Summary
Many human fungal pathogens infect people when they are inhaled as spores. Despite the serious impact of fungal spores on human health, little is known about their basic properties or how they interact with the host. This is particularly true for Cryptococcus neoformans, a human fungal pathogen that causes more than 600,000 deaths annually. Spores of C. neoformans have not been well characterized previously because of technical challenges in isolating them; however, recent advances in spore isolation have lead to the first direct analyses of spores. Novel insights into the spore-host interaction, specifically how spores interact with alveolar macrophages, have provided a new model of cryptococcosis that could have broad implications for human fungal pathogenesis.
Introduction
Sporulation is a strategy used by many organisms, including bacteria, fungi, protozoa, algae, and ferns to survive conditions that are too harsh to sustain vegetative growth. Survival is generally facilitated by developing specialized cells (spores) with physical properties that confer resistance to environmental assault. Many organisms also produce spores on specialized structures that are adapted for efficient dispersal via wind or water currents [1]. Through these adaptations, sporulation is an effective mechanism to either persist until local conditions improve or disperse to new environments conducive for growth.
For pathogenic microbes, favorable growth conditions are often found in a mammalian host, resulting in serious consequences for human health. For example, spores of protozoan parasites, such as the oocytes of Cryptosporidium sp., can be found in untreated or fecal waste-contaminated water and have been estimated to cause >50% of water-borne parasitic disease worldwide, including major outbreaks in the United States [2]. Spores of bacterial pathogens, such as those produced by Bacillus anthracis, are extremely resistant to physical and chemical insult, making B. anthracis a potentially devastating biological weapon [3]. In fungi, spores are thought to be the infectious particles of many fungal pathogens. This has been shown rigorously for a number of plant fungal pathogens, such as the wheat rusts, Puccinia sp., which disperse globally on an annual basis and cause damage to food crops totaling ~3 billion dollars per year [1,4].
Among human fungal pathogens, spores are presumed infectious particles for many organisms. The infection-causing potential of spores from human fungal pathogens is exemplified by Coccidioides immitis, as few as 10 spores can establish disease and cause fatal disease [5]. Because these highly infectious spores are adapted for wind dispersal, C. immitis spores, similar to spores from Bacillus anthracis, have been postulated to be serious threats as biological weapons [6]. Despite the demonstrated capacity of spores from human fungal pathogens to infect mammalian hosts, the specific roles that spores play in establishing disease are less clear.
Many human fungal pathogens are free-living in the environment and infect humans via a respiratory route. For example, Aspergillus fumigatus, Histoplasma capsulatum, Blastomyces dermatitidis, Penicillium marneffei, Coccidioides immitis, Paracoccidioides brasiliensis, and Sporothrix schenckii all grow filamentously in the environment and produce conidia (asexual spores) that are dispersed easily by wind currents [7,8]. Conidia are presumed infectious particles because they are small relative to filamentous hyphal cells, thus increasing their chances of being deposited in the alveoli of the lung and causing an infection [9]. Despite the high likelihood that airborne conidia are natural infectious particles for these human fungal pathogens, relatively little is known about the molecular properties of conidia or how they interact with the host.
Another human fungal pathogen for which there is limited information with respect to spores is Cryptococcus neoformans. C. neoformans is distributed worldwide and causes meningoencephalitis, primarily in immunocompromised individuals, resulting in approximately one million cases and 600,000 deaths annually [10]. C. neoformans is a free-living budding yeast that resides in the environment. The natural route of infection has been inferred from a preponderance of clinical evidence, which indicates that fungal cells (likely yeast or spores) are inhaled by a human host, causing an asymptomatic infection within the lung. In immunocompromised individuals, C. neoformans can disseminate from the lung to other tissues, including the central nervous system [11]. The resulting disease is uniformly fatal without treatment [12].
