Abstract
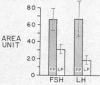
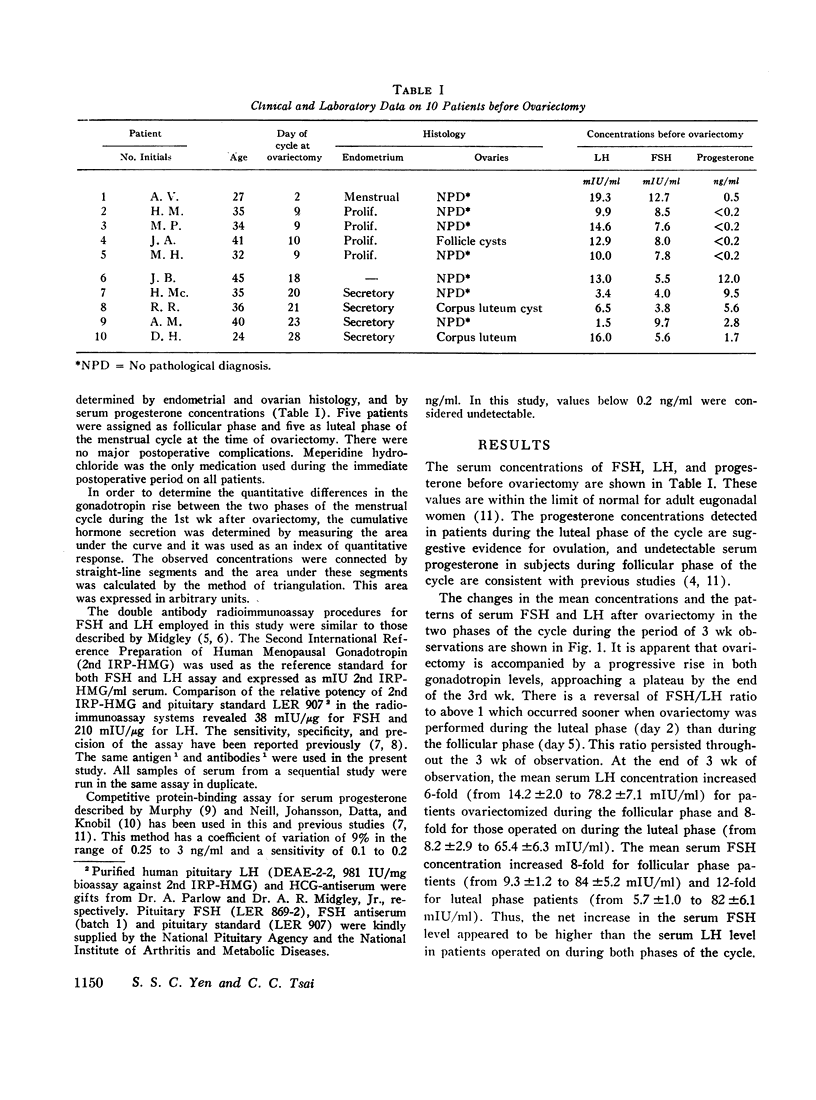
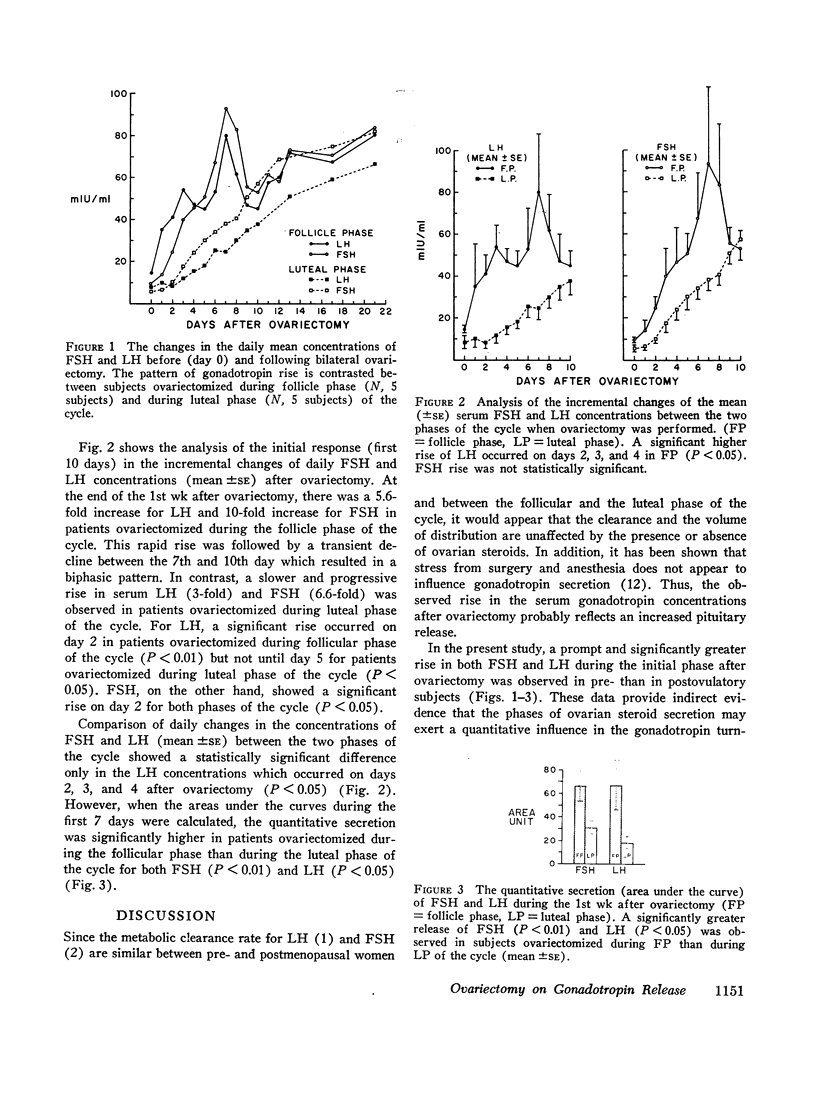
The sequential changes in the concentration and pattern of circulating luteinizing hormone (LH) and follicle-stimulating hormone (FSH)1 following bilateral ovariectomy were determined in 10 premenopausal women. The initial (1st wk) and delayed (3 wk) secretory responses of serum LH and FSH as related to the phases of the menstrual cycle were examined. Ovariectomy during follicular phase was accompanied by a prompt and much greater rise in both LH and FSH during the 1st wk. This rapid rise was followed by a transient decline between the 7th and 10th day which resulted in a biphasic pattern. In contrast, a slower and progressive rise in serum LH and FSH was observed in subjects ovariectomized during luteal phase of the cycle. The quantitative secretion (area under the curve) during the 1st wk after ovariectomy was significantly greater in patients operated on during the follicular phase than during the luteal phase for both LH (P < 0.05) and FSH (P < 0.01). Thereafter, a similar pattern of gonadotropin rise was observed for patients ovariectonized during either phase of the cycle and reached a plateau by the end of the 3rd wk. At this time, the mean LH concentration increased 6-fold for follicular phase surgery and 8-fold for luteal phase surgery. The mean serum FSH concentration increased 8-fold for follicular phase surgery and 12-fold for luteal phase surgery. The net increase in serum FSH level was higher than that in the serum LH level after surgery in both phases of the cycle and thus a reversal of FSH/LH ratio occurred. These data provide indirect evidence that the phase of ovarian steroid secretion may exert a quantitative influence on the gonadotropin turnover rate within the hypothalamic-pituitary system. The augmented gonadotropin release and the reversal of FSH/LH ratio following ovariectomy presumably could be due to an increased gonadotropin net synthesis which is more pronounced for FSH than for LH.