Abstract
The use of end-to-side neurrorhaphy remains a controversial topic in peripheral nerve surgery. The authors report the long-term functional outcome following a modified end-to-side motor reinnervation using the spinal accessory to innervate the suprascapular nerve following a C5 to C6 avulsion injury. Additionally, functional outcomes of an end-to-end neurotization of the triceps branch to the axillary nerve and double fascicular transfer of the ulnar and medial nerve to the biceps and brachialis are presented. Excellent functional recoveries are found in respect to shoulder abduction and flexion and elbow flexion.
Electronic supplementary material
The online version of this article (doi:10.1007/s11552-009-9242-3) contains supplementary material, which is available to authorized users.
Keywords: End-to-side, Neurorrhaphy, Collateral sprouting, Neurotization, Avulsion
Introduction
The use of end-to-side neurorrhaphy remains a controversial topic in peripheral nerve surgery. The premise of end-to-side neurorrhaphy is reinnervation of an injured recipient nerve by collateral sprouting from an intact donor nerve. Recent work has primarily focused upon mechanisms to induce collateral sprouting from the donor nerve and the interactions of sprouting axons with basal laminar layers [5, 10, 12, 13, 15, 28, 29, 35]. Most authors would agree that end-to-side coaptation is not an ideal strategy for optimal reinnervation when other options exist; yet, in the appropriate setting (e.g., reinnervation of noncritical sensory deficits), good results can be obtained. An increased utilization and understanding of the potential of nerve transfers has facilitated improved patient outcomes [7, 16, 18, 21, 22, 30]. Despite these results, nerve transfers are not always feasible and do carry some risk for loss of function to the donor nerve and muscle. End-to-side neurorrhaphy is useful in large nerve gaps or in injuries where the proximal stump of the injured nerve is unavailable. Autografting may be necessary when dissection cannot expose sufficient nerve length to permit a tension-free coaptation. We have recently investigated a definitive end-to-side repair mouse model, in which axons fluoresce and can be accurately traced to the motor neuron pool [12]. We have shown that collateral motor sprouting will not occur without injury, while collateral sensory sprouting will occur without injury. A crush injury will produce some motor regeneration, but a neurectomy is required for robust motor regeneration. We report a modified case of an end-to-side motor reinnervation of the suprascapular nerve using the spinal accessory nerve following a C5 to C6 avulsion injury. In this case, a partial neurectomy was made in the spinal accessory nerve at the location of the end-to-side repair prior to an induced crush injury proximal to the repair site.
Case Report
Preoperative Course
A 29-year-old right-hand dominant man was involved in a motorcycle accident in August 2003. The patient sustained a C5 to C6 nerve root avulsion leaving him with 0/5 shoulder and elbow motor strength and diminished sensation in the right thumb, index finger, and lateral humeral region. Notably, the patient had good trapezius motor function. The patient was referred to our institution for surgical evaluation approximately 12 weeks following his injury and on exam demonstrated no clinical or electrodiagnostic improvement in symptoms.
Operative Procedure
Reconstruction of the axillary and suprascapular nerves was performed, as is frequently done since these nerves are both compromised in upper trunk injuries. In addition, a double fascicular nerve transfer to restore elbow flexion was performed. A triceps to axillary nerve transfer was used for axillary reconstruction, employing the triceps branch (to medial head) of the radial nerve as the donor. The branch to the medial head is the longest triceps branch, allowing for a tension-free transfer while minimizing the need for intramuscular dissection to free the donor.
Given that the brachialis is the most powerful flexor of the arm, a double fascicular transfer procedure was performed using redundant fascicles from both the ulnar and median nerves to reinnervate both the biceps and the brachialis branches of the musculocutaneous nerve.
Both the triceps to axillary and double fascicular transfers have been well described previously, as illustrated in the thorough reviews by Mackinnon et al. and others [7, 14, 16, 17].
To first describe the end-to-side spinal accessory to suprascapular nerve transfer procedure, a supraclavicular incision in the neck was utilized while the patient was in the supine position. The distal accessory nerve was identified along the free border of the trapezius muscle. The plexus was followed distally to the suprascapular nerve. A second incision was made in the medial arm and dissection proceeded down through the soft tissue to identify the median, ulnar, musculocutaneous, and lateral antebrachial cutaneous (LABC) nerves. A 3-cm portion of the LABC was harvested for grafting and sutured end-to-end to the distal stump of the identified suprascapular nerve and end-to-side to the accessory nerve. A partial neurectomy over approximately half of the accessory nerve was performed (Fig. 1). After securing the graft in place, a 30-s crush injury with jewelers forceps was applied to the accessory nerve proximal to the end-to-side anastomosis.
