Summary
The adult hippocampal dentate gyrus (DG) exhibits cell proliferation and neurogenesis throughout life. We examined the effects of daily administration of eszopiclone (Esz), a commonly used hypnotic drug and GABA agonist, compared to vehicle, on DG cell proliferation and neurogenesis, and on sleep-wake patterns. Esz was administered during the usual sleep period of rats, to mimic typical use in humans. Esz treatment for 7 days did not affect the rate of cell proliferation, as measured by 5-bromo-2’-deoxyuridine (BrdU) immunostaining. However, twice daily Esz administration for two weeks increased survival of newborn cells, by 46%. Most surviving cells exhibited a neuronal phenotype, identified BrdU-NeuN double-labeling. NeuN (Neuronal nuclei) is a marker of neurons. NREM sleep was increased on day one, but not on days 7 or 14 of Esz administration. Delta EEG activity was increased on days 1 and 7 of treatment, but not on day 14.
There is evidence that enhancement of DG neurogenesis is a critical component of the effects of antidepressant treatments of major depressive disorder (MDD). Adult born DG cells are responsive to GABAergic stimulation which promotes cell maturation. The present study suggests that Esz, presumably acting as a GABA agonist, has pro-neurogenic effects in the adult DG. This result is consistent with evidence that Esz enhances antidepressant treatment response of MDD patients with insomnia.
Keywords: sleep, hippocampus, adult neurogenesis, GABA, hypnotic, MDD
Introduction
Sleep disturbance is an established harbinger and correlate of major depressive disorder (MDD) (Ford and Chamber, 1989). A recent double-blind study showed that adjunctive treatment of MDD patients with insomnia with a hypnotic, eszopiclone (Esz), in conjunction with fluoxetine (Flu) substantially accelerated and enhanced the antidepressant response to Flu (Fava et al., 2006). The study described here is focused on a mechanistic link between the effects of this hypnotic and its use for adjunctive treatment of MDD. It is now recognized that new neurons are produced throughout life in the subgranular zone of the hippocampal dentate gyrus (DG) (reviewed in Kempermann et al., 2004b; Ming and Song, 2005). Adult hippocampal neurogenesis is found in humans (Eriksson et al., 1998) as well as several other mammalian species (Kempermann et al., 2004b). Newborn cells mature into functional DG neurons and contribute to hippocampal-dependent cognitive functions (e.g.,(Saxe et al., 2006; Winocur et al., 2006)). There is evidence that enhancement of adult hippocampal DG neurogenesis is a critical factor in the treatment of MDD. In preclinical studies, the efficacy of antidepressant agents such as Flu depended on enhanced DG neurogenesis (Dranovsky and Hen, 2009; Sahay and Hen, 2007).
DG neurogenesis requires a series of processes, including initiation of the cell cycle and cell division, herein called proliferation, as well as generation of a vascular niche, expression of genes determining cell phenotype and supporting growth of axons, dendrites, and receptors, and cell migration (Kempermann et al., 2004a; Lledo et al., 2006). Each stage of adult neurogenesis may be modulated, either positively or negatively, by particular physiological and behavioral events. Many proliferating cells do not survive to maturity. Thus, survival of proliferating cells is a key endpoint in the neurogenic process, and investigation of factors that affect survival has been a focus of recent research (Epp et al., 2007; Tashiro et al., 2007). Sleep fragmentation and sleep restriction strongly suppress adult DG neurogenesis (Guzman-Marin et al., 2007; Hairston et al., 2005; Mueller et al., 2008; Roman et al., 2005; Tung et al., 2005). Stress, an important etiological factor in MDD, also suppresses DG neurogenesis (Cameron and Gould, 1994; Gould et al., 1992). Sleep deprivation increases subsequent stress responses (Meerlo et al., 2002). Thus, use of hypnotics to reduce sleep fragmentation and restriction and to reduce awake stress responses could facilitate adult neurogenesis.
Recent studies suggest an additional mechanism underlying possible effects of sedative hypnotics on adult neurogenesis. Shortly after birth, proliferating DG cells express GABAA receptors and respond to GABA (Ge et al., 2006; Mayo et al., 2005; Wadiche et al., 2005; Wang et al., 2005). Treatment with GABA agonists increases maturation and survival of proliferating cells (Ge et al., 2006; Tozuka et al., 2005). In adult newborn cells, GABA is excitatory (Tozuka et al., 2005; Wadiche et al., 2005), due to early expression of the Na+-K+-2Cl− transporter (NKCC1), a Cl− importer (Ge et al., 2006). The excitatory response to GABA is critical to the pro-maturation effects of GABAergic stimulation (Ge et al., 2006; Tozuka et al., 2005).
Like other non-benzodiazepine hypnotics, Esz binds to the benzodiazepine-GABA receptor complex, acting as an allosteric modulator (Hanson et al., 2008). Based on these concepts, we hypothesized that Esz would facilitate the rate of proliferation and the survival in proliferating cells in the adult DG, in conjunction with facilitation of sleep. In 3 experiments, Esz was administered during the usual sleep period, to mimic its typical use in humans.
Methods
All protocols were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Animals Care and Use Committee at the V.A. Greater Los Angeles Healthcare System. Forty-four male Sprague-Dawley rats (Harlan, IN), initially 220–250 g, were used for this study. The ages of animals at the beginning of the study were 60–70 days. Animals were housed in individual Plexiglas cages (27×29×30 cm) in a 12:12 hr light/dark cycle (lights on at 06.00h, denoted as ZT0) with access to food and water ad libitum. In two studies, rats were surgically prepared for sleep-wake cycle monitoring in aseptic conditions using Ketamine 80 mg (kg body weight)−1i.p and xylazine 10 mg (kg body weight)−1i.p to induce anesthesia. Six stainless steel gold-plated electrodes were implanted in the frontal and parietal bones of the skull for electroencephalogram (EEG) recording, and three stainless steel gold-plated wires were inserted into nuchal muscles for electromyogram (EMG) recording. All electrodes were then connected with a plug and fixed to the skull with dental cement. Post-surgically, the rats received 1 injection/day of 5.0 ml/kg Carprofen, once daily, for 3 days. Rats were allowed 10 – 12 days for post-surgical recovery before initiating treatments.
