Abstract
The Na-K-2Cl cotransporter (NKCC2) regulates sodium transport along the thick ascending limb of Henle’s loop and is important in control of sodium balance, renal concentrating ability and renin release. To determine if there are sex differences in NKCC2 abundance and/or distribution, and to evaluate the contribution of ovarian hormones to any such differences, we performed semiquantitative immunoblotting and immunoperoxidase immunohistochemistry for NKCC2 in the kidney of Sprague Dawley male, female and ovariectomized (OVX) rats with and without 17-β estradiol or progesterone supplementation. Intact females demonstrated greater NKCC2 protein in homogenates of whole kidney (334%±29), cortex (219%±20) and outer medulla (133%±9) compared to males. Ovarian hormone supplementation to OVX rats regulated NKCC2 in the outer medulla only, with NKCC2 protein abundance decreasing slightly in response to progesterone but increasing in response to 17-β estradiol. Immunohistochemistry demonstrated prominent NKCC2 labeling in the apical membrane of thick ascending limb cells. Kidney section NKCC2 labeling confirmed regionalized regulation of NKCC2 by ovarian hormones. Localized regulation of NKCC2 by ovarian hormones may have importance in controlling sodium and water balance over the lifetime of women as the milieu of sex hormones varies.
Keywords: Gender, estrogen, progesterone, ovariectomy, tubule, kidney
Introduction
Sex differences in renal concentrating ability and sodium handling have been noted in both humans and rodents [7, 13, 17, 24]. The Na-K-2Cl cotransporter (NKCC2) regulates sodium transport along the thick ascending limb of Henle’s loop and is important in the regulation of sodium balance and urinary concentrating ability. Furthermore, NKCC2 is also localized in the macula densa cells where it is involved in signaling for tubuloglomerular feedback and renin release [14, 18, 19]. The extent to which NKCC2 contributes to the above-mentioned sexual dimorphisms is unknown. Since most studies on the regulation of NKCC2 have been conducted in the male, the impact of gender and/or ovarian hormones on the normal expression of NKCC2 is not clear. Of note, estrogen and progesterone receptors have been found in the kidney [2, 21].
The overall aim of this study was to gain deeper insight into sex- and ovarian hormone-mediated control of salt and water homeostasis by specifically investigating regulation of the NKCC2 cotransporter. Accordingly we conducted a series of studies to determine how sex and ovariectomy (OVX), with and without 17-β estradiol and progesterone supplementation, influence the abundance and distribution of NKCC2 in the thick ascending limb of Henle’s loop.
Experimental Procedure
Animals were housed in the Virginia Commonwealth University (VCU) animal facility. All animal protocols were approved by the Institutional Animal Care and Use Committee (VCU IACUC) and in accord with the NIH Guide for animal use. Young adult male, intact female and ovariectomized (OVX) Sprague Dawley (SD) rats from Harlan (Indianapolis, Indiana) were used for these experiments. All rats were placed in metabolic cages for 3 days and fed a rationed gelled diet containing water (25ml/200 g body weight (BW)/day), agar (0.125 g BW/day), and rat chow (15 g/200 g BW/day) (Zeigler Rodent NIH-31 Open Formula, Ziegler Bros, Inc, Gardners, PA) from which sodium intake was calculated (Na Intake = (Gel food intake) * (% rat chow in gel food (37.4%)) * (% Na in rat chow (0.28 %)) * (1000)/23 (FW Na)). A final 24 hour urine collection was made from which sodium excretion (UNaV = V × UNa) was determined. Sodium balance was then calculated (Na intake – Na excretion (fecal Na was not determined)). A terminal blood sample from the abdominal vena cava was collected and analyzed for 17-β estradiol and progesterone by RIA analysis (DPC, Los Angeles, CA). Urine samples were analyzed for Na (Electrolyte 16+ analyzer, Nova Biomedical, Waltham, MA).
