Abstract
Recent studies have suggested Fas-mediated elimination of antigen-presenting cells as an important mechanism down-regulating the induction of autoimmune responses. It remains unknown whether this mechanism restricts the magnitude of immune responses to non-self antigens. We used a mouse model of a cutaneous CD8+ T cell-mediated immune response (contact hypersensitivity, CHS) to test if CD4+CD25+ T cells expressing FasL regulate hapten-specific effector CD8+ T expansion through elimination of Fas-expressing hapten-presenting dendritic cells. In wild type mice, attenuation of CD4+CD25+ T regulatory cell activity by anti-CD25 mAb increased hapten-presenting dendritic cell numbers in skin-draining lymph nodes that led to increased effector CD8+ T cell priming for CHS responses. In contrast, CD4+CD25+ T cells did not regulate hapten-specific CD8+ T cell priming and CHS responses initiated by Fas-defective, lpr, dendritic cells. Thus, restricting dendritic cell priming functions through Fas-FasL interactions is a potent mechanism employed by CD4+CD25+ regulatory cells to restrict CD8+ T cell-mediated allergic immune responses in the skin.
Keywords: CD4+CD25+ T Cells, dendritic cells, contact hypersensitivity
Introduction
The development of antigen-specific effector T cells during the induction of immune responses must be tightly regulated to prevent excessive damage of tissues and organs. Recent studies have identified elimination of antigen-presenting cells (APC), including dendritic cells (DC) and B cells, as an important mechanism restricting T cell-mediated immune responses [1–4]. Several have reported that APC elimination is mediated through apoptosis induced by CD4+ T cells reactive to antigen/class II MHC complexes presented by the DC [2, 3, 5]. Importantly, Fas-mediated elimination of DC has been recently implicated as a mechanism regulating the initiation of autoimmune responses [4]. The role of this mechanism in regulating priming of T cells to exogenous antigens remains unclear.
Contact hypersensitivity (CHS) is a skin allergy that is the most frequently observed dermatosis in industrialized countries [6]. CHS responses occur in response to epicutaneous sensitization and challenge with haptens including urushiol, 2,4-dinitrofluorobenzene (DNFB) and oxazolone (Ox) [7, 8]. These responses are mediated by IFN-γ and IL-17-producing CD8+ T cells primed by hapten-presenting Langerhans cells (LC) and dermal dendritic cells migrating from the sensitized skin to the draining lymph nodes [9–12]. The numbers and persistence of hapten-presenting DC in these lymph nodes during effector T cell priming is restricted through Fas-FasL interactions [1]. Although CD4+ T cells are not required to mediate CHS as effector or helper cells, regulatory CD4+CD25+ T cells restrict hapten-specific CD8+ T cell expansion for CHS responses [13, 14]. Whether the role of Fas-FasL-mediated regulation is associated with CD4+CD25+ T cells remains untested. Two approaches were used to directly test whether these regulatory T cells induce FasL-mediated DC apoptosis to limit the duration of antigen presentation and expansion of the CD8+ effector T cells in CHS responses. First, the impact of CD4+CD25+ T cells on the survival of hapten-presenting DC in the lymph node priming site was evaluated in vivo and the ability of these regulatory T cells to enhance FasL-mediated apoptosis of hapten-presenting DC was tested in vitro. Second, Fas-sufficient (wild type) and Fas-defective (lpr) DC were compared for induction of CD8+ T cell and CHS responses and the potential influence of CD4+CD25+ T cells on the priming capabilities of these DC was tested. The results strongly support the hypothesis that CD4+CD25+ T cells regulate CD8+ T cell-mediated immune responses in the skin by inducing FasL-mediated apoptosis of skin-derived antigen-presenting DC.
Results
Attenuation of CD4+CD25+ regulatory cell activity increases the presence of hapten-bearing DC in the priming site
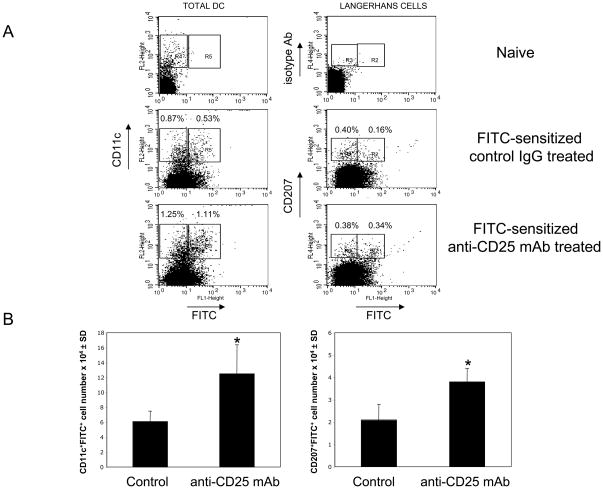
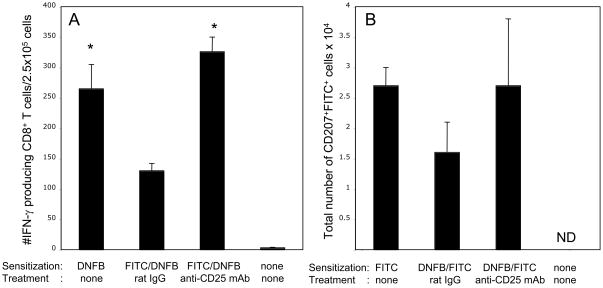
Recent studies from this laboratory indicated increased hapten-specific CD8+ T cell development and subsequent increases in the magnitude and duration of CHS responses to hapten challenge when CD4+CD25+ T cells were inhibited during hapten priming [13]. Here we investigated the mechanism of CD4+CD25+ T cell-mediated regulation by testing if increased numbers of hapten-presenting DC (hpDC), including Langerhans cells, in skin-draining lymph nodes accompanies the increased effector CD8+ T cell development and CHS responses in anti-CD25 mAb treated mice. When anti-CD25 mAb was given before and during sensitization with FITC, the percentages of FITC-bearing DC identified as the CD11c+FITC+ population as well as the percentages of FITC-bearing LC identified as the CD207+FITC+ cells were increased two-fold on day 3 post-sensitization (Figure 1A, gate R5: 0.54±0.03% of FITC+ DC in control group vs. 1.10±0.02% in anti-CD25 mAb-treated group, and, gate R2: 0.22±0.04% vs. 0.40±0.05% of FITC+ LC respectively, P < 0.02). Likewise, the total numbers of FITC-presenting cells within both total DC and LC populations were increased two-fold in the skin-draining lymph nodes of FITC-sensitized mice treated with anti-CD25 mAb (Figure 1B, *P < 0.05). In contrast, anti-CD25 mAb treatment had no significant impact on the percentages of FITC-negative DC (Figure 1A, gates R4 and R3). Therefore, inhibition of regulatory CD4+CD25+ T cell activity increased the numbers of hapten-presenting DC in the T cell priming site.