Experimental Procedures
In all studies, Esz or vehicle (Veh) were administered during the light phase of the 24h light-dark cycle during the usual primary sleep period of rats. In all experiments, rats were adapted to individual enclosures within a temperature controlled incubator (23±0.5 °C) and in Experiment 1, were connected to overhead cables for sleep-wake recordings, for 3 days before beginning studies. Recording cables were counterweighted and attached to slip rings to minimize disturbance to animals.
In pilot studies we determined that a 5 mg/Kg Esz dose, administered at ZT3, when baseline sleep amounts are high, had no significant effects on the amounts of NREM or REM sleep. In all subsequent studies we studied effects of Esz at a dose of 10 mg/Kg, IP, or vehicle (Veh). Esz was dissolved in 50 mM acetate buffer (pH 4.5) and prepared in a concentration of 5 mg/mL, and diluted to an injected volume of 1.0 mL. To assess cell proliferation and neurogenesis, rats were injected IP with 5-bromo-2’-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO), freshly dissolved in warm sterile saline (15 mg/mL).
Exp. 1 assessed the effects of Esz compared to Veh on sleep-wake parameters. Esz or Veh were administered twice daily, at ZT3 and ZT9, for 14 consecutive days, in conjunction with continuous EEG and EMG recordings. EEG (2 channels) and EMG signals were filtered at 1.0 and 30 Hz and 30–300 Hz, respectively, and digitized at 128 Hz for EEG signals and 256Hz for EMG signals with the Cambridge Electronic Design Spike 2 (V 5.0) data acquisition system. Sleep–wake states were scored off-line in 10 s epochs on the basis of the predominant state within the epoch, with the aid of the Sleep Sign software. Wake was identified by low amplitude, high-frequency EEG activity and sustained neck muscle tone. High-amplitude, low frequency EEG with decreased muscle activity defined non-rapid eye movement (NREM) sleep whereas rapid eye movement (REM) sleep was defined by higher frequency, low amplitude EEG with dominant theta frequency (4–8 Hz), combined with minimum tonic muscle tone. The percentages of each state were calculated for 4 24-hour periods (Baseline, day 1, day 7, and day 14). In addition, we measured the duration of sleep bouts. EEG spectral power in the low (0.75–2.0 Hz) and high (2.1–4.0 Hz) delta ranges within NREM sleep was quantified as a percent of total power within NREM during Esz or Veh treatment.
Exp. 2 assessed the effects of Esz on survival of proliferating cells. BrdU (200 mg/Kg, IP) was administered at ZT9 for three consecutive days. Beginning two days after the last BrdU administration, Esz or Veh was administered for twice daily for 14 consecutive days, at ZT3 and ZT9, exactly as in Exp. 1. Animals were then returned to their home cages and left undisturbed for another 14 days. They were sacrificed at ZT 11, 33 days after the beginning of BrdU injection. Esz- and Veh- treated animals gained 40.5 and 41.8 gms, respectively, during the course of the study.
Exp. 3 assessed the effects of Esz treatment on the proliferative stage of adult neurogenesis. Rats were administered Esz or Veh at ZT 3 for seven consecutive days. BrdU (200 mg/Kg, IP) was administered at ZT12 on days 3, 5, and 7 of Esz or Veh treatment. Rats were sacrificed at ZT15 on day 7. We note that BrdU labels cells in the “S”- or DNA synthesis phase of the mitotic cell cycle. The S phase in the adult rat DG is estimated to last about 9 hrs, and BrdU was found to be available for 2 hrs after injection (Cameron and McKay, 2001). Thus, BrdU injections at ZT12 label cells that enter the S-phase between ZT3 and ZT14.
Two daily doses of Esz were administered in experiments 1 and 2 because a single daily dose had little sustained effect on sleep. Experiments 1 and 2 focused on the possible sustained or cumulative effect of Esz on cell survival and correlated changes in sleep. In experiment 3, one dose was used because the potential effects of drug on the initial stage of proliferation would be expected to occur acutely during the over-lapping time windows of Esz availability and BrdU labeling of the S phase.
Perfusion, Immunohistochemistry, and Cell Counting
Animals from Exps. 2 and 3 were deeply anesthetized (Nembutal 100 mg kg−1), perfused transcardially with phosphate buffer (PB) 0.1 M followed by ice cold paraformaldehyde (4%); brains were removed and stored in 10% and 30% sucrose at 4 °C until they sank. Brains were cut in 40 µm coronal sections. Sections encompassing the hippocampus were preserved in a cryoprotectant solution containing sucrose, polyvinyl-pyrrolidone (PVP-40, Sigma, St. Louis, MO, USA) and ethylene glycol dissolved in PB pH 7.2, which provides long-term protection of the tissue. Sections were processed for BrdU immunohistochemistry or double immunofluorescence labeling to identify cell phenotype. As primary antibodies we used: mouse-anti-BrdU (1:200, BD Bioscience, San Jose, CA, USA) or rat anti-BrdU (1:200, Accurate, Westbury, NJ, USA), and mouse anti-NeuN (1:300, Chemicon, Temecula, CA, USA).
For single labeling, to visualize the expression of the BrdU alone we used the peroxidase method (ABC system, Vectastain, Vector Laboratories, Burlington, CA, USA). Immunohistochemistry was performed simultaneously on sections from Esz- and Veh-treated rats to maximize the reliability of comparisons across groups. Staining was performed on slide-mounted sections. Slides were placed in pre-stirred boiling citric acid (0.01 M, pH = 6.0) for 40 min. After cooling, slides were rinsed in water (X5) and then PBS (X5 after this and each subsequent step unless otherwise specified). Sections were pretreated for BrdU immunostaining by DNA denaturation (2 N HCl at 37 °C for 30 min) followed by 10 min in borate buffer (pH 8.5). Tissue was rinsed in Tris-buffered saline (TBS) 0.1 M. Sections were then incubated with a mouse anti-BrdU primary antibody in PBS containing 0.5% Tween 20 for 48 h at 4°C. Tissue from Esz- and Veh-treated groups was treated with aliquots from the same batch of antibodies. Sections were subsequently incubated with a biotinylated horse anti-mouse IgG (1:200, Vector Laboratories), then acted with avidin–biotin complex (1:100, Vector Elite) and developed with diaminobenzidine tetrahydrochloride (DAB) in hydrogen perioxide (Sigma kit). Absence of the primary antibody resulted in an absence of specific nuclear staining (samples available on request).