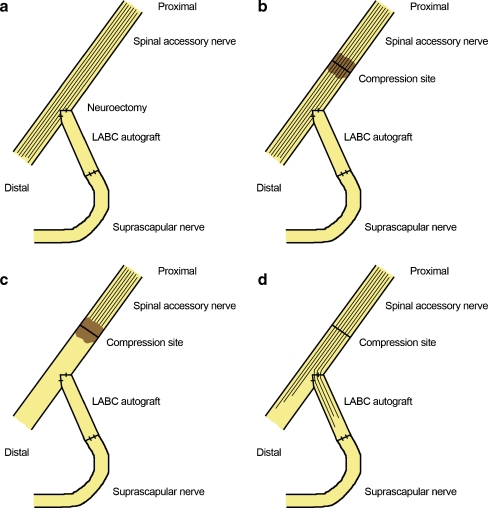
Figure 1.
Reinnervation mechanism of an end-to-side neurotization of the suprascapular nerve to the spinal accessory nerve to restore supra/infraspinatus function and preserve trapezius function. a In this case, a neuroectomy at the coaptation site of the spinal accessory nerve was created in order to acquire reinnervation by the motor fibers. b In addition, an axonotmetic injury was created proximal to the end-to-side neurotization through a compression to induce reinnervation of the suprascapular nerve. c Wallerian degeneration proceeds distal to the axonotmetic injury following the compression. d Afterwards, axonal regeneration proceeds through the native donor and into the recipient pathway through the end-to-side repair. The LABC graft was measured at 3 cm for this case.
Postoperative Course
Postoperatively, the patient participated in active physical therapy and motor re-education. He was seen back in the immediate postoperative period at 3, 9, 16 months, and 5.5 years. At 16 months, the patient had regained full strength in both elbow flexion and shoulder abduction with some atrophy of the deltoid. At 5.5 years, the patient demonstrated the same full strength in both elbow flexion (Fig. 2; Video 1) and shoulder abduction (Fig. 3a–c) without atrophy. Reduced strength in shoulder flexion was also apparent with the inability to flex farther than 120° with a 5-lb weight (Fig. 3f). Without the weight, the patient demonstrated the ability to flex the shoulder to its full range of motion (Fig. 3d). Stabilization of the scapula during these planar movements appeared to be proper (Video 2). Active external rotation had improved to 4/5 motor strength. The patient did have a persistent sensory deficit in the thumb and index finger but declined any further operative intervention. The patient was able to return to all his normal activities of daily living.
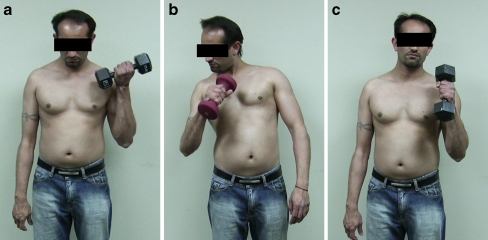
Figure 2.
Restoration of elbow flexion 5.5 years following end-to-end neurotization of the ulnar nerve (flexor carpi ulnaris fascicle) to the brachialis nerve and median nerve (flexor carpi radialis fascicle) to the biceps branch of the musculocutaneous nerve. a Normal flexion on the unoperated left side is observed with a 25-lb weight. b Restoration of elbow flexion is observed on the operated right side with a 7-lb weight. A degree of pronation is also evident during this range of motion with the weight in addition to the assistance of compensatory accessory muscles. c Normal flexion while pronated is demonstrated with a 25-lb weight.
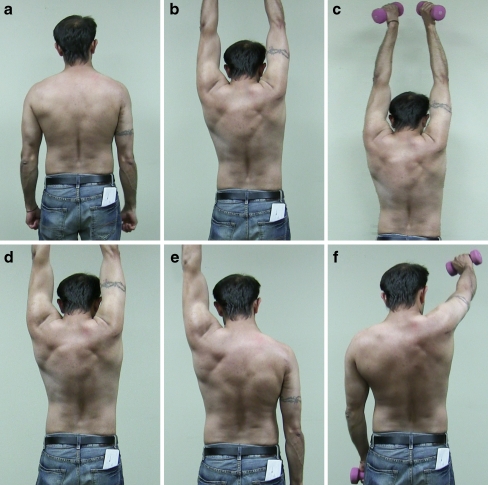
Figure 3.