To investigate sex differences in NKCC2 protein expression and cellular localization, in a first series we used semiquantitative immunoblotting and immunoperoxidase immunohistochemistry in intact male and intact female rats. All rats were sexually mature, young adults. Rats were weight-matched in an attempt to control sodium intake with male rats harvested at ~9 weeks of age and female rats at ~12 weeks. In a second series to determine the specific effect of 17-β estradiol on NKCC2 regulation, intact female and OVX rats were used (~9 weeks at harvest). Rats were ovariectomized at Harlan, transported to VCU ~1 week following surgery. Rats were permitted ~1 week to acclimate prior to metabolic cage studies and hormone replacement. Kidney harvest and euthanasia was performed ~1 week following hormone replacement. Rats were anesthetized with 2–5% isofluorane anesthesia and implanted subcutaneously (SC) at the nape of the neck with either a pellet containing 17-β estradiol (0.05 mg, 21 day release) or placebo pellet (Innovative Research of America, Sarasota, FL) and studied after seven days. In a 3rd series intact female and OVX rats were studied to determine the effect of progesterone on NKCC2 regulation (~10 weeks at harvest). The OVX rats were randomly assigned to groups to be given either SC injections of vehicle (200 μl polyethylene Glycol (PEG 200 MW) (Sigma Aldrich, St. Louis, MO) or progesterone (Sigma Aldrich, St. Louis, MO) (2.5 mg/200 μl PEG) daily for seven days. The intact female rats received a daily needle stick.
The dose of hormones used was a modification of the protocol used by Campbell and Febbraio [3]. Briefly, we implanted 17-β estradiol time-release pellet (2.5 ug/day) from Innovative Research of America in OVX rats (SC). Since our animals were ovariectomized at the breeders we implanted pellets following the acclimatization period (~1 week following transport, ~2 weeks after OVX). However, our protocol resulted in higher circulating levels of 17-β estradiol than reported by Campbell and Febbraio [3]. We attribute the higher serum levels of 17-β estradiol, at least in part, to the fact that our rats were smaller (~20%). We have previously shown that in response to experimental alterations, abundance changes of sodium transporter proteins are seen between 3 and 10 days [10]. Thus, we chose 7 days to optimize appearance of changes in sodium transporter protein in this study.
At sacrifice all rats were anesthetized with isoflurane (5%) and the left kidney was harvested intact (whole kidney, WK) and the right kidney was dissected into cortex (CTX) and inner stripe of the outer medulla (OM). For Western blot analysis the WK, CTX and inner stripe of the OM sections were homogenized in a chilled isolation solution containing 10 mM triethanolamine, 250 mM sucrose (Mallinckrodt Baker Inc., Phillipsburg, NJ) and protease inhibitors, phenylmethyl sulfonyl fluoride (25mg/ml isopropyl alcohol) (Biochemical Corp, Lakewood, NJ) and leupeptin (1mg/ml dd H2O) (Novabiochem/EMD Chemicals, Inc., Gibbstown, NJ). Protein concentrations of homogenates were determined by the BCA protein assay kit (Pierce Biotechnology, Rockford, IL). All samples were solubilized at 60 ° C in a Laemmli sample buffer. SDS-PAGE was performed on Criterion precast 10% Tris-HCl gels (Bio-Rad Laboratories, Inc., Hercules, CA). An initial gel was stained with GelCode Blue Reagent (Pierce Biotechnology, Rockford, IL) as described previously to confirm equal loading [22]. Figure 1 is a representative image of a loading gel. For immunoblotting, each sample was loaded into individual lanes, the proteins were transferred electrophoretically to nitrocellulose membranes. The blots were blocked with 5 g/dl non-fat dry milk (Bio-Rad Laboratories, Hercules, CA) and probed with primary antibody over night. Blots were incubated with peroxidase-conjugated secondary antibodies (Pierce Biotechnology, Rockford, IL, no. 31458 & 31452), followed by an enhanced luminol reagent, Western Lightning Chemilumnescence Reagent Plus (Perkin Elmer LAS, Inc., Boston, MA). Blots were exposed to X-ray film and band densities were quantified by VersaDoc Imaging System (VersaDoc Bio-Rad, Bio-Rad Laboratories, Hercules, CA). To facilitate comparisons, we normalized the densitometry values, defining the mean for the control group as 100%. Thus the control group for each blot was designated as 100% and the experimental condition either increased or decreased band density from control as previously described [4, 8, 9, 12].
Figure 1.