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAHN R. C., LORENZ N., BENNETT W. A., ALBERT A. Gonadotropins of the pituitary of postmenopausal women. Endocrinology. 1953 Oct;53(4):455–457. doi: 10.1210/endo-53-4-455. [DOI] [PubMed] [Google Scholar]
- Barraclough C. A., Haller E. W. Positive and negative feedback effects of estrogen on pituitary LH synthesis and release in normal and androgen-sterilized rats. Endocrinology. 1970 Mar;86(3):542–551. doi: 10.1210/endo-86-3-542. [DOI] [PubMed] [Google Scholar]
- Charters A. C., Odell W. D., Thompson J. C. Anterior pituitary function during surgical stress and convalescence. Radioimmunoassay measurement of blood TSH, LH, FSH and growth hormone. J Clin Endocrinol Metab. 1969 Jan;29(1):63–71. doi: 10.1210/jcem-29-1-63. [DOI] [PubMed] [Google Scholar]
- Coble Y. D., Jr, Kohler P. O., Cargille C. M., Ross G. T. Production rates and metabolic clearance rates of human follicle-stimulating hormone in premenopausal and postmenopausal women. J Clin Invest. 1969 Feb;48(2):359–363. doi: 10.1172/JCI105992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay V. L., Bogdanove E. M. Plasma and pituitary LH and FSH in the castrated rat following short-term steroid treatment. Endocrinology. 1969 May;84(5):1132–1142. doi: 10.1210/endo-84-5-1132. [DOI] [PubMed] [Google Scholar]
- Gay V. L., Midgley A. R., Jr Response of the adult rat to orchidectomy and ovariectomy as determined by LH radioimmunoassay. Endocrinology. 1969 Jun;84(6):1359–1364. doi: 10.1210/endo-84-6-1359. [DOI] [PubMed] [Google Scholar]
- Kohler P. O., Ross G. T., Odell W. D. Metabolic clearance and production rates of human luteinizing hormone in pre- and postmenopausal women. J Clin Invest. 1968 Jan;47(1):38–47. doi: 10.1172/JCI105713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley A. R., Jr Radioimmunoassay: a method for human chorionic gonadotropin and human luteinizing hormone. Endocrinology. 1966 Jul;79(1):10–18. doi: 10.1210/endo-79-1-10. [DOI] [PubMed] [Google Scholar]