Five years and 6 months following an end-to-side neurotization of the suprascapular nerve to the spinal accessory nerve and end-to-end neurotization of the triceps branch to the axillary nerve on the right side. a At rest, the patient does not demonstrate scapular winging on the previously injured right side. b Restoration of the deltoid and supra/infraspinatus is evident with the ability to abduct the right arm to 180° without weights. In addition, hypertrophy of the lower trapezius is seen on the right side with proper scapular stabilization. c To determine the extent of these functional outcomes, the patient demonstrates proficient abduction to 180° with a 5-lb weight. d Flexion was also examined and the patient was able to flex both arms to their full range of motion of 180° without weights. e With a 5-lb weight, the patient was able to demonstrate proper flexion to 180° on the left side. f In comparison, on the right side with a 5-lb weight, the patient was only able to flex to approximately 120°.
Discussion
The precise mechanism of axonal regeneration following end-to-side neurrorhaphy remains controversial with both experimental and clinical studies reporting mixed results [4, 6, 8, 11, 12, 19, 20, 23–25, 33–37]. Several authors have concluded that axonal damage to the donor nerve is required to induce motor collateral sprouting but may not be required to induce sensory sprouting in end-to-side neurorrhaphy [1, 12, 28, 29]. In a recent review by Dvali et al., the authors concluded that the degree of motor axonal sprouting following end-to-side repairs depends upon the degree of axonal damage, while sensory sprouting was less dependent on the presence of injury [8]. After reviewing both the basic science and clinical literature, they concluded that end-to-side repair without deliberate nerve injury should be limited to repair of noncritical sensory deficits. In a similar review by Rovak et al., the authors also concluded that collateral sprouting depended not only upon axonal damage but also was highly dependent on the extent of injury, with more collateral sprouting associated with increased damage [26]. While several authors have reported excellent functional results with facial nerve reinnervation using an end-to-side repair using the hypoglossal nerve as a donor [2, 3, 9, 27, 31, 32], the most definitive experimental study on end-to-side repair was recently reported by Hayashi et al. using Thy1-GFP mice that express green fluorescent protein in their motor axons [12]. This study utilized the femoral nerve as a donor and a transected sciatic nerve as the recipient. A nerve graft taken from the median nerve was sutured end-to-side into the femoral nerve and into the distal stump of the sciatic nerve. Both atraumatic model and epineurotomy group were used, with and without a proximal compression injury to the donor (femoral) nerve. Confocal imaging, histomorphometry, and retrograde labeling demonstrated the most rapid and robust regeneration in the epineurotomy group that had also received a proximal compression injury.
Our report corroborates the laboratory findings of Hayashi et al. by illustrating several important technical points regarding end-to-side repairs. As described in the operative procedure, it is critical to introduce a neurectomy into the donor nerve, creating a small degree of axonal disruption while removing basal laminar layers (Fig. 1a). After securing either the recipient stump or in this case an intervening autograft, an axonotmetic crush injury was induced proximal to the end-to-side repair site on the donor nerve (Fig. 1b). Wallerian degeneration proceeds distal to the axonotmetic injury (Fig. 1c). Following recovery from axonotmetic injury, axonal regeneration proceeds down both the native donor nerve and into the recipient pathway through the end-to-side repair (Fig. 3d). While neurectomy alone has been shown to cause some injury to the donor nerve, an additional crush would result in a greater net degree of injury, which animal studies have shown to result in greater motor recovery (as described above). Therefore, in our patient, the crush injury and the neurectomy to the donor nerve both likely play an important role in enhancing regeneration.
The results from this end-to-side suprascapular nerve to axillary nerve were outstanding, especially in the fact that we did not downgrade trapezius muscle function evident with proper scapular stabilization (Video 2). We did need a graft, however, and there may well be situations where it is not feasible to find a satisfactory distal end of suprascapular nerve in the supraclavicular exposure to allow this procedure to be performed. In those situations, the posterior approach of the distal accessory to suprascapular nerve would be the best option. However, in situations where the distal suprascapular nerve is available in the supraclavicular exposure, we believe that this offers the best opportunity to reinnervate the suprascapular nerve without downgrading trapezius muscle function.