C. neoformans has been found in association with trees, soil, and bird droppings, but sources of spores in the environment have not been identified [13]. In the laboratory, spores can be produced via two distinct pathways: sexual development and monokaryotic fruiting. Sexual development occurs when haploid yeast cells of opposite mating types (a and α) encounter one another under appropriate environmental conditions and fuse. The resulting binucleate a+α cell initiates a new developmental program and grows filamentously off of the substrate. In response to unknown signals, the terminal hyphal cells produce fruiting structures, called basidia, in which nuclear fusion and meiosis occur [14]. The resulting products are replicated and packaged into spores that are budded onto the basidium surface to produce long chains of spores [15]. The other sporulation pathway, known as monokaryotic fruiting, occurs when α cells in nutrient limiting conditions produce hyphae, basidia, and spore chains in the absence of a cells [16]. In nature, sexual development and monokaryotic fruiting have not been observed directly; however, population genetics studies show that recombination occurs in some regions of the world, and in other areas both clinical and environmental samples show a strong bias in favor of the α mating type [17–19]. These findings support the hypothesis that spores exist in nature.
However, because sexual and fruiting structures have yet to be observed outside the laboratory, it has been proposed that the infectious propagules in C. neoformans infections are desiccated yeast [20]. Desiccated yeast in the environment are approximately 1–5 μm in size, making them small enough to lodge in the alveoli of the lung [21,22]. One downside to this proposal is that desiccated yeast are not particularly robust. The yeast tend to die over time, making them less appealing as infectious agents [23]. On the other hand, spores are quite robust and fall into the 1–2 μm range, ideal for alveolar deposition [24]. Because both desiccated yeast and spores are small enough to establish infection within the lower airway, the C. neoformans system provides an excellent opportunity to compare the abilities of spores and yeast to cause disease. Ultimately, differences between spore-mediated and yeast-mediated disease in animal models may inform the identities of natural infectious particles.
A direct comparison of the properties of spores and yeast has never been conducted because, until recently, it has been impossible to isolate spores away from yeast and filamentous cells in large numbers. Breakthroughs in density gradient centrifugation now provide a reliable method for isolating large numbers of pure spores [24]. Direct analyses of the physiological, biochemical, and virulence properties of isolated spores implicate C. neoformans spores as infectious particles.
C. neoformans spores are adapted to withstand external stress
One key feature of microbial spores, and an important difference between spores and vegetative cells, is that spores are adapted to withstand harsh conditions. C. neoformans spores are more resistant than yeast to oxidative stress, high temperatures, chemical insult, and desiccation [24]. Because C. neoformans spores are produced on basidia that grow away from the substrate, and spores have been shown to be aerosolized by air currents generated under laboratory conditions, it is likely that spores are aerially dispersed in nature [25]. It is therefore important that spores be adapted to withstand desiccating conditions that they might encounter during aerial dispersal.
Broad stress resistance is a feature of many spores, and in a number of fungi, such as Saccharomyces cerevisiae, it has been linked to a thick spore coat [26]. The spore coat acts as a barrier preventing water from leaving spores and keeping deadly compounds out of the spore, which protects the contents from damage [27,28]. One major difference between C. neoformans spores and yeast is a thick spore coat, which is distinct from the yeast cell wall (Fig. 1) [24]. This coat likely contributes to the broad stress resistance of C. neoformans spores. By protecting the spore from damage, the coat could provide an advantage for spores in surviving dispersal conditions and thus increase their chances of encountering a susceptible host.
Figure 1. C. neoformans spores are covered by a thick spore coat.
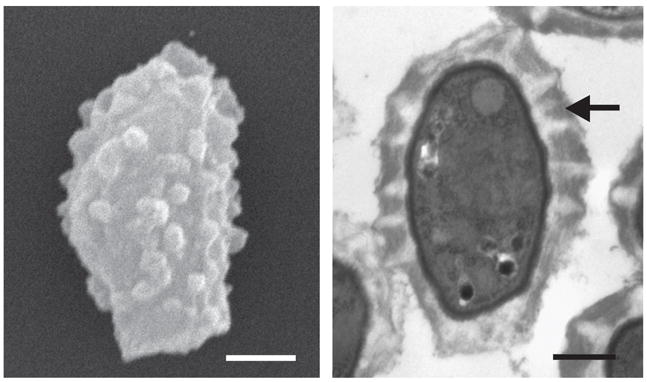
The left panel is a scanning electron micrograph of a C. neoformans spore, showing the characteristic polar morphology with a stalk-like structure on the bottom and a crenulated surface (Bar = 500 nm). The right panel shows a transmission electron micrograph displaying a cross section of a C. neoformans spore. The arrow indicates the thick spore coat, which is a heterogeneous structure containing striations of varying electron density (Bar 500 nm).