BrdU-positive cells were counted using a 40 × objective (Nikon E600, Nikon, Tokyo, Japan) in one-in-six series of sections from the full rostrocaudal extent of the DG. A modified optical fractionator method was used for counting. Briefly, in every section, the contours of SGZ/GLC and hilus areas were first delineated for counting using the tracing function of the MicroBrightfield Stereo Investigator stage control system software. The SGZ was defined as a layer 2 cell diameters thick adjacent to the GCL. Following this, the optical fractionator software component was activated. Due to the sparse and sporadic nature of BrdU-labeling, we used exhaustive sampling, counting all labeled cells in the selected sections. The precision of estimates of the number of cells was expressed using the coefficient of error (CE) and stereological sampling was considered acceptable when CE was ≤ 0.05 (West et al., 1991). In the present study, the ventral SGZ/GCL was identified, in frontal sections, from about AP −4.8 to −6.0 (Paxinos and Watson, 1998), in which it is seen as a separate island and can be unambiguously identified. Counts from more posterior sections, in which the SGZ/GCL is seen as a single structure, were included in the dorsal SGZ/GCL.
The phenotype of the newly generated BrdU-positive cells was determined in 1-in-12 series of sections from animals of each group using double immunolabeling with antibodies to BrdU and NeuN. After pretreatment (see above) and blocking with goat serum and Triton-X 10% in TBS, sections were incubated in a mixture with antibodies against BrdU (monoclonal from rat) and NeuN (monoclonal from mouse) in TBS for 3 days at 4 °C. After rinsing with TBS and blocking for 1 h, sections were incubated in an antibody mixture of Alexa 488 goat anti-rat and Alexa 555 goat anti-mouse, both at 1:300 (Molecular Probes, Eugene, OR, USA) in TBS for 2 h. Sections were mounted and coverslipped with Vectastain (Vector) mounting medium.
The phenotype of BrdU-labeled cells was assessed in 25–68 cells per animal (100 cells in one animal) by visualization of occurrence of red (NeuN) fluorescence in green (BrdU) fluorescent cells (Nikon E-600). Co-localization was confirmed in a sub-sample by z-series analysis (z-step, 0.36 µm) through the cell nucleus and three-dimensional reconstruction of cells in the x–z and y–z planes. An individual blinded to the experimental conditions did all counting.
Statistical Methods