Representative control loading gel stained with GelCode Blue. Prior to Western blotting control loading gels were run to confirm equality of loading in each lane. Shown are homogenates from the inner stripe of the outer medulla from the progesterone study. Each lane was loaded with homogenate from a different rat. To ensure equality of loading, three representative bands were quantified by densitometry.
For the 17-β estradiol study, the previously characterized [4] rabbit polyclonal antibody directed to NKCC2 (a kind gift from Mark Knepper) was used. For all other studies we used the NKCC2 antibody that was developed in this laboratory. The same method for antibody development, affinity purification and characterization was used as previously described [4].
For immunohistochemistry additional animals were used and the kidneys were perfusion fixed, and sections prepared as described previously [12]. Immunohistochemistry staining for NKCC2 was performed according to manufacturers instructions by using a staining kit (Vectastain Elite ABC Kit, Vector Laboratories UK). This kit is specific for the species used for antibody development (rabbit). We used two sets of slides from separate groups of rats, to confirm the immunohistochemical results. Using this approach, sections were blocked with serum blocking solution then incubated sequentially with anti-NKCC2 (1:400), biotinylated secondary antibody (1:200), and streptavidin-peroxidase conjugate. Peroxidase was visualized by instructions addition of 3, 3-diaminobenzidine tetrahydrochloride substrate.
Statistical Analysis
Data are given as mean±standard error (SE). SPSS statistical program was used for all analysis (SPSS 12.0, Chicago, Illinois). An independent t-test or Mann-Whitney was used for the sex difference studies. A one-way analysis of variance (ANOVA) with a bonferroni post hoc was used for the progesterone and 17-β estradiol studies. The null hypothesis was rejected at p<0.05.
Results
Physiological Data
As shown in Table 1 the initial body weights at the beginning of the study were similar in male and female rats. There was a modest increase in body weight (BW) in male versus female rats by the end of the study (~40 g). However, there was no significant gender difference in Na intake, Na excretion or Na balance. There was no statistically significant difference between the sexes in mean serum osmolarity. As expected, serum estradiol and progesterone levels were much higher in female rats than in male rats
Table 1.
Physiologic data for gender difference study
Summary of physiological data from metabolic cage analysis of male and female rats. At time of harvest male rats were ~9 weeks old and female rats were ~12 weeks old. Initial body weight (BW) at beginning of study, final BW at end of study, BW difference (final BW-initial BW), Na intake, urine output (V), urine Na concentration, Na excretion (UNaV), Na balance (Na intake – UNaV), serum osmolarity, serum progesterone and serum 17 β estradiol. An unpaired T-test was performed.
| Male (n=6) | Female (n=6) | |
|---|---|---|
| Initial Body Weight (g) | 209 ± 5 | 207 ± 3 |
| Final Body Weight (g) | 249 ± 4 | 216 ± 2* |
| Difference in Body Weight (g) | 40 ± 5 | 9 ± 3* |
| Intake of Na (mM) | 2.16 ± 0.11 | 1.98 ± 0.02 |
| Urine | ||
| Urine output (mL/day) | 14.3 ± 1.2 | 15.4 ± 0.7 |
| Na (mM) | 106.2 ± 6.4 | 95.7 ± 2.0 |
| Na excretion (mmol/day) | 1.49 ± 0.09 | 1.47 ± 0.08 |
| Na balance | 0.62 ± 0.06 | 0.46 ± 0.06 |
| Serum | ||
| Osmolarity (mmol/kg) | 1035 ± 48 | 1111 ± 35 |
| Estradiol (pg/ml) | 7.25 ± 0.45 | 53.77 ± 17.68* |
| Progesterone (ng/ml) | 2.89 ± 1.14 | 18.96 ± 5.86* |
Mean ± standard error (SE),
vs male, p< 0.05.
Table 2 compares female rats intact, OVX, and OVX + 17 β-estradiol. OVX caused a significant increase in BW while 17 β-estradiol treatment returned BW to a level not significantly different from that of intact females. Intake of Na was modestly higher in OVX rats with no difference in Na excretion, resulting in a slight increase in Na balance. As anticipated, OVX female rats had lower plasma levels of the ovarian hormones than intact females, and 17-β estradiol treatment significantly increased circulating levels to above the value seen in the intact female.