C. neoformans spores cause disease in a mouse model of cryptococcosis
If spores act as infectious particles in nature, one would anticipate that they could cause disease in an animal model. Decades of studying the virulence properties of C. neoformans yeast have provided a reliable murine model of cryptococcosis. In this model, yeast are inoculated via an intranasal route leading to an infection within the lungs. The yeast then proliferate and are capable of disseminating to the brain, which appears to mimic the proposed natural route of infection and dissemination in humans [29]. When mice in this model are infected with spores, they also develop fatal cryptococcosis [30–32]. An inoculum of as few as 500 spores is lethal in this model, indicating that C. neoformans spores are effective infectious particles [25,32].
In addition, when mice are infected with similar numbers of either spores or yeast, the mice become moribund at the same time (~30 days post infection). Despite this identical survival time, the fungal burden within the lung of spore-infected mice 6 days post infection is 10-fold lower than the fungal burden of yeast-infected mice [32]. Through an unknown mechanism, C. neoformans spores are able to overcome significantly lower numbers early during disease progression and cause death at the same time as yeast. These data suggest a potentially significant difference between infections caused by spores and infections caused by yeast.
Thus far, the infectious nature of C. neoformans spores has been explored using established type strains that have been selected for laboratory use because they are highly virulent in the mouse model of infection. Yeast from many clinical and environmental isolates harbor varying abilities to cause morbidity in the mouse model [33]. It is possible that many strains that are not particularly virulent as yeast may produce spores that are better suited to cause disease. Additional studies of spores from a diverse population of strains may facilitate the development of a sensitized system for studying the natural route of infection. This system would be ideal for studying the differences between spores and yeast in establishing infection within the host and provide insights into how spores may be better adapted to cause disease.
Spore and yeast interactions with the host are distinct
One striking difference between C. neoformans spores and yeast is their interactions with macrophages in culture. A long-standing observation is that C. neoformans yeast are not phagocytosed by macrophages in culture in the absence of opsonization [34]. Spores on the other hand are readily phagocytosed by both cultured murine macrophages and primary alveolar macrophages in the absence of opsonins [25,32]. Once inside the macrophages, spores germinate into yeast and grow. These yeast can withstand the reactive oxygen and nitrogen intermediates (ROI and RNI, respectively) produced during the macrophage killing response, proliferate, and escape [35].
This scenario is similar to the one in H. capsulatum, in which conidia are readily phagocytosed by alveolar macrophages, and can germinate into yeast that are resistant to the anti-microbial activities of the innate immune system [36]. In this case, phagocytosis of H. capsulatum conidia provides a means of entry for the fungus into host macrophages, where rapid germination produces yeast that are resistant to the anti-microbial activities of host immune cells, thus initiating an intracellular parasitic lifestyle.
This proposed mechanism is similar to what is observed for many intracellular bacterial pathogens and is known as the Trojan Horse Model. Using alveolar macrophages as Trojan horses, intracellular pathogens are ferried out of the alveoli and into the bloodstream, thus facilitating dissemination [37]. The mechanisms of dissemination of C. neoformans from the lung to the central nervous system are not known. It is possible that the phagocytosis of C. neoformans spores provides a means for the fungus to leave the lung and disseminate to the brain or remain dormant until conditions in the host support dissemination.
Spore survival is a race between germination and activation
Although spores can be phagocytosed and grow inside macrophages in culture, this is possible only when the macrophages are not activated [32]. In culture, macrophages are not subject to the natural activation responses of the host, but this response can be mimicked by exposing macrophages to lipopolysaccharide (LPS) and interferon-γ (IFN-γ) [38]. When macrophages in culture are activated with LPS and IFN-γ prior to exposure to C. neoformans spores, the spores are phagocytosed and then killed rapidly in a manner that is dependent on ROI and RNI produced by the macrophages [32]. In contrast, opsonized yeast that are phagocytosed by activated macrophages are resistant to macrophage killing mechanisms. Because dormant spores are more resistant to environmental stress than yeast, one might predict that spores would also be more resistant to macrophage killing. However, given the sensitivity of spores to ROI and RNI, it appears that germination is a period of vulnerability when C. neoformans is susceptible to the innate immune response.