Figure 1. Attenuation of CD4+CD25+ T cells increases survival of hapten-presenting DC in skin-draining lymph nodes.
(A) Mice were sensitized with 0.5% FITC on day 0 and were given i. p. injections of 250 μg of anti-CD25 mAb or control rat IgG on days −1, 0 and +1. On day +3 (72 h) post-sensitization LNC suspensions were stained with PE-labeled anti-CD11c mAb (total DC) or were permeabilized and stained with AlexaFluor647-labeled anti-CD207/Langerin (Langerhans cells) mAb or with isotype control Ab. CD11c-positive or CD207-positive cells were gated and analyzed for FITC expression. LNC suspensions from naïve mice were used to distinguish FITC+ cells (right and left upper panels, gates R2 and R5) from FITC-negative cells (right and left upper panels, gates R3 and R4). The experiments were repeated three times with similar results.
(B) Total numbers of CD11c+FITC+ or CD207+FITC+ cells in the skin-draining lymph nodes of each individual mouse were calculated based on the percentage of these cells in each analyzed cell aliquot. Six mice were analyzed per each group in two independent experiments with similar results each time, *P < 0.05 by two-tailed Students’ t test.
Hapten-presenting DC express high levels of Fas, while CD4+CD25+ T cells express FasL and increase apoptosis of hapten-presenting DC during in vitro culture
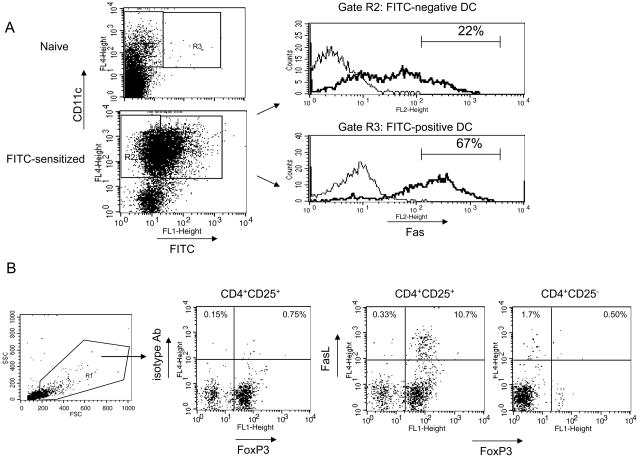
Our previous studies indicated that the survival of hapten-presenting DC in skin-draining lymph nodes during T cell priming is restricted through Fas-FasL interactions [1]. To begin to study the contribution of CD4+CD25+ regulatory T cells to this mechanism, we tested the expression of Fas on hapten-presenting DC activated during hapten sensitization vs. residential DC in the lymph nodes. Total DC were purified from the skin-draining lymph nodes of FITC-sensitized mice 24 h post-sensitization using positive selection of CD11c+ cells. During co-culture these purified DC activated hapten-specific, but not naïve, CD8+ T cells to produce IFN-γ indicating the presence of hapten-presenting DC in this cell population (not shown). Purified DC were stained with PE-labeled anti-Fas mAb and then CD11c+FITC− cells or CD11c+FITC+ cells were gated using CD11c+FITC− cells from naïve mice as a control (Figure 2A, gates R2 and R3 respectively) and then the levels of Fas expression by FITC-positive and FITC-negative DC were quantified as mean fluorescence intensity (MFI) of the PE channel. The majority of DC isolated from the lymph nodes of sensitized mice expressed Fas, however, the expression of Fas was increased more than four fold on FITC-presenting DC when compared to FITC-negative residential DC (MFI = 434.0 ± 11.3 for FITC-positive DC vs. 92.7 ± 6.9 for FITC-negative DC, p < 0.01). The percentages of DC expressing high levels of Fas were increased three-fold in the FITC-positive DC population (67%) in comparison to the FITC-negative DC (22%) (Figure 2A).
Figure 2. Hapten-bearing DC up-regulate Fas expression and CD4+CD25+ T cells express FasL in the skin-draining lymph nodes.
(A) DC were purified from LNC of FITC-sensitized mice by positive selection using magnetic beads and 3 × 105 cell aliquots were stained with anti-CD11c mAb and anti-Fas mAb. CD11c-positive FITC− cells (gate R2) or FITC+ cells (gate R3) were gated using FITC-negative DC from naïve mice as a control (upper dot plot) and each DC population was analyzed for Fas expression. Fas expression for FITC− or FITC+ DC is shown as a solid histogram. Staining of DC with PE-labeled isotype control Ab is shown as a thin histogram. The numbers in the histograms indicate percentages of cells expressing high levels of Fas.
(B) CD4+CD25+ or CD4+CD25− T cells were purified using magnetic beads. The purity of each cell fraction was approximately 80–90% as assessed by flow cytometry. Purified CD4+CD25+ and CD4+CD25− T cells were stained with biotin-labeled anti-FasL mAb + streptavidin-APC. Then cells were permeabilized and stained with FITC-labeled anti-FoxP3 mAb. Cells stained with biotin-labeled isotype control Ab + streptavidin-APC and with anti-FoxP3 mAb were used as a negative control. To exclude dead cells and to reduce autofluorescence, viable cells were gated based on the forward scatter/side scatter (gate R1) and CD25-positive or CD25-negative cells within this gate were analyzed for FasL expression. Percentages of CD25-positive or CD25-negative cells expressing FasL are shown in the dot plots. The experiments were repeated two times with similar results.
