin Arthritis Rheum. 2004;33(4):231–8. doi: 10.1053/j.semarthrit.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Karpiński L, Płaksej R, Kosmala W, Witkowska M. Serum levels of interleukin-6, interleukin-10 and C-reactive protein in relation to left ventricular function in patients with myocardial infarction treated with primary angioplasty. Kardiol Pol. 2008 Dec;66(12):1279–85. [PubMed] [Google Scholar]
- 33.Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, Wassenberg S, Zink A. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008 Mar;58(3):667–77. doi: 10.1002/art.23281. [DOI] [PubMed] [Google Scholar]