Sleep state percentages were evaluated by two-way ANOVA (factors Esz or Veh treatment and sleep stage), followed by Holm-Sidak post-hoc multiple comparisons. EEG spectral data were evaluated with a one-way ANOVA followed by Holm-Sidak post-hoc multiple comparisons or Kruskal-Wallis one-way ANOVA on ranks, with factor Esz or Veh treatment. Effects of Esz on proliferation and survival of proliferating cells were assessed by Students t test. Counts are shown as Mean ± SEM.
Results
Exp. 1
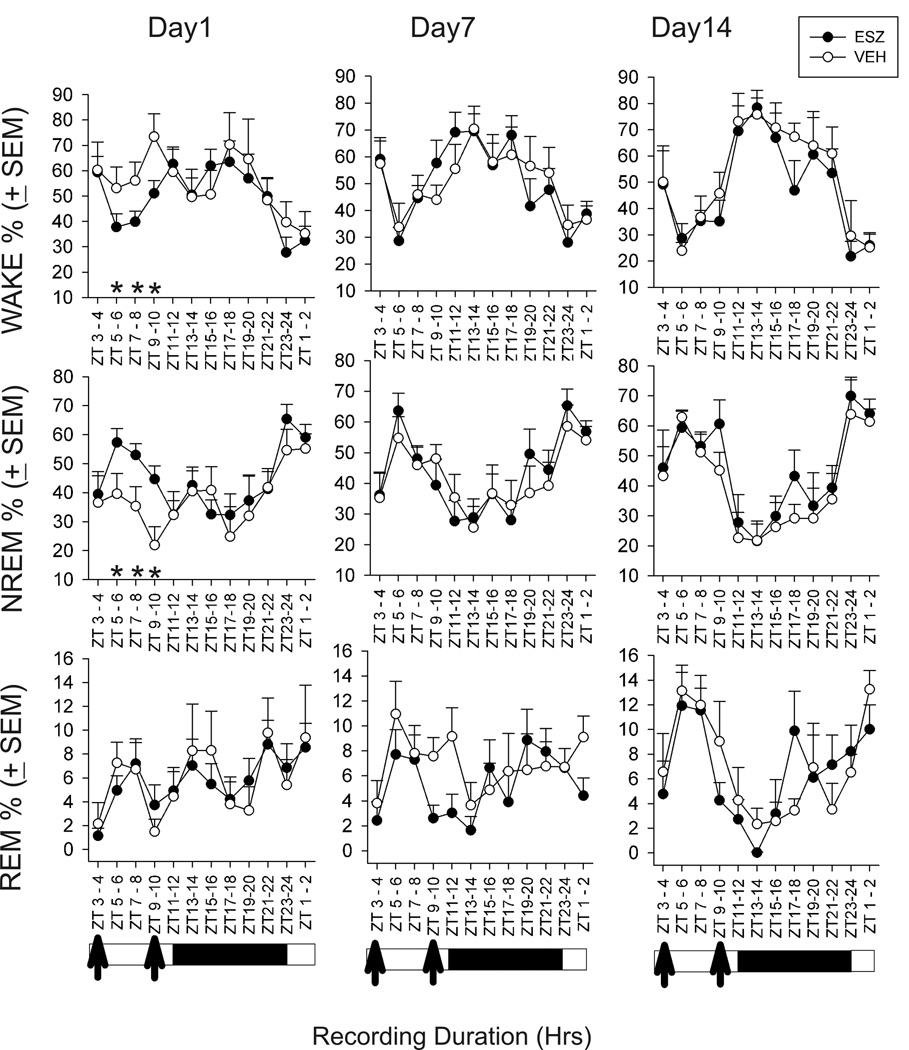
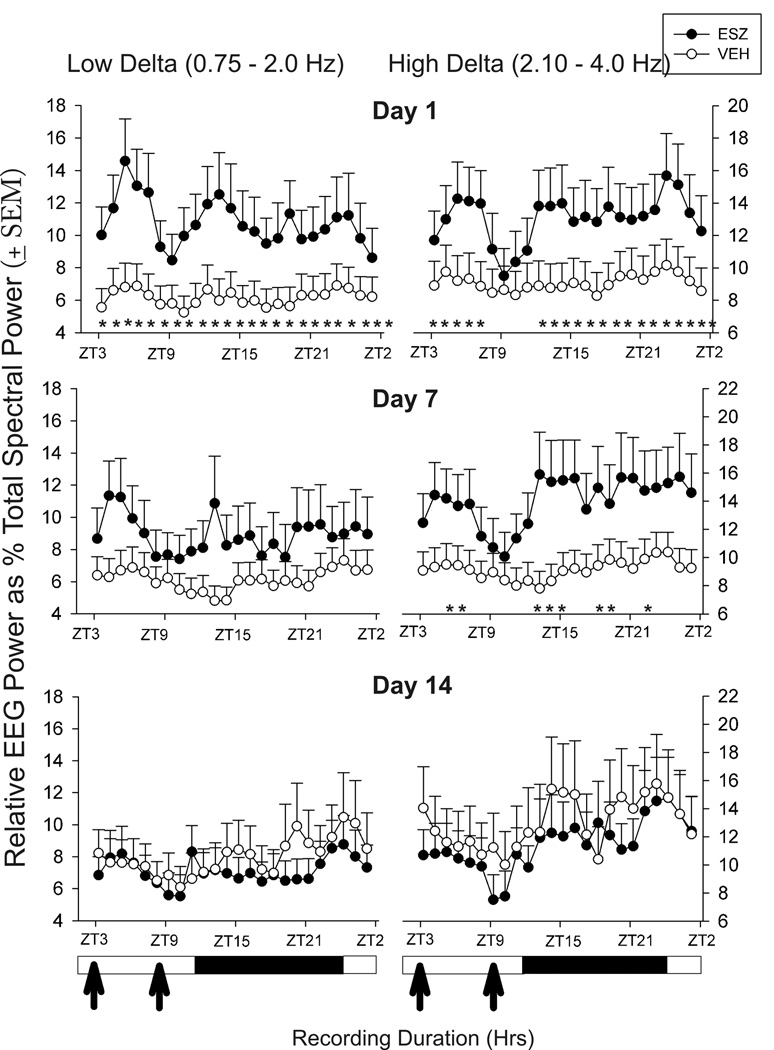
Effects of Esz on sleep were assessed on drug treatment days 1, 7, and 14. Compared to Veh, Esz significantly increased NREM sleep percent while decreasing wake for 6 hrs on day 1, but had no sleep stage effects on days 7 or 14 (Fig 1). Both low frequency (0.75–2.0 Hz) and higher frequency (2.1–4.0 Hz) delta power within NREM sleep were increased in Esz-treated animals throughout most of the 24 h recording on day 1 (Fig. 2). By day 7, only higher frequency delta was increased, and primarily during the 2 or 3 hrs following Esz administration, although a significant increase persisted some hrs after the second injection. On day 14, Esz no longer significantly affected delta power. We also compared sleep and wake bout durations, but these were not significantly changed by Esz treatment on any of the three days analyzed (data not shown).
Fig. 1.
Twenty-four hour sleep-wake state effects on days 1, 7, and 14, of twice daily treatment with Esz (10 mg/Kg), compared to Veh. Esz was administered at ZT 3 and 9. Esz treatment resulted in significant increases in NREM and decreases in wake for 6 hrs on day 1 of treatment, from ZT 5 through ZT10. On day 1, from ZT0 to ZT12, total NREM was: Esz, 44.9±1.3%, Veh, 34.4±1.8% (p < 0.001) and wake was: Esz, 50.2±1.1, Veh, 58.7±1.4 (p<0.001). No significant effects of Esz on sleep-wake stages were found after ZT12 on day 1, or on either days 7 or 14. (N=8 in both Esz and Veh groups)
Fig. 2.
Changes in low band (0.75–2.0 Hz) and high band (2.1–4.0 Hz) delta activity, within NREM sleep, on days 1, 7, and 14 induced by twice daily Esz treatment. On day 1, both low and high band delta were significantly increased for most of the 24 hrs following injections; the increase in high band delta was transiently suppressed following the second injection. On day 7 of Esz treatment, high band delta was increased for 2 hrs following the first injection, and for 6 partially non-continuous hrs following the second injection. By day 14, no effects of Esz on delta power were found.