Although the precise mechanisms underlying end-to-side regeneration remain unresolved, this case provides a clinical example in which end-to-side repair was used with a good clinical outcome. As discussed above, this case utilized both an axonotmetic injury and direct neurectomy to the donor nerve, both of which are likely critical to allow adequate motor nerve regeneration for an end-to-side nerve transfer.
Electronic Supplementary materials
(WMV 2355 kb)
(WMV 10354 kb)
References
- 1.Akeda K, et al. Regenerating axons emerge far proximal to the coaptation site in end-to-side nerve coaptation without a perineurial window using a T-shaped chamber. Plast Reconstr Surg. 2006;117:1194–1203. doi: 10.1097/01.prs.0000201460.54187.d7. [DOI] [PubMed] [Google Scholar]
- 2.Asaoka K, Sawamura Y. Hypoglossal-facial nerve side-to-end anastomosis. J Neurosurg. 1999;91:163–164. [PubMed] [Google Scholar]
- 3.Asaoka K, Sawamura Y, Nagashima M, Fukushima T. Surgical anatomy for direct hypoglossal-facial nerve side-to-end "anastomosis". J Neurosurg. 1999;91:268–275. doi: 10.3171/jns.1999.91.2.0268. [DOI] [PubMed] [Google Scholar]
- 4.Bertelli JA, Ghizoni MF. Nerve repair by end-to-side coaptation or fascicular transfer: a clinical study. J Reconstr Microsurg. 2003;19:313–318. doi: 10.1055/s-2003-42499. [DOI] [PubMed] [Google Scholar]
- 5.Bontioti E, Kanje M, Lundborg G, Dahlin LB. End-to-side nerve repair in the upper extremity of rat. J Peripher Nerv Syst. 2005;10:58–68. doi: 10.1111/j.1085-9489.2005.10109.x. [DOI] [PubMed] [Google Scholar]
- 6.Brenner MJ, Dvali L, Hunter DA, Myckatyn TM, Mackinnon SE. Motor neuron regeneration through end-to-side repairs is a function of donor nerve axotomy. Plast Reconstr Surg. 2007;120:215–223. doi: 10.1097/01.prs.0000264094.06272.67. [DOI] [PubMed] [Google Scholar]
- 7.Colbert SH, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand (N Y) 2006;1:71–77. doi: 10.1007/s11552-006-9004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvali LT, Myckatyn TM. End-to-side nerve repair: review of the literature and clinical indications. Hand Clin. 2008;24:455–460. doi: 10.1016/j.hcl.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Godefroy WP, Malessy MJ, Tromp AA, Mey AG. Intratemporal facial nerve transfer with direct coaptation to the hypoglossal nerve. Otol Neurotol. 2007;28:546–550. doi: 10.1097/mao.0b013e31804301b8. [DOI] [PubMed] [Google Scholar]
- 10.Goheen-Robillard B, Myckatyn TM, Mackinnon SE, Hunter DA. End-to-side neurorrhaphy and lateral axonal sprouting in a long graft rat model. Laryngoscope. 2002;112:899–905. doi: 10.1097/00005537-200205000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi A, et al. Collateral sprouting occurs following end-to-side neurorrhaphy. Plast Reconstr Surg. 2004;114:129–137. doi: 10.1097/01.PRS.0000129075.96217.92. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi A, et al. Axotomy or compression is required for axonal sprouting following end-to-side neurorrhaphy. Exp Neurol. 2008;211:539–550. doi: 10.1016/j.expneurol.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess JR, Brenner MJ, Myckatyn TM, Hunter DA, Mackinnon SE. Influence of aging on regeneration in end-to-side neurorrhaphy. Ann Plast Surg. 2006;57:217–222. doi: 10.1097/01.sap.0000215258.57614.89. [DOI] [PubMed] [Google Scholar]
- 14.Liverneaux PA, Diaz LC, Beaulieu JY, Durand S, Oberlin C. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg. 2006;117:915–919. doi: 10.1097/01.prs.0000200628.15546.06. [DOI] [PubMed] [Google Scholar]