Table 2.
Physiologic data for 17-β estradiol study
Summary of physiological data from metabolic cage analysis of female, ovariectomized (OVX), and OVX + 17 β estradiol rats. At time of harvest rats were ~ 9 weeks old. Body weight (BW) at end of study, Na intake, urine output (V), urine Na concentration, Na excretion (UNaV), Na balance (Na intake – UNaV), serum progesterone, and serum 17 β estradiol. A one-Way ANOVA with Bonferroni Post Hoc was performed. Mean ± standard error (SE), p< 0.05.
| Intact (n=10) | OVX (n=6) | OVX + 17-β estradiol (n=7) | |
|---|---|---|---|
| Body Weight (g) | 176 ± 1 | 192 ± 3* | 172 ± 1# |
| Intake of Na (mM) | 1.64 ± 0.01 | 1.72 ± 0.02* | 1.68 ± 0.01 |
| Urine | |||
| Urine output (mL/day) | 13.1 ± 0.4 | 13.1 ± 0.4 | 13.9 ± 0.5 |
| Na (mM) | 92.6 ± 2.3 | 87.7 ± 1.3 | 90.7 ± 1.3 |
| Na excretion (mmol/day) | 1.30 ± 0.03 | 1.24 ± 0.02 | 1.36 ± 0.04 |
| Na balance | 0.34 ± 0.03 | 0.48 ± 0.04* | 0.32 ± 0.03# |
| Serum | |||
| Estradiol (pg/ml) | 14.61 ± 4.23 | 5.06 ± 0.31 | 204.07 ± 39.28*# |
| Progesterone (ng/ml) | 24.33 ± 4.03 | 2.78 ± 0.52* | 3.48 ± 0.85*# |
Significance is denoted as follows:
vs female and
vs OVX.
Physiological data of female intact, OVX and OVX + progesterone treated rats are shown in Table 3. BW, intake of Na, and excretion of Na was higher with OVX, but no difference was seen in Na balance between the three groups. OVX decreased serum progesterone levels while progesterone administration to OVX rats increased levels.
Table 3.
Physiologic data for progesterone study
Summary of physiological data from metabolic cage analysis of female, ovariectomized (OVX), and OVX + progesterone rats. At the time of harvest rats were ~ 10 weeks old. Body weight (BW) at end of study, Na intake, urine output (V), urine Na concentration, Na excretion (UNaV), Na balance (Na intake – UNaV), and serum progesterone. A one-Way ANOVA with Bonferroni Post Hoc was performed. Mean ± standard error (SE), p< 0.05.
| Intact (n=6) | OVX (n=6) | OVX + progesterone (n=6) | |
|---|---|---|---|
| Body Weight (g) | 189 ± 2 | 211 ± 4* | 211 ± 2* |
| Intake of Na (mM) | 1.72 ± 0.04 | 1.91 ± 0.05* | 1.91 ± 0.14* |
| Urine | |||
| Urine output (mL/day) | 13.2 ± 0.5 | 12.5 ± 0.8 | 12.7 ± 0.4 |
| Na (mM) | 109.67 ± 1.71 | 135.17 ± 5.07* | 133.67 ± 4.60* |
| Na excretion (mmol/day) | 1.65 ± 0.07 | 1.91 ± 0.07* | 1.92 ± 0.02* |
| Na balance | 0.27 ± 0.05 | 0.24 ± 0.06 | 0.22 ± 0.02 |
| Serum | |||
| Progesterone (ng/ml) | 17.15 ± 5.17 | 5.78 ± 1.67 | 52.36 ± 3.88*# |
Significance is denoted as follows:
vs female and
vs OVX.
Impact of ovarian steroids on NKCC2 protein abundance
To examine regional differences in NKCC2 protein abundance, we performed immunoblotting of homogenates of WK, CTX and OM. Intact males showed weak NKCC2 band densities in WK and CTX with the greatest concentration of NKCC2 in OM (Figure 2). Intact females exhibit more robust band densities in all regions with 300% and 200% increases in NKCC2 abundance in WK and CTX respectively, and 30% greater abundance in OM compared to males. We did not restrict the use of intact female rats to one stage of estrus cycle. Figure 2 shows that the individual band densities from each female rat were higher compared to male rats irrespective of the stage of the estrus cycle.