Next, we evaluated the expression of FasL on regulatory CD4+CD25+ T cells vs. CD4+CD25− T cells. CD4+CD25+ or CD4+CD25− T cells were purified from the skin-draining lymph nodes of naïve mice, with the purity of each cell fraction 80–90% as assessed by flow cytometry. To test whether CD4+CD25+ T cells constitutively express FasL, freshly isolated CD4+CD25+ T cells and CD4+CD25− T cells were tested for co-expression of FoxP3 and FasL by flow cytometry. The majority of cells expressing FasL were detected within the FoxP3-positive population of CD4+CD25+ T cells (Figure 2B, 10.1 ± 2.8% of CD4+CD25+ T cells co-expressed FoxP3 and FasL, n = 3 samples tested), while CD4+CD25− T cells did not express FoxP3 and only a small population of these cells (1.9 ± 0.1%, n = 3) expressed FasL. The results indicate a population of CD4+CD25+FoxP3+ cells that constitutively expresses FasL.
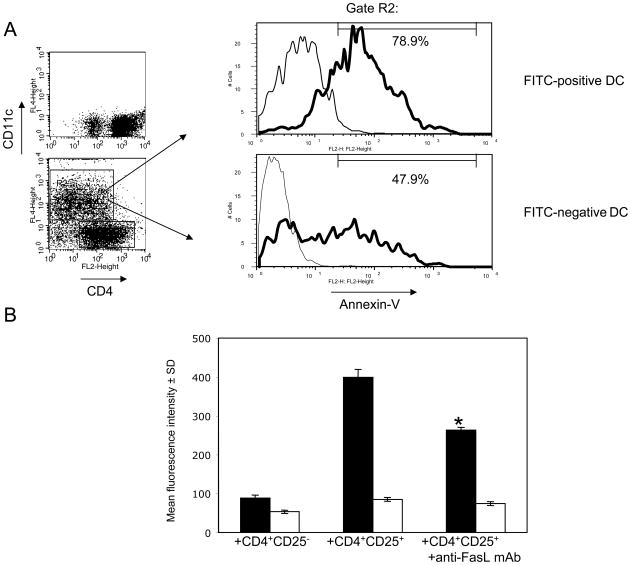
To test the potential killing of hapten-presenting DC by regulatory CD4+CD25+ T cells through Fas-FasL interactions, DC purified from FITC-sensitized mice were cultured with CD4+CD25+ T cells purified from skin-draining lymph nodes of naïve mice. Following 4 h of culture, DC were gated as a CD11c-positive cell population (Figure 3A, gate R2) and analyzed for apoptosis by staining with Annexin-V. First, FITC+ and FITC − DC were gated as described above in Figure 2A and tested for Annexin-V staining after culture with CD4+CD25+ T cells. To normalize the intensity of Annexin-V staining, the autofluorescence of unstained DC was subtracted from the MRI of each DC population stained with Annexin-V. The intensity of the Annexin-V staining was significantly increased in the FITC+ DC population when compared to FITC− DC (MFI = 113.0 ± 3.3 for FITC+ vs. MFI = 71.6 ± 2.9 for FITC− DC, n = 3, P < 0.01). While both FITC+ and FITC− DC populations contained Annexin-V-positive cells, the percentages of these cells were significantly increased in the FITC+ DC population (Figure 3A, 80.7 ± 2.6% vs. 52.3 ± 5.1%, n = 3, p < 0.01). The considerable proportion of Annexin-V-positive cells within the FITC− DC population is likely due to the spontaneous death of DC in vitro which has been reported in studies using similar cultures of DC alone or with CD4+ T cells [2]. Overall, the results indicated the increased death of hapten-presenting DC during culture with CD4+CD25+ T cells in comparison to the death of non-presenting DC under the same culture conditions. These results correlated with our in vivo studies indicating that the numbers of FITC+, but not FITC −, DC significantly increased in the priming site when CD4+CD25+ T cells were attenuated by anti-CD25 mAb.
Figure 3. CD4+CD25+ T cells kill FITC+, but not FITC− DC through Fas-FasL interactions during co-culture.
(A)2 × 105 cell aliquots of purified CD4+CD25+ T cells were co-cultured in triplicate with 105 DC purified from skin-draining lymph nodes of FITC-sensitized mice. After 4 h of culture, cells were stained with APC-labeled anti-CD11c mAb and with Annexin-V-PE. Dot plots show control aliquots of CD4+ T cells cultured alone (upper panel) or with DC (lower panel) and stained with anti-CD4 and anti-CD11c mAb. CD11c-positive cells were gated (gate R2) and then FITC+ or FITC− DC within this gated population were analyzed by histogram for Annexin-V staining (solid histograms). The numbers in histograms indicate the percentages of Annexin-V+ DC depicted by the gate that excludes autofluorescent DC that were not stained with Annexin-V (thin histograms). The experiments were repeated three times with similar results.
(B)Purified CD4+CD25+ T cells or CD4+CD25− T cells were cultured in triplicate with DC purified from FITC-sensitized mice for 4 h, and then stained with APC-labeled anti-CD11c mAb and with Annexin-V-PE. FITC+ DC (■) and FITC − DC (□) were gated as described in Figure 2A and analyzed by histograms for the fluorescence intensity of Annexin-V staining. Purified anti-FasL mAb was added to CD4+CD25+ T cells and DC cultures at 25 ug/ml. * P < 0.05 as compared to CD4+CD25+ T cells and DC co-cultures without anti-FasL mAb. The experiments were repeated two times with similar results.