Exp. 2
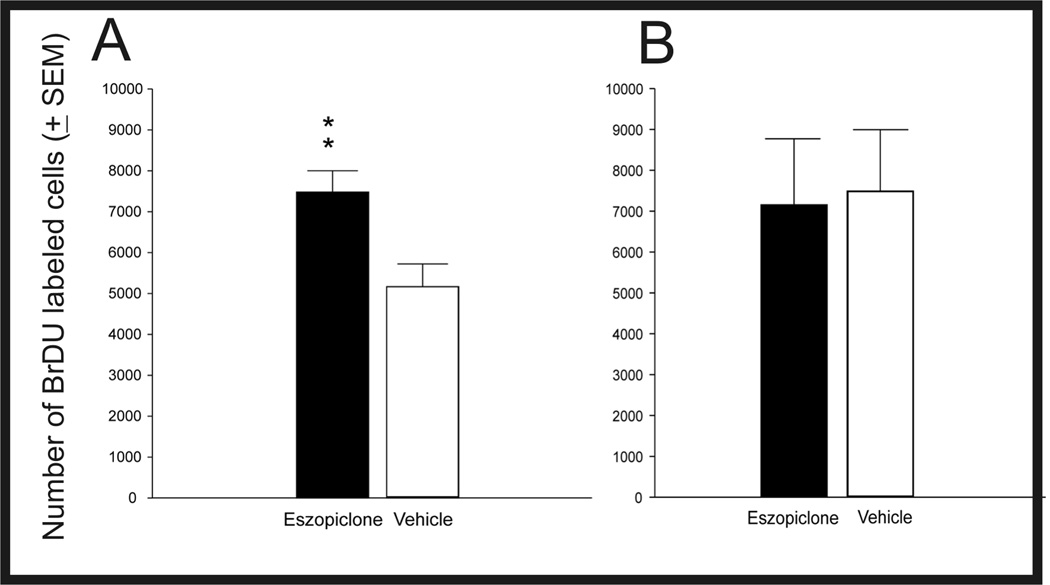
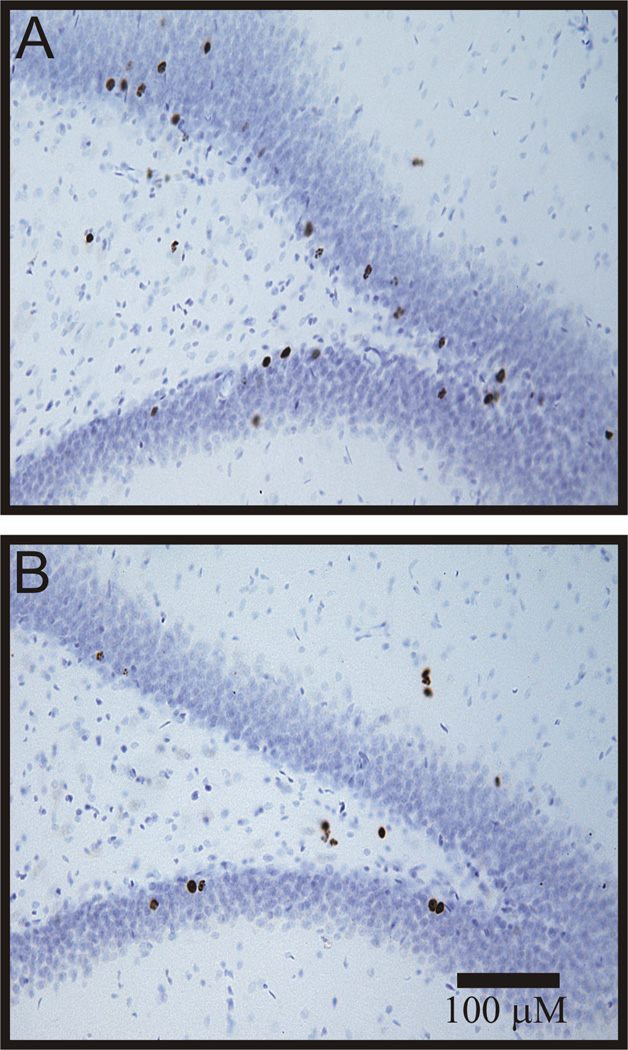
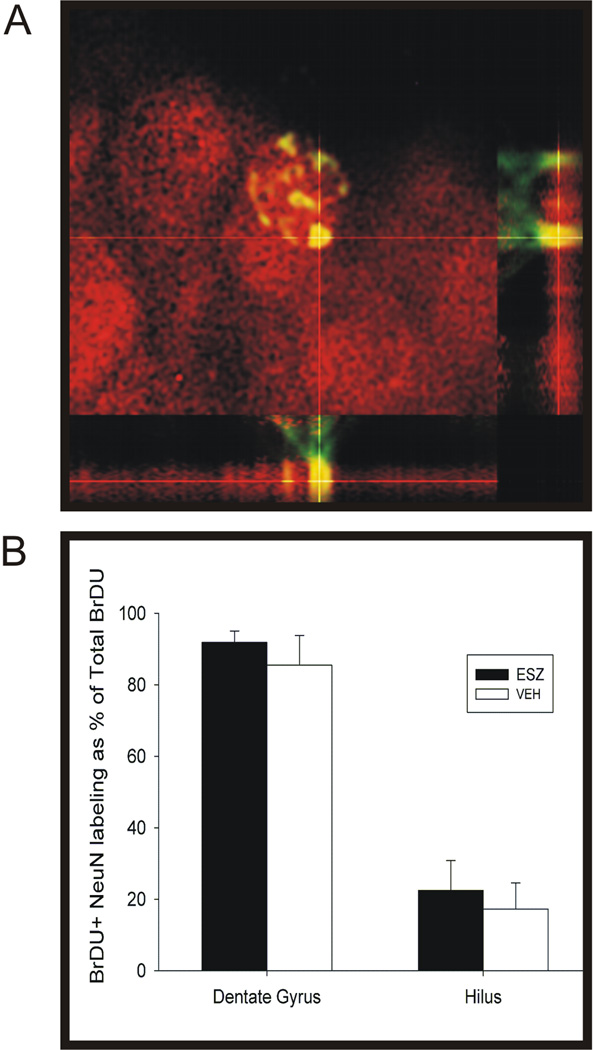
This study compared the effects of 14 consecutive days of twice daily Esz administration, compared to Veh, on survival of proliferating cells labeled by BrdU injections on days 5, 4, and 3 days before treatments. The Esz and Veh administration protocol was identical to that used in Exp. 1. The numbers of BrdU-labeled cells were determined 33 days after the first BrdU injection. As shown in Fig 3A, Esz treatment significantly increased the numbers of BrdU-labeled cells in the SGZ/GCL, by 46% (Esz: 7482 ± 520, Veh: 5168 ± 553, t= 3.05, p< 0.01). Sample sections showing BrdU labeling following sustained Esz or Veh treatment are shown in Fig. 4. BrdU-labeled cell counts were significantly increased in dorsal/posterior SGZ/GCL (t=3.14, p<0.01), but not in ventral SGZ/GCL. We also examined the phenotype of labeled cells by assessing the expression of the mature neuronal marker, NeuN in strongly BrdU-labeled cells (Fig 5). There were no significant differences between Esz- and Veh-treated groups in percentages of BrdU-labeled cells expressing NeuN in either SGZ/GCL (91.8 vs 85.4%) or hilus (22.5 vs 17.3%).