Figure 2.

Semiquantitative immunoblots showing sex differences of NKCC2 abundance. For each blot, each lane was loaded with homogenate from a different rat. A preliminary Coomasssie-stained gel demonstrated equality of loading among the lanes. (A) Whole kidney (WK) homogenates compared 6 male and 6 female rats. B) Homogenates from cortex (CTX) and inner stripe of the outer medulla (OM) compared 4 male and 4 female rats. C) Bar graphs summarizes quantification of band densities. Band densities were normalized to control males with males set at 100%. An unpaired T-test was performed. Mean±standard error (SE), * p<0.05
To determine if ovarian hormones mediate the sex difference of NKCC2 expression, we performed immunoblotting of homogenates of WK, CTX and OM in female and OVX rats (Figure 3). 17-β estradiol supplementation in OVX rats increased the NKCC2 protein by ~30 % in WK (upper bands of Figure 3A) but did not alter levels in CTX (middle bands of Figure 3A). 17-β estradiol increased NKCC2 expression by ~65% above OVX in OM (lower band of Figure 4A. In contrast, compared to OVX rats progesterone supplementation had no impact on WK or CTX NKCC2 but led to a small decrease in NKCC2 abundance (~15 %) in the OM (Figure 4).
Figure 3.

Semiquantitative immunoblots showing effect of 17-β estradiol on NKCC2 abundance. A) Homogenates from whole kidney (WK), cortex (CTX) and inner stripe of the outer medulla (OM) compared 8 intact female, 6 ovariectomized (OVX)+placebo pellet (SC, 21 day pellet – for 7 days) female and 7 OVX +17 β estradiol (SC 0.05 mg, 21 day pellet, for 7 days) rats. B) Band densities are quantified and summarized as bar graphs. Band densities were normalized to intact females with females set at 100%. A one-way ANOVA with a bonferroni post hoc was used to compare groups. Mean±standard error (SE), * versus intact female, # versus OVX; p<0.05
Figure 4.

Semiquantitative immunoblots showing effect of progesterone on NKCC2 regulation. Whole kidney (WK), cortex (CTX) and inner stripe of the outer medulla (OM) homogenates from different rats were loaded in each lane. (A) Immunoblots compared intact females, ovariectomized (OVX)+vehicle (SC 200 μl PEG for 7 days) and OVX+progesterone (SC 2.5 mg/200 μl PEG for 7 days). B) Quantification of band densities were normalized to intact females with intact females set at 100%. Band densities are summarized as bar graphs. A one-way ANOVA with a bonferroni post hoc was used to compare groups. Mean±standard error (SE), * versus intact female, # versus OVX; p<0.05
Impact of 17-β estradiol actions on NKCC2 localization
We performed immunoperoxidase labeling of kidney sections probed for NKCC2. Microscope settings and labeling conditions were identical for each set of slides examined. Figure 5 shows immunoperoxidase labeling from cortex of male and female rats. Specific NKCC2 staining is seen in cortical thick ascending limbs. Stronger labeling is seen in female rats compared to male rats. This is consistent with findings seen from Western blotting (Figures 2).
Figure 5.
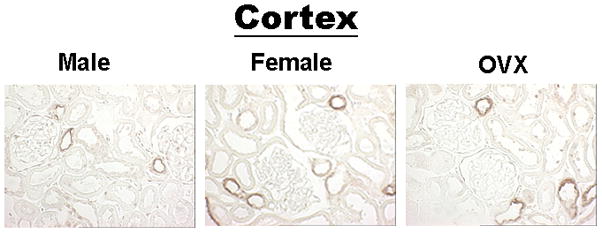
Immunoperoxidase NKCC2 labeling of cortex (CTX). NKCC2 labeling from rats using Vectastain Elite ABC Kit, (rabbit-specific stain). Kidney sections from male, female and ovariectomized (OVX) rats.
Figure 6 shows immunoperoxidase staining from a representative set of rats. Predominant apical labeling of NKCC2 in OM TAL cells from males, intact females and OVX females with and without 17-β estradiol supplementation is seen. There is clearly more NKCC2 labeling in OM of intact females vs intact males. OVX reduces OM NKCC2 protein abundance to male level while 17-β estradiol supplementation increases NKCC2 protein abundance.