While apoptosis of FITC+ DC was increased when these DC were cultured with CD4+CD25+ T cells, we did not detect this increase after DC co-culture with CD4+CD25 − T cells (Figure 3B). Furthermore, addition of anti-FasL mAb to the co-cultures of DC and CD4+CD25+ T cells resulted in a significant decrease of FITC+ DC apoptosis (Figure 3B, * P < 0.05), while this FasL blockade had no effect on the death of FITC-negative DC. This inhibitory effect was dose-dependent as lower concentrations of anti-FasL mAb resulted in less inhibition of DC apoptosis mediated by CD4+CD25+ T cells (not shown). Collectively, these results indicate that CD4+CD25+ T cells mediate killing of hapten-presenting DC in vitro at least in part through Fas-FasL-dependent interactions.
Fas-defective DC induce increased and sustained expansion of hapten-specific CD8+ T cells
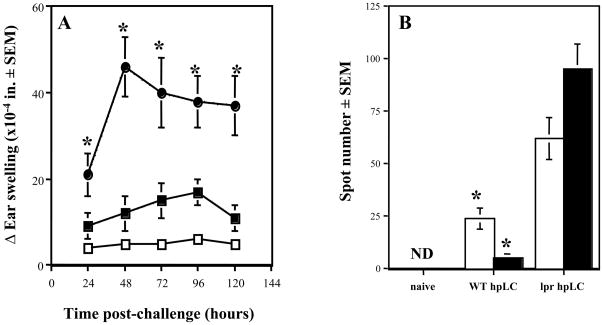
As a second approach to test our hypothesis, we compared the capability of cutaneous DC that do or do not express functional Fas to prime effector CD8+ T cells for CHS responses. DC were purified from the skin-draining lymph nodes of DNFB-sensitized wild type or lpr mice and were transferred intra-dermally into naïve wild type mice as previously described [15,16]. The magnitude of CHS responses induced by transfer of DC isolated from DNFB-sensitized wild type mice decreased to background levels at 120 h post-challenge. In contrast, the magnitude of CHS responses in mice receiving DC from Fas-defective lpr mice was markedly increased and sustained (Figure 4A, *P < 0.05). The characteristics of these CHS responses correlated with the magnitude of hapten-specific CD8+ T cell development in the skin-draining lymph nodes of DC-transferred mice. At day +5 post-transfer, hapten-specific CD8+ T cells producing IFN-γ were easily detectable in mice primed with wild type DC, but within two days (i.e. day +7 post-transfer), the number of these CD8+ T cells decreased more than three-fold (Figure 4B). In contrast, considerably higher numbers of hapten-specific CD8+ T cells producing IFN-γ were observed on day +5 in the lymph nodes of mice primed with lpr DC (Figure 4B, WT DC vs. lpr DC, *P < 0.05), and these numbers continued to increase by day +7 post-transfer. Thus, the augmented and prolonged ear swelling responses observed in mice primed with Fas-defective DC correlated with increased and sustained numbers of hapten-specific CD8+ T cells producing IFN-γ in the lymph nodes. These results were consistent with negative regulation of DC priming functions in CHS responses through Fas-FasL.
Figure 4. Fas-defective DC induce enhanced development of hapten-specific CD8+ T cells and CHS responses in wild type recipient mice.
(A) DC purified from DNFB-sensitized wild type (■) or lpr (●) mice were transferred by intradermal injection into naïve wild type mice. Five days later DC recipient or naïve (□) mice were challenged with 0.2% DNFB and the change in ear thickness (swelling) was measured at 24 h intervals. The mean increase in ear thickness ± SEM is shown for each group of three mice. *P < 0.01 by two-tailed Students’ t test when compared to recipients of wild type DC. The experiments were repeated two times with similar results.
(B) CD8+ T cells were enriched from LNC of naïve, wild type DC recipient (WT DC) or lpr DC recipient (lpr DC) mice on day +5 (□) and day +7 (■) post-transfer. CD8+ T cell aliquots (5 × 105 cells) were cultured with DNBS-labeled splenocytes (5 × 105 cells) on anti-IFN-γ mAb-coated ELISPOT plates. Numbers of IFN-γ producing CD8+ T cells were evaluated by ELISPOT. ND – not detected. *P < 0.05 by two-tailed Students’ t test as compared to recipients of wild type DC. The experiments were repeated two times with similar results.
CD4+CD25+ T cells restrict hapten-specific CD8+ T cell activation through a Fas-dependent mechanism
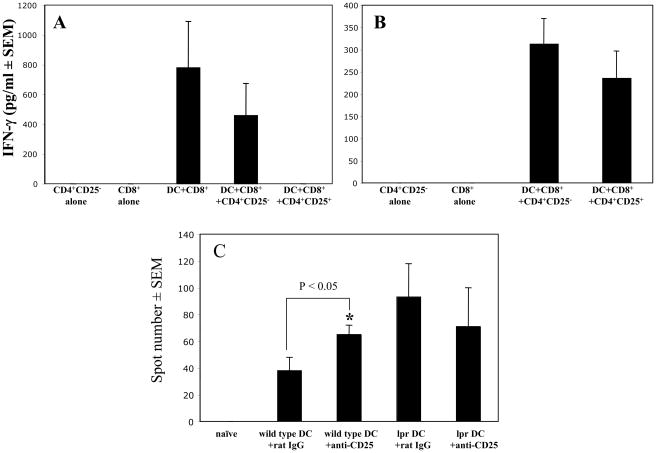
To directly test whether regulatory CD4+CD25+ cells utilize Fas-FasL interactions to inhibit activation of hapten-specific CD8+ T cells by Fas-expressing DC, immune CD8+ T cells from sensitized wild type mice were cultured with hapten-presenting DC purified from sensitized wild type or lpr mice in the presence of naïve wild type CD4+CD25+ or CD4+CD25− cells and IFN-γ production by the immune CD8+ T cells was assessed by ELISA. To assess the possibility that CD4+CD25− or CD4+CD25+ cells produce IFN-γ during this culture, we tested IFN-γ production by immune CD8+ T cells cultured with hapten-presenting DC only. The results indicated that additional amounts of IFN-γ were not produced when CD8+ T cells were cultured with DC and CD4+CD25− T cells when compared to CD8+ T cell/DC cultures (Figure 5A). In fact IFN-γ production was slightly decreased in CD8+ T cell/DC/CD4+CD25− T cell cultures, although this was not a significant decrease and most likely due to competition between the T cells for access to the DC. When CD4+CD25+ cells purified from lymph nodes of naïve mice were co-cultured with wild type DC and hapten-primed CD8+ T cells, IFN-γ production was inhibited when compared to cultures of wild type DC, hapten-specific CD8+ T cells and naïve CD4+CD25− cells (Figure 5A). In contrast, addition of CD4+CD25+ cells had no significant effect on the ability of lpr DC to induce IFN-γ production by hapten-specific wild type CD8+ T cells under the same culture conditions (Figure 5B). Thus, CD4+CD25+ cells inhibited the activation of effector CD8+ T cells indirectly through effects on Fas-expressing hapten-presenting DC.