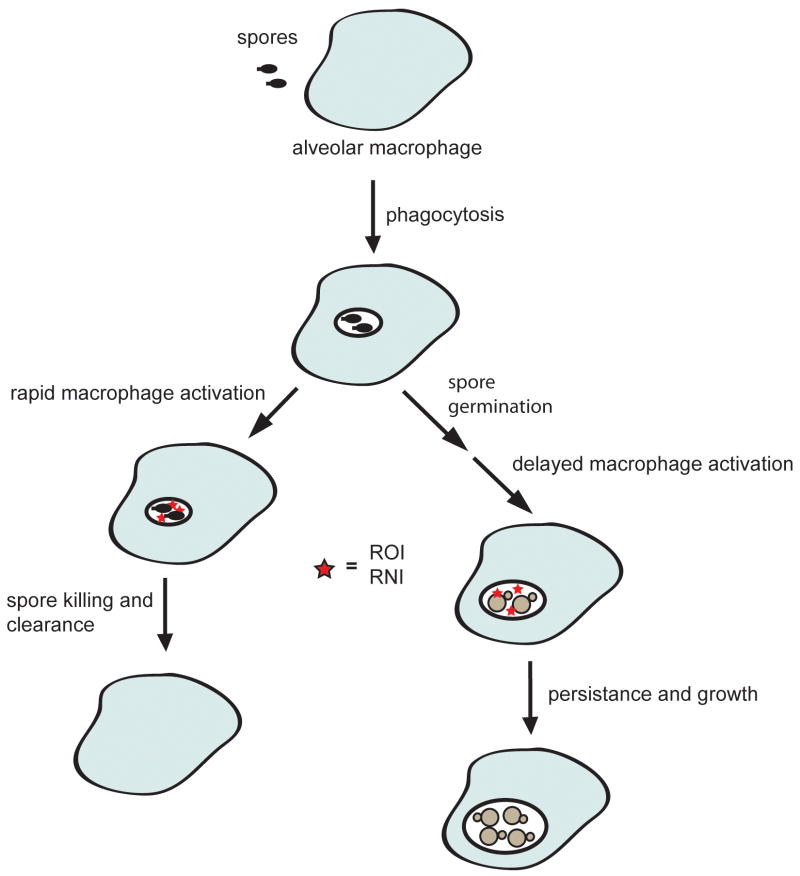
Because germinating spores are susceptible to the anti-microbial activities of macrophages, it is paradoxical that spores cause disease as efficiently as yeast in mice. One possible explanation for this finding invokes a kinetic model, which balances competition between spore germination and macrophage activation. In this scenario, although the majority of spores are killed and cleared by activated macrophages, a small number of spores germinate before macrophage activation responses can kill them. These spores (now yeast) can grow in the protected environment of the macrophage and use macrophages as Trojan horses to disseminate (Fig. 2). Because yeast may not be phagocytosed as efficiently as spores in vivo, dissemination in spore-infected mice may be more efficient, leading to the same time-to-disease with fewer persisting cells.
Figure 2. The kinetic model of C. neoformans spore-mediated infections.
Inhaled spores are phagocytosed by alveolar macrophages. Rapid activation of the macrophage leads to the production of ROI and RNI (stars) before the spores have completed germination, thus killing and clearing the spores. Delayed activation of the macrophages allows the spores to germinate into yeast, which are capable of withstanding the ROI and RNI. These yeast then persist and grow within the macrophage.
This model highlights the importance of the kinetics of spore germination and macrophage activation for determining whether C. neoformans causes disease. As a result, the kinetics of macrophage activation within a host could be an important factor in understanding susceptibility to cryptococcosis. Perhaps a rapid immune response from immunocompetent individuals is sufficient to kill and clear the vast majority of germinating spores; as a result, C. neoformans spores are unable to persist within this host. However, individuals with immune system defects may not mount a sufficiently early immune response to attack C. neoformans spores during the window of germination. In particular, people infected with HIV show dysfunctional cytokine signaling due to viral infection of CD4+ T lymphocytes and alveolar macrophages that can decrease or delay the innate immune response within the lung [39]. This delayed response could allow spores to germinate into yeast and proliferate, leading to disseminated disease.