Figure 6.

Immunoperoxidase NKCC2 labeling of inner stripe of outer medulla (OM). NKCC2 labeling from rats using Vectastain Elite ABC Kit, (rabbit-specific stain). Kidney sections from male, female and ovariectomized (OVX) rats treated with either placebo pellets (SC, 21 day pellet, for 7 days) or with 17-β estradiol (SC 0.05 mg, 21 day pellet, for 7 days) were used.
Discussion
The major novel findings of this study are: (1) NKCC is more abundant in WK, CTX and OM of intact females compared to intact males; (2) OVX suppresses this sex difference in WK; (3) Ovarian hormone supplementation in OVX rats alters NKCC2 abundance only in OM; (4) In OVX rats 17-β estradiol increased while progesterone decreased NKCC2 abundance in the OM.
The present study focused on ovarian hormone regulation of NKCC2 expression in normotensive rats and indicates a sex difference in NKCC2 abundance with a greater transporter density in the female. This sex-dependent difference in NKCC2 is apparent in both CTX and OM. Cortical NKCC2 is also involved in macula densa signaling via detection of luminal NaCl delivery [14], and therefore plays an important role in tubuloglomerular feedback (TGF) control of glomerular filtration rate [18, 19] and in regulating renal renin secretion as part of the volume sensing and control system [12]. The importance of NKCC2 in the macula densa is demonstrated by comparison of NKCC2 knock out mice with Na hydrogen exchanger – type 3 (NHE3) knock out mice [20]. The salt wasting that is seen in the NKCC2 knock out mice is far more severe than that seen in the NHE3 knock out model. This is likely due to the fact that without NKCC2 in the macula densa cells the TGF mechanism is compromised. The higher levels of NKCC2 in females relative to males noted in the current study could lead to greater macula densa NaCl concentration that attenuates renin secretion. Activation of the renin angiotensin aldosterone system (RAAS) is important in renal retention of Na and the maintenance of Na balance. Although the resolution of the immunoperoxidase immunohistochemistry labeling in our study was not high enough to definitively identify macula densa labeling, it is important to note that Western blotting and immunoperoxidase immunohistochemistry both indicate that ovariectomy does not reverse the sexual dimorphism in cortical NKCC2 (Figure 3, 4 and 5). Interestingly, plasma renin activity (PRA) is elevated in men compared to premenopausal women [16]. This is also consistent with the finding that plasma renin in postmenopausal women is not elevated, and that it does not change 17-β estradiol supplementation [5]. Further, the young adult female rat kidney is vasoconstricted relative to the male [11] which could reflect increased macula densa NKCC2 abundance and TGF-mediated vasoconstriction. These observations are consistent with the possibility that the lower NKCC2 seen in CTX in the male rat is androgen dependent. However, further studies are required to rigorously test this hypothesis.
In contrast to what we see in the cortex we observed that ovarian hormones influence NKCC2 protein abundance in OM. NKCC2 in the medulla plays an important role in establishing the medullary concentration gradient, thereby regulating water excretion via the countercurrent multiplier mechanism. Additionally it is involved in diluting the tubular fluid that is presented to the distal tubule and collecting duct [9]. Male rats and OVX females have similar OM NKCC2 levels and 17-β estradiol supplementation in OVX females raises OM NKCC2 protein as indicated by Western blotting and immunoperoxidase immunohistochemistry (Figure 3 and 6). This indicates that regional differences exist within the kidney with respect to the action of 17-β estradiol on the abundance of the transporter. Our immunohistochemistry data show that NKCC2 is mostly in the apical membrane in males, intact females and OVX females with or without 17-β estradiol supplementation and confirms our Western blotting findings that male and OVX rats have less NKCC2 labeling in the OM compared to either intact female or OVX females with 17-β estradiol supplementation. Therefore, the differences in NKCC2 abundance that we report likely reflect functional differences. As mentioned above, NKCC2 is involved in generating the cortocmedullary osmotic gradient that is critical for the concentration of urine by the collecting, thereby controlling water balance. In the thick ascending limb cells, NKCC2 is involved in generating the corticomedullary osmotic gradient. This gradient is critical for the concentration of urine by the collecting duct thereby controlling water balance. A greater NKCC2 abundance in intact females suggests that females may have a better renal concentrating ability than males. This may amplify the impact of the recently reported increase in arginine vasopressin V2 receptor that would also act to enhance maximum urine osmolarity [6]. Future studies should investigate this possibility further since as pointed out by Liu et al [6] the combination of these effects may render premenopausal women more vulnerable to hyponatremia.