Figure 5. CD4+CD25+ T cells inhibit in vitro and in vivo stimulatory functions of Fas-sufficient, but not Fas-defective DC.
(A-B) CD8+ T cells enriched from LNC of sensitized wild type mice were cultured alone (no DC) or with DC purified from sensitized wild type mice (A) or lpr mice (B). CD4+CD25+ T cells or CD4+CD25− T cells purified from LNC of naïve wild type mice were added to these cultures. After 72 h, supernatants were collected and tested for IFN-γ by ELISA. * P < 0.05. The experiments were repeated three times with similar results observed each time.
(C) DC purified from DNFB-sensitized wild type mice (wild type DC) or lpr mice (lpr DC) were transferred by intradermal injection into naïve wild type mice that were treated with 250 μg of control rat IgG or anti-CD25 mAb on days −1, 0 and +1 of transfer. Five days later, CD8+ T cells were enriched from LNC of naïve or DC recipient mice and were cultured with DNBS-labeled splenocytes on anti-IFN-γ mAb-coated ELISPOT plates. Numbers of IFN-γ roducing CD8+ T cells were evaluated by ELISPOT. *P < 0.05 by two-tailed Students’ t test as compared to the culture of CD8+ T cells with DC from wild type mice treated with control rat IgG. The experiments were repeated two times with similar results.
To test the FasL-dependent regulatory activity of CD4+CD25+ cells in vivo, naïve mice were primed by intra-dermal injection of DC from sensitized wild type or lpr mice. The development of hapten-specific IFN-γ producing CD8+ T cells was markedly increased in mice primed by wild type DC and treated with anti-CD25 mAb when compared to control mice treated with rat IgG (Figure 5C, *P < 0.05). In contrast, anti-CD25 mAb treatment of mice primed by Fas-defective DC did not increase the development of hapten-specific CD8+ T cells when compared to the control group (Figure 5C). Collectively, these results indicated that the priming activity of hapten-presenting DC expressing functional Fas is restricted during induction of CHS response by CD4+CD25+ regulatory T cells, while the priming activity of Fas-defective DC is not.
CD4+CD25+ regulatory T cells can restrict CHS responses in a non-specific manner
The data presented to this point suggest a model in which hapten application to the skin induces the emigration of DC from the skin to the draining lymph nodes where the hapten-presenting DC express Fas and subsequently activate and/or engage CD4+CD25+FasL+ T cells that mediate apoptosis of the DC, limiting the duration and magnitude of hapten-reactive CD8 T cell priming. This model predicts that at times when this CD4+CD25+ T regulatory cell activity is in operation to mediate apoptosis of the hapten-presenting DC, the active CD4+CD25+ T cells may also mediate the apoptosis of DC presenting other haptens that enter the skin draining lymph node. This activity would result in decreased CD8 T cell responses to these other haptens. Therefore, we tested if CD4+CD25+ regulatory T cells activated to suppress the CHS response to a specific hapten were also capable of suppressing the response to subsequent sensitization with a different hapten.
Mice were first sensitized with FITC to induce a FITC-specific CHS response and then sensitized with DNFB five days later to activate DNFB-specific CD8+ T cells. Distinct areas of the skin (on the back and on the abdomen) were sensitized with FITC or with DNFB to exclude the possibility that cutaneous DC from the sensitized skin present both haptens to the two populations of hapten-specific effector CD8+ T cells. Induction of DNFB-specific IFN-γ producing CD8+ T cells was reduced two-fold in mice pre-sensitized with FITC when compared to control mice sensitized with DNFB only (Figure 6A). This non-specific regulation was completely abrogated by treatment with anti-CD25 mAb at the time of pre-sensitization with FITC, as the numbers of DNFB-specific IFN-γ producing CD8+ T cells in anti-CD25 mAb-treated group were similar to the numbers in the control group sensitized with DNFB only (Figure 6A, *P < 0.01 when compared to mice pre-sensitized with FITC and treated with control rat IgG). The results indicated that CD4+CD25+ T cell-mediated negative regulation induced by FITC sensitization suppressed the subsequent activation of DNFB-specific CD8+ T cells in the skin-draining lymph nodes. Consistent with the results of this report, CD4+CD25+ T cell-mediated negative regulation of the activation of CD8+ T cells specific to a second hapten (FITC) correlated with decreased total numbers of LC presenting this hapten in the lymph nodes of mice pre-sensitized with DNFB and treated with control rat IgG at the time of first sensitization (Figure 6B). The numbers of FITC-presenting LC were increased to the level observed in the control group when mice were given anti-CD25 mAb at the time of the first sensitization with DNFB.
Figure 6. CD4+CD25+ T cells can suppress activation of hapten-specific CD8+ T cells in a non-specific manner.
(A) Mice were sensitized with 1% FITC on day 0 and were given i. p. injections of 250 μg of control rat IgG or anti-CD25 mAb on days 0, +1 and +2. On days +5 and +6 post-sensitization mice were sensitized with 0.25% DNFB. Five days after DNFB sensitization, CD8+ T cells enriched from LNC of sensitized mice were cultured with DNBS-labeled splenocytes and the numbers of DNFB-specific IFN-γ producing CD8+ T cells were evaluated by ELISPOT. Mice sensitized with DNFB without FITC pre-sensitization and non-sensitized mice were used as positive and negative controls. *P < 0.01 by two-tailed Students’ t test as compared to the group of mice sensitized with FITC and DNFB and treated with rat IgG.