Fig. 3.
Effects of Esz treatment, compared to Veh, on hippocampal cell survival (A) and proliferation (B). Cell survival was assessed 33 days after the first of 3 daily BrdU injections. Esz was administered twice daily for 14 days after BrdU injections. The total number of BrdU-labeled cells was increased by 46% (P<0.008). Cell proliferation was assessed by counting BrdU-labeled cells following seven days Esz treatment; BrdU was administered on days 3, 5, and 7. Significant changes in proliferation were not found. (Survival study: N=8 for both Esz and Veh groups. Proliferation study; N=6 in both groups.)
Fig. 4.
Examples of surviving BrdU-labeled cells in the dentate gyrus in Esz (A) and Veh (B) treated animals (100×). Esz treatment increased the numbers of surviving cells in the subgranular zone and dentate gyrus. We counted both solid and punctate-labeled cells using exhaustive sampling, in a one of six series, at 400X, using the MicroBrightfield Stereoinvestigator system, focusing through the depths of sections.
Fig. 5.
Determination of neuronal phenotype of surviving BrdU-labeled cells as assessed by visualization of coincidence of red (NeuN) fluorescent in strongly-labeled green (BrdU) fluorescent cells A. Orthogonal reconstruction of fluorescent signals derived from a stack of 56 images at 0.36 µm intervals. B. There was no significant difference between Esz- and Veh-treated groups in the percentages of strongly BrdU-labeled cells also exhibiting NeuN-labeling in either the GCL/SGZ or hilus.
Exp. 3
This study compared the numbers of cells labeled by BrdU, administered on days 3, 5, and 7, following 7 days Esz or Veh treatment. There were no differences between groups in the numbers of BrdU-labeled cells counted in the SGZ/GCL immediately following treatments (Fig. 3B , Esz: 7152±1614, Veh:7488±1505). Separate analyses showed no significant effects of Esz in dorsal/posterior or ventral portions of the SGZ or the hilus (data not shown). Thus, under the conditions of this experiment, the proliferative stage of neurogenesis was not altered by Esz treatment.
Discussion
Our study examined the effects of treatment with the hypnotic drug, Esz, administered during the usual rat sleep period, on two stages of neurogenesis in the adult rat dentate gyrus. We found that Esz administration for 7 days had no effect, compared to Veh, on the numbers of newly-proliferated cells in the SGZ/GCL . However, 14 day Esz treatment strongly enhanced the survival of proliferating cells labeled before Esz administration. We suggest that this finding is best explained by recent evidence that GABAergic input to proliferating cells facilitates their survival and maturation. Newly-proliferative cells express GABAA receptors, and exhibit excitatory responses to GABA (Tozuka et al., 2005; Wadiche et al., 2005; Wang et al., 2005). GABAergic stimulation of newly-proliferative cells induced NeuroD, a positive regulator of DG neuronal differentiation (Tozuka et al., 2005). The excitatory response to GABA reflects the early expression in immature neurons of the Na+-K+-2Cl− transporter, a Cl− importer (Ge et al., 2006; Tozuka et al., 2005). Knock-down or knock-out of this importer resulted in impaired development of dendritic arborization in new neurons (Ge et al., 2006). Seven days treatment with the partial GABA agonists, phenobarbital or pentobarbital, increased survival of BrdU-labeled neurons at 28 days, and 7 day antagonist treatment reduced survival (Tozuka et al., 2005). The pro-maturational effects of excitatory responses to GABA in adult born cells is congruent with similar mechanisms during early brain development (Ben-Ari, 2002).
Since our study did not include a cage control group, we must consider the possibility that the Veh control group exhibited reduced cell survival due to the stresses of injections, and that Esz did not directly facilitate cell survival, but only counteracted the effects of stress. The following evidence argues against that interpretation. Studies in rats (Cowen et al 2008, Takase et al, 2009, Su et al, 2009) or mice (Païzanis et al, 2009, Wentz and Magavi, 2009) have shown that daily injections do not necessarily affect cell survival.
A recent study (Su et al, 2009) also examined the effects of Esz (10 mg/Kg, as in our study) as well as fluoxetine (Flu, 5 mg/Kg) on cell proliferation and survival. Neither drug nor the combination significantly affected proliferation assessed following 21 day drug treatments, but the combination significantly increased cell survival in the dorsal GLC. Esz alone resulted in an approximately 25% increase in survival in the whole GLC, but this was not significant. The larger and significant increase in survival found in the present study may be due to our twice daily Esz treatment. Among other differences between studies were the duration of Esz treatment (28 days in Su et al vs 14 days in our study) and the timing and dose of BrdU (at ZT1, 150 mg/Kg for 3 days in Su et al vs at ZT 9, 200 mg/Kg for 3 days in our study).
Treatment with another hypnotic, zolpidem, for 21 days failed to affect survival of proliferating cells (Takase et al., 2009). However, the response of newborn cells to a particular GABA agonist will depend on the receptor subunit binding of the agonist and the receptor subunit expression in the cells. Although newborn cells are responsive to GABAA agonists, the receptor lacks α1 subunits (Wadiche et al., 2005), which could limit responses to zolpidem, but not Esz (Hanson et al., 2008). Thus, the potential use of particular GABA agonists to enhance neurogenesis must be confirmed experimentally.