Recent findings from Sartori et al [17] have shown that young adult normotensive Sprague Dawley rats demonstrate a gender difference in baseline blood pressure. Male rats had mean blood pressures ~5mmHg higher than age matched female rats, which was statistically significant. These findings suggest that normotensive Sprague Dawley rats begin to demonstrate gender specific alterations in blood pressure and Na handling at a relatively early age. Our study was performed in sexually mature, young adult Sprague Dawley rats that were weight-matched to ensure a similar Na intake in both sexes. There were no differences in Na excretion or Na balance between males and females. Interestingly in spite of the fact that we controlled Na intake, which is known to effect Na transporter regulation [4, 8], there was a 300% increase in the protein abundance of NKCC2 in the WK of female compared to male rats. Further study is needed to adequately understand these gender specific differences in Na transporter regulation.
Progesterone did not restore NKCC2 levels in any region of the OVX kidney and in fact progesterone supplementation to OVX females reduced OM NKCC2 further. Weaker and colleagues identified progesterone receptors in the thick ascending limb [25], which may regulate NKCC2 levels (Figure 4).
There have been several other studies on the effect of sex steroids on NKCC2 that have yielded variable results. Tiwarri and colleagues [23] recently reported that in OM but not CTX NKCC2 was higher in young adult female vs male mice [23]. In contrast to our findings Riazi and colleagues reported no change in NKCC2 expression in the CTX and a significant down-regulation in OM with 17-β estradiol replacement following OVX in lean Zucker rats [15]. There were differences between our experimental protocols and those of Riazi et al [15]. These investigators gave 17-β estradiol injections at 4 day intervals for 12 weeks, whereas we used slow release pellets to deliver 17-β estradiol continuously for one week. Moreover, the circulating levels of 17-β estradiol that we achieved with 17-β estradiol supplementation were higher, and NKCC2 may be regulated by 17-β estradiol in a dose-dependent and/or time-dependent manner. Bandoli et al [1] reported no difference in cortical or medullary NKCC2 expression between male and female rat kidneys. This may be due to the large difference in BW and corresponding greater intake of food (and sodium) as well as water in the males. Studies have previously shown that differences in sodium and water intake can influence sodium transporter and channel abundance [4, 8]. Rats for the present study arrived weight-matched and were placed on a ration feeding protocol, to control for sodium and water intake. Following the acclimatization period and duration of study, there was only a modest difference in weights between groups (~40 g). Another difference between ours and the Brandoni study is that Brandoni et al [1] dissected the entire medulla and thus included regions of the kidney where NKCC2 is not expressed whereas we dissected the inner stripe of the OM, the region where NKCC2 is preferentially expressed.
In conclusion, our studies demonstrate increased CTX and OM NKCC2 protein expression in females compared to males and show that ovarian hormones regulate NKCC2 expression in a region- and steroid- specific fashion. These findings emphasize the need for further study of the specific actions and interactions of sex hormones on renal tubular function.