(B) Mice were sensitized with 0.25% DNFB on days 0 and +1 and were given i. p. injections of 250 μg of control rat IgG or anti-CD25 mAb on days 0, +1 and +2. On day +5 post-sensitization mice were sensitized with 1% FITC. On day +3 after FITC sensitization, LNC suspensions were stained with PE-labeled anti-CD11c mAb and AlexaFluor647-labeled anti-CD207 mAb. FITC-presenting LC were identified as CD207+FITC+ cells within the CD11c+ population. Total numbers of CD207+FITC+ cells in the skin-draining lymph nodes of each individual mouse were calculated based on the percentage of these cells in each analyzed cell aliquot. ND – not detected.
Discussion
Antigen-specific CD8+ T cells undergo rapid expansion within the lymphoid priming site in response to pathogen infection and the number of these effector cells rapidly declines following antigen clearance [17, 18]. One critical factor regulating antigen-specific CD8+ T cell expansion is the duration of CD8+ T cell exposure to antigen and co-stimulatory signals provided by the APC. In vitro models have indicated apoptosis of APC during culture with antigen-specific effector CD4+ T cells suggesting this elimination as a mechanism affecting the magnitude and duration of T cell-mediated immune responses [2, 19]. In vivo studies have identified Fas-dependent elimination of APC as a mechanism restricting systemic autoimmune disorders such as lymphoproliferation and production of autoimmune antibodies [4]. Langerhans cells resistant to apoptosis through a deficiency in Bid or Fas induced stronger CD4+ T cell-mediated immune responses than wild type DC [2, 3]. The increased lifespan of DC and B cells in mice with a targeted FasL gene deletion in T cells suggests that FasL-expressing T cells down-regulate autoimmune responses by controlling APC numbers [20]. It remains unclear, however, whether the same mechanism down-regulates CD8+ T cell-mediated immune responses to antigens deposited in the skin as well as the identity of the cells mediating this negative regulation.
Our previous studies suggested that FasL-expressing CD4+ T cells regulate hapten-presenting DC activation of effector CD8+ T cells for CHS [1]. We had also reported that attenuation of the regulatory CD4+CD25+ T cell compartment by anti-CD25 mAb treatment during initiation of CHS responses enhanced the magnitude of hapten-specific CD8+ T cell expansion and subsequently increased the magnitude and duration of CHS responses mediated by these effector CD8+ T cells [13]. This suggested that CD4+CD25+ T cells might negatively regulate CD8+ T cell-mediated CHS responses through FasL-dependent mechanisms. In the current study, anti-CD25 mAb treatment of mice during hapten sensitization resulted in more than a two-fold increase in the numbers of hapten-presenting DC in the skin-draining lymph nodes of sensitized mice but had little effect on the numbers of DC not presenting the hapten (e.g. resident DC). In support of our hypothesis that regulatory CD4+CD25+ T cell eliminate hapten-presenting DC through Fas-FasL interactions, the majority of FasL-expressing T cells were detected within the CD4+CD25+FoxP3+ cell population while constitutive expression of FasL on CD4+CD25− T cells was at low to undetectable levels. Furthermore, hapten-bearing DC expressed higher levels of Fas than did hapten-negative DC. Finally, hapten-presenting DC experienced increased apoptosis during culture with CD4+CD25+ T cells than with CD4+CD25− T cells and this apoptosis was blocked by anti-FasL mAb. It is worth noting that even high concentrations of anti-FasL mAb (25 ug/ml) did not completely inhibit the DC apoptosis mediated by CD4+CD25+ T cells in vitro, suggesting that cytotoxic mechanisms other than Fas-FasL may also be involved. Human regulatory CD4+CD25+ T cells activated in vitro have been reported to utilize granzyme A and perforin-dependent cytotoxicity to kill autologous target cells, including both mature and immature DC [21].
Negative regulation of effector T cell expansion and CHS responses by FasL-mediated apoptosis of DC has been suggested by several studies. First, the clearance of hapten-bearing DC is delayed in the lymph nodes of sensitized gld and lpr mice [22]. Second, the LC-derived cell line XS52 is eliminated by agonist anti-Fas mAb or by CD4+ T cells through Fas-FasL engagement in vitro [2]. Third, regulatory T cells induced in CHS by UV irradiation require Fas-FasL to down-regulate CHS responses and to induce DC apoptosis during in vitro culture [23]. Finally, studies from this laboratory have indicated that the unregulated expansion of hapten-specific CD8+ T cells and CHS responses in FasL-defective gld mice was down-regulated by adoptively transferred CD4+ T cells from wild type mice [1].
It is worth noting that we did not observe increased hapten-specific CD8+ T cell development or CHS responses in Fas-defective lpr mice when compared to wild type animals (A. Gorbachev, unpublished observations). One possible explanation is that Fas-FasL interactions play a dual role in immune responses. While functions of APC are negatively regulated by Fas-induced apoptosis, FasL expressed by T cells may deliver co-stimulatory signals during CD8+ T cell activation [24, 25]. To dissect the influence of Fas/FasL on DC and T cell functions in CHS responses, the effector CD8+ T cell and CHS responses were compared in naïve mice that had received transferred hpLC from sensitized wild type or lpr donors. Consistent with previous findings suggesting negative regulation of hpLC functions through Fas-FasL interactions [1, 2, 22], the expansion of hapten-specific CD8+ T cells and CHS responses were markedly increased and prolonged in wild type mice receiving Fas-defective lpr DC when compared to recipients of wild type DC. It was unlikely that Fas-defective DC were more stimulatory than Fas-sufficient DC as hapten-presenting DC from lpr and wild type mice activated similar levels of hapten-specific CD8+ T cells in vitro (A. Gorbachev, unpublished observations). Anti-CD25 mAb treatment of mice receiving wild type DC increased hapten-specific CD8+ T cell activation, while blockade of CD4+CD25+ T cell activity did not affect hapten- specific CD8+ T cell activation in recipients of lpr DC. Finally, CD4+CD25+ T cells suppressed the activation of hapten-specific CD8+ T cells cultured with wild type but not lpr DC, indicating that negative regulation of effector CD8+ T cell activation was mediated through effects on Fas-expressing DC but not on Fas-expressing CD8+ T cells. Together these results indicate that CD4+CD25+ T cells regulate the priming functions of hapten-presenting DC in CHS through Fas-FasL interactions.