Other studies of the effects of GABA agonists on the proliferative phase of neurogenesis have been inconsistent. Administration of phenobarbital for 3 days reduced numbers of cells in the early proliferative state, designated as type 2 (nestin-expressing) cells (Tozuka et al., 2005). In another study, a single dose of diazepam increased numbers of BrdU+ cells after 24 h (Petrus et al., 2009), but had no clear effect on numbers of type 2a rapidly amplifying cells. In the same study diazepam could oppose an increase in the number of proliferating cells expressing calretinin, an early marker of neuronal lineage, induced by an NMDA antagonist. Our failure to find an effect of 7 days Esz treatment on proliferation is consistent with a report that 2 days treatment with zolpidem, another hypnotic, had no effects on proliferation in younger mice and suppressed proliferation in older mice (Takase et al., 2009), although, as noted above, the lack of the α1 subunit in the GABAA receptor in proliferating cells, may limit the response to zolpidem. Anesthetics that bind to GABA receptors also do not acutely affect proliferation (Tung et al., 2008). Proliferation is currently understood to consist of distinct developmental steps (Kempermann et al., 2004a), which may have differential sensitivity to GABA agonists (Petrus et al., 2009). In this context, one factor in the varying findings may be the number of treatments; more than a single treatment seems to be ineffective or, in the case of phenobarbital, to have anti-proliferative effects, possibly reflecting opposing effects on initial and later stages of proliferation. In our study, Esz was given for 7 days; BrdU was given on 3 of those 7 days. We also have not excluded the possibility that two daily Esz doses, like those used in the survival study, could significantly alter proliferation.
Considering the effects of Esz on sleep, to our knowledge, this is the first study to report effects of sustained administration of Esz in an animal model. Previous studies reported sleep changes during the first 2–6 hrs of treatment (Alexandre et al., 2008; Gauthier et al., 1997; Noguchi et al., 2004; Xi and Chase, 2008), usually to zopiclone, the racemic analog of Esz. In agreement with these short term studies, we found an initial enhancement of NREM sleep (Alexandre et al., 2008; Noguchi et al., 2004) and delta activity within NREM sleep (Xi and Chase, 2008) on day 1 of treatment, and waking was decreased. However, REM sleep was not significantly changed. On the seventh treatment day, sleep stage amounts were no longer affected, but significant increases in the amount of 2.1–4.0 Hz delta activity were still observed. By the fourteenth day, delta EEG changes were no longer found. We emphasize that Esz was administered during the light phase, when sleep amounts and delta activity are normally high, and no insomnia-like characteristics were induced. In humans with chronic insomnia, during daily use for 6 months, Esz had sustained efficacy (Krystal et al., 2003; Walsh et al., 2007).
Although an association of suppression of adult DG neurogenesis with the etiology of MDD is unlikely, there is strong evidence that pharmacological enhancement of neurogenesis can play a positive role in treatment of MDD (Sahay and Hen, 2007). Diverse antidepressant treatments have in common the enhancement of neurogenesis. In preclinical studies, the effects of several antidepressant treatments, including administration of Flu, on depression-like behaviors are prevented if increases in adult neurogenesis are blocked (e.g. (Santarelli et al., 2003)). Further, to enhance neurogenesis, these treatments must be applied chronically, mimicking that requirement for clinical efficacy of antidepressant treatments (Malberg et al., 2000). The delay in the efficacy of antidepressant treatments can be explained by the time requirement for maturation of new DG neurons (Jacobs et al., 2000). In this context, the enhancement of neurogenesis by Esz could also confer an antidepressant benefit. As noted above, Su et al (2009) found that the combination of Esz and Flu increased survival in dorsal SGZ.
Our result and those of Su et al provides a mechanistic explanation for the finding that adjunctive treatment with Esz, in MDD patients with insomnia, increased speed and efficacy of the antidepressant response to Flu (Fava et al., 2006). Further, the benefits of Esz persisted after discontinuation of Esz cotherapy with Flu (Krystal et al., 2007), pointing to a possible role of underlying structural changes in brain, such as enhancement of DG neurogenesis. It must be emphasized that these possible benefits may not apply to all patient groups; in randomized trials of hypnotic vs. placebo, incidence of depression was slightly increased by hypnotics (Kripke, 2007).
We note that studies using proton magnetic resonance spectroscopy in medication-free MDD patients have shown that GABA levels are reduced in occipital cortex (Bhagwagar et al., 2007; Sanacora et al., 1999, 2004), and probably in prefrontal (Hasler et al., 2007) and cingulate cortex (Bhagwagar et al., 2008). Treatment with citalopram or fluoxetine or ECT increased occipital GABA levels in MDD patients (Bhagwagar et al., 2004; Sanacora et al., 2002; Sanacora et al., 2003). Possibly, these antidepressant treatments act, at least in part, through GABAergic stimulation of newborn DG cells. Esz could then have a synergistic action. However, there is limited information on hippocampal GABA in MDD. A post mortem study showed reduced expression of the GABA-synthesizing enzyme, glutamic acid decarboxylase (GAD65) in bipolar patients, including in dentate gyrus (Heckers et al., 2002), but another study found higher densities of GAD-immunoreactive neurons in both MDD and bipolar patients, but noted likely influences of prior medication (Bielau et al., 2007).
At least three mechanisms could underlie effects of Esz on adult DG neurogenesis. These include 1) facilitation of sleep, 2) prevention of sleep deprivation induced enhancement of stress responses (Meerlo et al., 2002), and 3) direct effects of Esz on GABA receptors on proliferating cells, as summarized above. The present findings primarily support the last possibility. As Esz- and Veh-treated animals in this study were treated identically, and no particular stressors were applied, it is unlikely that stress played a role in our outcome. The role of sleep in adult neurogenesis has been studied using severe sleep deprivation, fragmentation, or restriction (Guzman-Marin et al., 2005; Guzman-Marin et al., 2007; Hairston et al., 2005; Mueller et al., 2008; Roman et al., 2005; Tung et al., 2005). There is no evidence that, in normally-sleeping animals, modest Esz-induced increases in NREM sleep and delta activity above a normal background would have a direct neurogenesis-enhancing effect. Nevertheless, in MDD patients, who exhibit sleep fragmentation and who are susceptible to stress, Esz treatment could enhance DG neurogenesis through each of these three mechanisms.
This study did not address a number of issues. For example, we do not know 1) what receptor binding properties or doses of GABA agonists that would maximally enhance neurogenesis, 2) if the time of day of administration is important, or 3) what is an optimal treatment duration or cycle. Future studies should address the effects of hypnotic agents in models of insomnia, and control for co-variations in sleep.