Acknowledgments
This research was supported by grants from the National Heart, Lung, and Blood Institute (K22HL66994) and the Virginia’s Commonwealth Health Research Board. The authors thank Mary L Giebel, VCU- Internal Medicine Core Laboratory, for immunohistochemical staining of kidney sections and Itaf F Fahkry, VCU – Nephrology Core Laboratory, for measurements of sodium from urine and plasma samples and measurement of serum 17-β estradiol and progesterone. The authors also thank Rebecca Habenicht for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandoni A, Villar SR, Torres AM. Gender-related differences in the pharmacodynamics of furosemide in rats. Pharmacology. 2004 February;70(2):107–112. doi: 10.1159/000074675. [DOI] [PubMed] [Google Scholar]
- 2.Bumke-Vogt C, Bahr V, Diederich S, Herrmann SM, Anagnostopoulos I, Oelkers W, Quinkler M. Expression of the progesterone receptor and progesterone- metabolising enzymes in the female and male human kidney. J Endocrinol. 2002 November;175(2):349–64. doi: 10.1677/joe.0.1750349. [DOI] [PubMed] [Google Scholar]
- 3.Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol. 2002;282:E1139–E1146. doi: 10.1152/ajpendo.00184.2001. [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na(+)-K(+)-2Cl- cotransporter, BSC-1. Am J Physiol. 1996 September;271(3 Pt 2):F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa A, Sumino H, Ogawa T, Ichikawa S, Nitta K. Effects of long-term transdermal hormone replacement therapy on the renin-angiotensin- aldosterone system, plasma bradykinin levels and blood pressure in normotensive postmenopausal women. Geriatr Gerontol Int. 2008;8:259–64. doi: 10.1111/j.1447-0594.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Tam H, Zheng W, Ji H, Sharma N, Wu X, Manigrasso MB, Sandberg K, Verbalis JG. Sex Differences in Vasopressin V2 Receptor Expression and Vasopressin-Induced Antidiuresis. JASN. 2009 doi: 10.1152/ajprenal.00199.2010. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Sex and age differences of renal function in rats with reduced ANGII activity during the nephrogenic period. Am J Physiol Renal Physiol. 2007;293:F506–F510. doi: 10.1152/ajprenal.00066.2007. [DOI] [PubMed] [Google Scholar]
- 8.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999 October;104(7):R19. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masilamani S, Knepper MA, Burg MB. The Kidney. In: Brenner BM, editor. Urine concentration and dilution. 6. Philadelphia: Saunders; 2000. pp. 595–636. [Google Scholar]
- 10.Masilamani S, Wang X, Kim G-H, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002;283:F648–F657. doi: 10.1152/ajprenal.00016.2002. [DOI] [PubMed] [Google Scholar]
- 11.Munger KA, Baylis C. Sex differences in renal hemodynamic in rats. Am J Physiol. 1988;254:F223–F231. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol. 2002 November;283(5):F923–F933. doi: 10.1152/ajprenal.00015.2002. [DOI] [PubMed] [Google Scholar]
- 13.Perucca J, Bouby N, Valeix P, Bankir L. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Regul Integr Comp Physiol. 2007;292:R700–R705. doi: 10.1152/ajpregu.00500.2006. [DOI] [PubMed] [Google Scholar]
- 14.Peti-Peterdi J. Confocal imaging and function of the juxtaglomerular apparatus. Curr Opin Nephrol Hypertens. 2005 January;14(1):53–7. doi: 10.1097/00041552-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Riazi S, Maric C, Ecelbarger CA. 17-beta Estradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int. 2006 February;69(3):471–80. doi: 10.1038/sj.ki.5000140. [DOI] [PubMed] [Google Scholar]
- 16.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001 May;37(5):1199–208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 17.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hyperten. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 18.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998 February;274(2 Pt 2):R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 19.Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl. 1998 September;67:S40–S45. doi: 10.1046/j.1523-1755.1998.06708.x. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter’s syndrome. PNAS. 2000 May;97(10):5434–9. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000 February;24(1):145. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 22.Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996 August;271(2 Pt 2):F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex differences in adaptive downregulation of pre-macula densa sodium transporters with ANG II infusion in mice. Am J Physiol Renal Physiol. 2010;298:F187–95. doi: 10.1152/ajprenal.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y-X, Crofton JT, Miller J, Sigman CJ, Liu H, Huber JM, Brooks DP, Share L. Sex difference in urinary concentrating ability of rats with water deprivation. Am J Physiol Reg Integ Comp Physiol. 1996;270:R550–R555. doi: 10.1152/ajpregu.1996.270.3.R550. [DOI] [PubMed] [Google Scholar]
- 25.Weaker FJ, Herbert DC, Sheridan PJ. Progesterone--specific binding sites in the kidney of the female baboon. J Urol. 1984 Oct;132(4):792–4. doi: 10.1016/s0022-5347(17)49875-2. [DOI] [PubMed] [Google Scholar]