The ability of regulatory CD4+CD25+ T cells to express FasL and kill Fas-expressing target cells has been previously reported [19, 26, 27]. This report is the first, to our knowledge, demonstrating the ability of these regulatory cells to restrict DC priming functions in CD8+ T cell-mediated immune responses through a Fas-FasL-dependent mechanism. Furthermore, CD4+CD25+ T cells suppress CD8+ T cell-mediated CHS responses in a non-specific manner. CD4+CD25+ regulatory T cells activated by hapten sensitization restricted the ability of LC activated by subsequent sensitization with a non-related hapten to activate CD8+ T cells specific to the latter hapten. These results are consistent with studies demonstrating non-antigen-specific suppression of T cell-mediated autoimmune gastritis and viral responses by CD4+CD25+ regulatory T cells [28, 29]. The current report further supports the hypothesis that previously activated CD4+CD25+ regulatory T cells can exert non-specific suppressor functions [28].
Collectively, these studies reveal the restriction of cutaneous DC priming functions in the skin-draining lymph nodes through Fas-FasL interactions as a mechanism employed by CD4+CD25+ T cells to regulate effector CD8+ T cell development and expansion during cutaneous immune responses. The findings may be also applicable to the understanding of immunoregulation of other T cell-mediated immune responses.
Materials and methods
Mice
Wild type and lpr female mice on the C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed according to the National Institutes of Health Guides for the Care and Use of Laboratory Animals and all protocols were approved by the Institutional Animal Care Use Committee (IACUC) of The Cleveland Clinic.
Reagents and antibodies
DNFB and FITC were purchased from Sigma (Sigma Chemical Co., St. Louis, MO). Monoclonal antibodies (mAb) for the capture and detection of IFN-γ in ELISPOT assays, PE-labeled and biotin-labeled hamster isotype control Ab, anti-CD11c, anti-Fas and anti-FasL mAb MFL3, and streptavidin-APC, streptavidin-PE and streptavidin-FITC were purchased from BD Bioscience (San Diego, CA). AlexaFluor 647-labeled mAb RMUL.2 specific for mouse CD207, rat isotype control Ab, and FITC-labeled anti-FoxP3 mAb were purchased from eBioscience (San Diego, CA). The rat anti-mouse CD25 mAb PC 61.5.3 was purified from hybridoma culture supernatants by protein G chromatography. Control rat IgG was purchased from Sigma.
Hapten sensitization and elicitation of CHS
For sensitization to DNFB or FITC, mice were painted with the hapten on the shaved abdomen and footpads as previously described [10, 11]. To test the effects of CD25 blockade on hapten-presenting DC, mice were treated with intra-peritoneal injections of 250 ug of anti-CD25 mAb given on days −1, 0 and +1 of sensitization.
To induce CHS responses to DNFB by adoptive transfer of hapten-presenting DC, mice were sensitized with DNFB and DC were purified from cells suspensions of skin-draining lymph nodes harvested on day +2 post-sensitization using anti-CD11c mAb-coated microbeads (Miltenyi Biotec Inc., Auburn, CA). The purity of DC was always ≥ 80% as assessed by flow cytometry and 4 × 105 DC were injected intradermally into the lower abdominal area of each animal. On day +5 DC-transferred and, as a negative control, non-transferred mice were challenged with 10 μl of 0.2% DNFB on both sides of each ear. Ear thickness was measured in a blinded manner at 24 h intervals after challenge as previously described [10]. The magnitude of ear swelling responses is presented as the mean increase of each group of 3 mice (i.e. 6 ears) ± SEM over the thickness measured just prior to hapten challenge on day +5 post-transfer.
ELISPOT assays to enumerate hapten-specific T cells producing IFN-γ were performed as previously described [11, 13].
Flow cytometry
Skin draining LNC suspensions were prepared from FITC-sensitized mice on day +2 post-sensitization. Two-color flow cytometry analyses were performed as previously described [30]. To specifically detect hapten-bearing LC, LNC were obtained at 72 h after sensitization with FITC and were fixed, permeabilized and stained with AlexaFluor 647-labeled anti-CD207 mAb. CD11c+FITC+ or CD207+FITC+ cells were gated and their percentage in the total LNC population was evaluated for each analyzed sample. Total numbers of LC in the skin-draining lymph nodes of each mouse were calculated based on the percentage of LC in analyzed cell aliquot. To evaluate apoptosis of DC in vitro, lymph nodes were pooled from 5–10 FITC-sensitized mice at 24 h post-sensitization, and DC were purified from LNC suspensions using anti-CD11c mAb-coated microbeads. Then, 105 DC aliquots were cultured with 2 × 105 cell aliquots of purified CD4+CD25+ or CD4+CD25 − T cells for 4h or 16 h. The cells were then stained with APC-labeled anti- CD11c mAb, washed and incubated with Annexin-V-PE for 10 min at RT. The data were analyzed using CellQuest and FlowJo software.
Evaluation of hapten-specific CD8+ T cell activation by hapten-presenting DC in vitro
DC were purified from pooled LNC of sensitized wild type or lpr mice as described above. To purify CD4+CD25+ or CD4+CD25− T cells from pooled LNC of naïve mice, non-CD4+ cells were depleted first by combination of the cocktail of biotinylated mAb (anti-CD8a, anti-CD11b, anti-CD45R, anti-CD49b and anti-Ter-119) and anti-biotin MicroBeads, and then CD4+CD25+ or CD4+CD25− T cells were purified from remaining CD4+ T cell pool using a CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec Inc., Auburn, CA). The purity of CD4+CD25+ or CD4+CD25− cells was 80–90% as assessed by flow cytometry. Then, 5 × 104 aliquots of wild type or lpr DC were cultured in triplicate with 2.5 × 105 CD8+ T cells enriched from LNC of sensitized mice obtained at day +5 post-sensitization in complete RPMI-1640 media at 37°C, 5% CO2 and 5 × 104 aliquots of CD4+CD25+ T cells or CD4+CD25− T cells purified from lymph nodes of naïve mice were added to these cultures. After 72 h of culture, supernatants were collected and tested for IFN-γ using Quantikine Mouse IFN-γ Immunoassay Kit (R & D Systems Inc., Minneapolis, MN).