- Midgley A. R. Radioimmunoassay for human follicle-stimulating hormone. J Clin Endocrinol Metab. 1967 Feb;27(2):295–299. doi: 10.1210/jcem-27-2-295. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Neill J. D., Johansson E. D., Datta J. K., Knobil E. Relationship between the plasma levels of luteinizing hormone and progesterone during the normal menstrual cycle. J Clin Endocrinol Metab. 1967 Aug;27(8):1167–1173. doi: 10.1210/jcem-27-8-1167. [DOI] [PubMed] [Google Scholar]
- Ross G. T., Cargille C. M., Lipsett M. B., Rayford P. L., Marshall J. R., Strott C. A., Rodbard D. Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res. 1970;26:1–62. doi: 10.1016/b978-0-12-571126-5.50005-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Diebel N. D., Bogdanove E. M. Analysis of initial and delayed effects of orchidectomy and ovariectomy on pituitary and serum LH levels in adult and immature rats. Endocrinology. 1970 May;86(5):1102–1111. doi: 10.1210/endo-86-5-1102. [DOI] [PubMed] [Google Scholar]
- Yen S. C., Llerena L. A., Pearson O. H., Littell A. S. Disappearance rates of endogenous follicle-stimulating hormone in serum following surgical hypophysectomy in man. J Clin Endocrinol Metab. 1970 Mar;30(3):325–329. doi: 10.1210/jcem-30-3-325. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Llerena O., Little B., Pearson O. H. Disappearance rates of endogenous luteinizing hormone and chorionic gonadotropin in man. J Clin Endocrinol Metab. 1968 Dec;28(12):1763–1767. doi: 10.1210/jcem-28-12-1763. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Vela P., Rankin J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. J Clin Endocrinol Metab. 1970 Apr;30(4):435–442. doi: 10.1210/jcem-30-4-435. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Vela P., Rankin J., Littell A. S. Hormonal relationships during the menstrual cycle. JAMA. 1970 Mar 2;211(9):1513–1517. [PubMed] [Google Scholar]