Similarly, germination kinetics could be a critical factor in determining the fate of spores when phagocytosed. Perhaps, in immunocompetent people, C. neoformans spore germination is not as rapid as macrophage activation, and that is why this seemingly ubiquitous human fungal pathogen is capable of causing disease only in immunocompromised individuals. Conversely, it is possible that the spores of Cryptococcus gattii, a closely related fungus that causes disease in immunocompetent people, can germinate rapidly enough to escape macrophage killing and allow this fungus to establish infection even in the presence of a rapid immune response in healthy individuals.
Conclusions
Recent advances in spore isolation have led to the first direct analyses of the properties of C. neoformans spores and how these properties contribute to the virulence of this human fungal pathogen. C. neoformans spores are more stress-resistant than yeast, indicating a possible advantage during dispersal. Furthermore, the differences between the spore and yeast surfaces lead to different interactions with host immune cells, likely resulting in a fundamentally different disease process within the host.
Further analysis of the properties of C. neoformans spores will help elucidate the role of C. neoformans spores in the natural process of infection. In particular, uncovering the differences between spores produced by sexual development and spores produced by monokaryotic fruiting promises to provide valuable insights into the natural life cycle of C. neoformans and the extent to which each type of spore contributes to disease in humans. Ultimately, further understanding Cryptococcus spores will also help shed light on common mechanisms of fungal pathogenesis that will likely have broad implications for current virulence models and guide future attempts to develop novel therapeutic strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown JK, Hovmoller MS. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science. 2002;297:537–541. doi: 10.1126/science.1072678. [DOI] [PubMed] [Google Scholar]
- 2.Yoder JS, Beach MJ. Cryptosporidium surveillance and risk factors in the United States. Exp Parasitol. 124:31–39. doi: 10.1016/j.exppara.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Driks A. The Bacillus anthracis spore. Mol Aspects Med. 2009;30:368–373. doi: 10.1016/j.mam.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Bolton MD, Kolmer JA, Garvin DF. Wheat leaf rust caused by Puccinia triticina. Mol Plant Pathol. 2008;9:563–575. doi: 10.1111/j.1364-3703.2008.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Converse JL, Reed RE. Experimental epidemiology of coccidioidomycosis. Bacteriol Rev. 1966;30:678–695. doi: 10.1128/br.30.3.678-695.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A, Pirofski LA. The weapon potential of human pathogenic fungi. Med Mycol. 2006;44:689–696. doi: 10.1080/13693780600928503. [DOI] [PubMed] [Google Scholar]
- 7.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitman J, Edwards JE, Filler SG, Mitchell AP. Molecular principles of fungal pathogenesis. Washington, D.C: ASM Press; 2006. [Google Scholar]
- 9.Hatch TF. Distribution and deposition of inhaled particles in respiratory tract. Bacteriol Rev. 1961;25:237–240. doi: 10.1128/br.25.3.237-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 11.Driver JA, Saunders CA, Heinze-Lacey B, Sugar AM. Cryptococcal pneumonia in AIDS: is cryptococcal meningitis preceded by clinically recognizable pneumonia? J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:168–171. [PubMed] [Google Scholar]
- 12.Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16:837–874. v–vi. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 13.Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28:1642–1644. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon-Chung KJ. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia. 1976;68:821–833. [PubMed] [Google Scholar]
- 15.Idnurm A. A tetrad analysis of the basidiomycete fungus Cryptococcus neoformans. Genetics. doi: 10.1534/genetics.109.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc Natl Acad Sci U S A. 1996;93:7327–7331. doi: 10.1073/pnas.93.14.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bui T, Lin X, Malik R, Heitman J, Carter D. Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, α mating type populations. Eukaryot Cell. 2008 doi: 10.1128/EC.00097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvintseva AP, Marra RE, Nielsen K, Heitman J, Vilgalys R, Mitchell TG. Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot Cell. 2003;2:1162–1168. doi: 10.1128/EC.2.6.1162-1168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon-Chung KJ, Bennett JE. Distribution of a and α mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978;108:337–340. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- 20.Ellis DH, Pfeiffer TJ. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet. 1990;336:923–925. doi: 10.1016/0140-6736(90)92283-n. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz A, Bulmer GS. Particle size of airborne Cryptococcus neoformans in a tower. Appl Environ Microbiol. 1981;41:1225–1229. doi: 10.1128/aem.41.5.1225-1229.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz A, Fromtling RA, Bulmer GS. Distribution of Cryptococcus neoformans in a natural site. Infect Immun. 1981;31:560–563. doi: 10.1128/iai.31.2.560-563.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulmer GS. Twenty-five years with Cryptococcus neoformans. Mycopathologia. 1990;109:111–122. doi: 10.1007/BF00436791. [DOI] [PubMed] [Google Scholar]
- •24.Botts MR, Giles SS, Gates MA, Kozel TR, Hull CM. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot Cell. 2009;8:595–605. doi: 10.1128/EC.00352-08. This work reports the first successful large scale isolation of C. neoformans spores. Direct analysis of spores provides the first description of the basic properties of C. neoformans spores, including resistance to external stress, and morphology and composition of the spore coat. This work also shows that an intact capsule biosynthetic pathway is required for proper spore formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •25.Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR. Heitman J: Spores as infectious propagules of Cryptococcus neoformans. Infect Immun. 2009;77:4345–4355. doi: 10.1128/IAI.00542-09. The authors demonstrate that spores are virulent in both the murine and the Galleria models of infection, with as few as 500 spores capable of killing mice. This work also describes that C. neoformans spores are phagocytosed by macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coluccio AE, Rodriguez RK, Kernan MJ, Neiman AM. The yeast spore wall enables spores to survive passage through the digestive tract of Drosophila. PLoS One. 2008;3:e2873. doi: 10.1371/journal.pone.0002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebbink MF, Claessen D, Bouma B, Dijkhuizen L, Wosten HA. Amyloids--a functional coat for microorganisms. Nat Rev Microbiol. 2005;3:333–341. doi: 10.1038/nrmicro1127. [DOI] [PubMed] [Google Scholar]
- 28.Briza P, Ellinger A, Winkler G, Breitenbach M. Chemical composition of the yeast ascospore wall. The second outer layer consists of chitosan. J Biol Chem. 1988;263:11569–11574. [PubMed] [Google Scholar]
- 29.Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, D.C: ASM Press; 1994. [Google Scholar]
- 30.Zimmer BL, Hempel HO, Goodman NL. Pathogenicity of the basidiospores of Filobasidiella neoformans. Mycopathologia. 1984;85:149–153. doi: 10.1007/BF00440944. [DOI] [PubMed] [Google Scholar]
- 31.Sukroongreung S, Kitiniyom K, Nilakul C, Tantimavanich S. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med Mycol. 1998;36:419–424. [PubMed] [Google Scholar]
- ••32.Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun. 2009;77:3491–3500. doi: 10.1128/IAI.00334-09. This paper provides the first conclusive data that C. neoformans spores can cause disease in mammals. In addition, the authors detail spore-macrophage interactions, and the ability of activated macrophages to kill C. neoformans spores. This paper also links β-1,3-glucan in the spore coat to the dectin-1 receptor on macrophages, which contributes to the phagocytosis of spores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litvintseva AP, Mitchell TG. Most environmental isolates of Cryptococcus neoformans var. grubii (serotype A) are not lethal for mice. Infect Immun. 2009;77:3188–3195. doi: 10.1128/IAI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozel TR. Opsonization and phagocytosis of Cryptococcus neoformans. Arch Med Res. 1993;24:211–218. [PubMed] [Google Scholar]
- 35.Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 36.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the β-glucan receptor. Proc Natl Acad Sci U S A. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidi-Rontani C. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 2002;10:405–409. doi: 10.1016/s0966-842x(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 38.Lambert LE, Paulnock DM. Differential induction of activation markers in macrophage cell lines by interferon-γ. Cell Immunol. 1989;120:401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- 39.Nicol MQ, Mathys JM, Pereira A, Ollington K, Ieong MH, Skolnik PR. Human immunodeficiency virus infection alters tumor necrosis factor α production via Toll-like receptor-dependent pathways in alveolar macrophages and U1 cells. J Virol. 2008;82:7790–7798. doi: 10.1128/JVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]