Statistical analysis
Statistical analysis to assess differences between experimental groups was performed using two-tailed Students’ t test. Differences were considered significant when P < 0.05. Three mice per each group were used in all in vivo experiments. For in vitro experiments, three triplicate samples were analyzed for each group. All experiments were repeated at least two times with similar results.
Acknowledgments
The authors thank the staff of the Cleveland Clinic Biological Resources Unit for excellent animal care.
This work was supported by National Institutes of Health Grant RO1 AI45888 (RLF).
Abbreviations
- CHS
contact hypersensitivity
- DC
dendritic cells
- DNFB
2,4-dinitrofluorobenzene
- LC
Langerhans cells
- LNC
lymph node cells
Footnotes
Conflict of interest
Authors declare no financial or commercial conflicts of interest
References
- 1.Gorbachev AV, Fairchild RL. CD4+ T cells regulate CD8+ T cell-mediated cutaneous immune responses by restricting effector T cell development through a Fas ligand-dependent mechanism. J Immunol. 2004;172:2286–2295. doi: 10.4049/jimmunol.172.4.2286. [DOI] [PubMed] [Google Scholar]
- 2.Matsue H, Edelbaum D, Hartmann AC, Morita A, Bergstresser PR, Yagita H, Okumura K, Takashima A. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- 3.Pradhan S, Genebriera J, Denning WL, Felix K, Elmets CA, Timares L. CD4 T cell-induced, bid-dependent apoptosis of cutaneous dendritic cells regulates T cell expansion and immune responses. J Immunol. 2006;177:5956–5967. doi: 10.4049/jimmunol.177.9.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liden C. Legislative and preventive measures related to contact dermatitis. Contact Dermatitis. 2001;44:65–69. doi: 10.1034/j.1600-0536.2001.440201.x. [DOI] [PubMed] [Google Scholar]
- 7.Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens; the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473–487. doi: 10.1084/jem.95.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enk AH. Allergic contact dermatitis: understanding the immune response and potential for targeted therapy using cytokines. Mol Med Today. 1997;3:423–428. doi: 10.1016/S1357-4310(97)01087-3. [DOI] [PubMed] [Google Scholar]
- 9.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engeman TM, Gorbachev AV, Gladue RP, Heeger PS, Fairchild RL. Inhibition of functional T cell priming and contact hypersensitivity responses by treatment with anti-secondary lymphoid chemokine antibody during hapten sensitization. J Immunol. 2000;164:5207–5214. doi: 10.4049/jimmunol.164.10.5207. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J Immunol. 2008;180:3057–3064. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kish DD, Gorbachev AV, Fairchild RL. CD8+ T cells produce IL-2, which is required for CD4+CD25+ T cell regulation of effector CD8+ T cell development for contact hypersensitivity responses. J Leukoc Biol. 2005;78:725–735. doi: 10.1189/jlb.0205069. [DOI] [PubMed] [Google Scholar]
- 14.Kish DD, Gorbachev AV, Fairchild RL. Regulatory function of CD4+CD25+ T cells from Class II MHC-deficient mice in contact hypersensitivity responses. J Leukoc Biol. 2007;82:85–92. doi: 10.1189/jlb.0207089. [DOI] [PubMed] [Google Scholar]
- 14.Gorbachev AV, Fairchild RL. Activated NKT cells increase dendritic cell migration and enhance CD8+ T cell responses in the skin. Eur J Immunol. 2006;36:2494–2503. doi: 10.1002/eji.200636075. [DOI] [PubMed] [Google Scholar]
- 15.Gorbachev AV, Gasparian AV, Gurova KV, Gudkov AV, Fairchild RL. Quinacrine inhibits the epidermal dendritic cell migration initiating T cell-mediated skin inflammation. Eur J Immunol. 2007;37:2257–2267. doi: 10.1002/eji.200636708. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 18.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 19.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–4612. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 20.Mabrouk I, Buart S, Hasmim M, Michels C, Connault E, Opolon P, Chiocchia G, Levi-Strauss M, Chouaib S, Karray S. Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells. Immunity. 2008;29:922–933. doi: 10.1016/j.immuni.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–591. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura T, Azuma M, Kayagaki N, Shimada S, Yagita H, Okumura K. Fas/Fas ligand-mediated elimination of antigen-bearing Langerhans cells in draining lymph nodes. Br J Dermatol. 1999;141:201–205. doi: 10.1046/j.1365-2133.1999.02965.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Grabbe S, Grosse-Heitmeyer K, Roters B, Riemann H, Luger TA, Trinchieri G, Schwarz T. Ultraviolet light-induced immune tolerance is mediated via the Fas/Fas-ligand system. J Immunol. 1998;160:4262–4270. [PubMed] [Google Scholar]
- 23.Sun M, Ames KT, Suzuki I, Fink PJ. The cytoplasmic domain of Fas ligand costimulates TCR signals. J Immunol. 2006;177:1481–1491. doi: 10.4049/jimmunol.177.3.1481. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki I, Martin S, Boursalian TE, Beers C, Fink PJ. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J Immunol. 2000;165:5537–5543. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]
- 26.Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4 − Tregs, regulate effector T cells using FasL. J Immunol. 2007;178:4891–4900. doi: 10.4049/jimmunol.178.8.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 28.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 29.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorbachev AV, Heeger PS, Fairchild RL. CD4+ and CD8+ T cell priming for contact hypersensitivity occurs independently of CD40-CD154 interactions. J Immunol. 2001;166:2323–2332. doi: 10.4049/jimmunol.166.4.2323. [DOI] [PubMed] [Google Scholar]