Acknowledgements
Supported by Sepracor, Inc, and NIH MH 075076. We acknowledge the suggestions of Rob Mariani and the technical support of Maple Schrader, Keng-Tee Chew, Ramya Davis, and Ben Mitrani.
Footnotes
Disclosures:
Melvi Methippara received salary support from an investigator-initiated research grant from Sepracor, Inc for one year.
Tariq Bashir reported no biomedical financial interests or potential conflicts of interest.
Natalia Suntsova reported no biomedical financial interests or potential conflicts of interest.
Ronald Szymusiak received research support and consultant fees from an investigator-initiated research grant from Sepracor, Inc.
Dennis McGinty received research support and a consultant fee from an investigator-initiated research grant from Sepracor, Inc
Reference List
- Alexandre C, Dondal A, Aixendri R, Guzman A, Hamon M, Adrien J. Sleep-stabilizing effects of E-6199, compared to zopiclone, zolpidem, and THIP in mice. Sleep. 2008;31:259–270. doi: 10.1093/sleep/31.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, et al. Reduction in occipital cortex γ–aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol. Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, et al. Low GABA concentration in occipital cortex and anterior cingulate cortiex in medication-free recovered depresed patients. Intern. J. Neuropsychopharm. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Taylor M, Jessard P, Mathews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- Bielau H, Steiner J, Mawrin C, et al. Dysregulation of GABAergic neurotransmission in mood disorders. Ann. N Y Acad. Sci. 2007;1096:157–169. doi: 10.1196/annals.1397.081. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cowen DS, Takase LF, Fornal CA, Jacobs BL. Age-dependent decline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res. 2008;1228:14–19. doi: 10.1016/j.brainres.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hipppocampal neurogenesis: Regulation by stress and antidepressants. Biol. Psychiatry. 2009;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Sprityzer MD, Galea LAM. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neurosci. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;11:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol. Psychiatry. 2006;59:1052–1056. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ford DE, Chamber DB. Epidemiological study of sleep disturbance and psychiatic disorders: An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Gauthier P, Arnaud C, Stutzmann JM, Gottesmann C. Influence of zopiclone, a new generation hypnotic, on the intermediate stage and paradoxical sleep in the rat. Psychopharmacology. 1997;130:139–143. doi: 10.1007/s002130050221. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G-I, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Wooley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neurosci. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur. J. Neurosci. 2005;22:2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Little MTM, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hanson SM, Morlock EV, Satyshur KA, Czajkowski C. Structural requirements for eszopiclone and zolpidem binding to the aminobutyric acid type-A (GABAA) receptor are different. J. Mol. Chem. 2008;51:7243–7252. doi: 10.1021/jm800889m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Dreverts WC. Reduced prefrontal glutamate/glutamine and γ-aminobutyic acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder snd schizophrenia. Arch. Gen. Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Pragg H, Gage FH. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol. Psychiat. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004a;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004b;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kripke DF. Greater incidence of depression with hypnotic use than with placebo. BMC Psychiatry. 2007;7:42. doi: 10.1186/1471-244X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal A, Fava M, Rubens R, et al. Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J. Clin. Sleep Med. 2007;15:48–55. [PubMed] [Google Scholar]
- Krystal A, Walsch JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: Results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neuro. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W, Lemaire NV, Malaterre J, et al. Pregnenolone sulfate enhances neurogenesis and PSA-NCAM in young and aged hippocampus. Neurobiol. Aging. 2005;26:103–114. doi: 10.1016/j.neurobiolaging.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Koehl M, van der Borght K, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- Ming G-L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mueller A, Pollock MS, Lieblich SE, Epp JR, Galea LAM, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;94(5):R1693–R1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Kitazumi K, Mori M, Shiba T. Electroencephalograhic properites of zaleplon, a non-benzodiazepam sedative/hypnotic in rats. J. Pharmacol. Sci. 2004;94:246–251. doi: 10.1254/jphs.94.246. [DOI] [PubMed] [Google Scholar]
- Païzanis E, Renoir T, Lelievre V, et al. Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptor-impaired mice. Int J Neuropsychopharmacol. 2009;24:1–16. doi: 10.1017/S1461145709990514. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Petrus DS, Fabel K, Kronenberg G, Winter C, Steiner B, Kempermann G. NMDA and benzodiazepam receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis. Eur. J. Neurosci. 2009;29:244–252. doi: 10.1111/j.1460-9568.2008.06579.x. [DOI] [PubMed] [Google Scholar]
- Roman V, van der Borght K, Leemburg SA, van der Zee EA, Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson N, et al. Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am. J. Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentraions in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason ST, Rothman DL, et al. Reduced cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J-W, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su XW, Li XY, Banasr M, Duman RS. Eszopiclone and fluoxetine enhance the survival of newborn neurons in the adult rat hippocampus. Int J Neuropsychopharmacol. 2009;12(10):1421–1428. doi: 10.1017/S1461145709990629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase LF, Fornal CA, Jacobs BL. Effects of the hypnotic drug zolpidem on cell proliferation and survival in the dentate gyrus of young and old rats. Brain Res. 2009;1259:26–31. doi: 10.1016/j.brainres.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3525–3529. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tung A, Herrera S, Fornal CA, Jacobs BL. The effect of prolonged anesthesia with isoflurane, propofol, dexmedetomide, or ketamine on neural cell proliferation in the adult rat. Anesth. Analg. 2008;106:1772–1777. doi: 10.1213/ane.0b013e31816f2004. [DOI] [PubMed] [Google Scholar]
- Tung A, Takase L, Fornal C, Jacobs BL. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neurosci. 2005;134:721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Wadiche LO, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signalling to newborn neurons in dentate gyrus. J. Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effects on sleep, quality of life, and work limitations. Sleep. 2007;30:959–968. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-P, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Moll. Cell. Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wentz CT, Magavi SS. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology. 2009;56(6–7):994–1000. doi: 10.1016/j.neuropharm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Xi M, Chase MH. Effects of eszopiclone and zolpidem on sleep and waking states in the adult guinea pig. Sleep. 2008;31:1043–1051. [PMC free article] [PubMed] [Google Scholar]