- 15.Lundborg G, Zhao Q, Kanje M, Danielsen N, Kerns JM. Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis? J Hand Surg Br. 1994;19:277–282. doi: 10.1016/0266-7681(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 16.Mackinnon SE. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg. 2006;118:1273. doi: 10.1097/01.prs.0000238220.56097.4e. [DOI] [PubMed] [Google Scholar]
- 17.Mackinnon SE, Colbert SH. Nerve transfers in the hand and upper extremity surgery. Tech Hand Up Extrem Surg. 2008;12:20–33. doi: 10.1097/BTH.0b013e31812714f3. [DOI] [PubMed] [Google Scholar]
- 18.Mackinnon SE, Roque B, Tung TH. Median to radial nerve transfer for treatment of radial nerve palsy. case report. J Neurosurg. 2007;107:666–671. doi: 10.3171/JNS-07/09/0666. [DOI] [PubMed] [Google Scholar]
- 19.Noah EM, Williams A, Fortes W, Terzis JK. A new animal model to investigate axonal sprouting after end-to-side neurorrhaphy. J Reconstr Microsurg. 1997;13:317–325. doi: 10.1055/s-2007-1006410. [DOI] [PubMed] [Google Scholar]
- 20.Noah EM, Williams A, Jorgenson C, Skoulis TG, Terzis JK. End-to-side neurorrhaphy: a histologic and morphometric study of axonal sprouting into an end-to-side nerve graft. J Reconstr Microsurg. 1997;13:99–106. doi: 10.1055/s-2007-1000224. [DOI] [PubMed] [Google Scholar]
- 21.Novak CB, Mackinnon SE. Treatment of a proximal accessory nerve injury with nerve transfer. Laryngoscope. 2004;114:1482–1484. doi: 10.1097/00005537-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Novak CB, Mackinnon SE, Tung TH. Patient outcome following a thoracodorsal to musculocutaneous nerve transfer for reconstruction of elbow flexion. Br J Plast Surg. 2002;55:416–419. doi: 10.1054/bjps.2002.3859. [DOI] [PubMed] [Google Scholar]
- 23.Okajima S, Terzis JK. Ultrastructure of early axonal regeneration in an end-to-side neurorrhaphy model. J Reconstr Microsurg. 2000;16:313–323. doi: 10.1055/s-2000-7339. [DOI] [PubMed] [Google Scholar]
- 24.Papalia I, Geuna S, D'Alcontres FS, Tos P. Origin and history of end-to-side neurorrhaphy. Microsurgery. 2007;27:56–61. doi: 10.1002/micr.20303. [DOI] [PubMed] [Google Scholar]
- 25.Ray WZ, Mackinnon SE. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol (2009), doi:10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed]
- 26.Rovak JM, Cederna PS, Kuzon WM., Jr Terminolateral neurorrhaphy: a review of the literature. J Reconstr Microsurg. 2001;17:615–624. doi: 10.1055/s-2001-18817. [DOI] [PubMed] [Google Scholar]
- 27.Singh N, Robertson B, Dellon L. Use of an innervated tongue flap to rehabilitate the tongue after hypoglossal-to-facial nerve transfer. Plast Reconstr Surg. 2002;109:2439–2442. doi: 10.1097/00006534-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 28.Tarasidis G, et al. End-to-side neurorrhaphy resulting in limited sensory axonal regeneration in a rat model. Ann Otol Rhinol Laryngol. 1997;106:506–512. doi: 10.1177/000348949710600612. [DOI] [PubMed] [Google Scholar]
- 29.Tarasidis G, et al. End-to-side neurorraphy: a long-term study of neural regeneration in a rat model. Otolaryngol Head Neck Surg. 1998;119:337–341. doi: 10.1016/S0194-5998(98)70074-9. [DOI] [PubMed] [Google Scholar]
- 30.Tung TH, Liu DZ, Mackinnon SE. Nerve transfer for elbow flexion in radiation-induced brachial plexopathy: a case report. Hand (N Y) 2009;4:123–128. doi: 10.1007/s11552-008-9136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda K, et al. Combination of hypoglossal-facial nerve jump graft by end-to-side neurorrhaphy and cross-face nerve graft for the treatment of facial paralysis. J Reconstr Microsurg. 2007;23:181–187. doi: 10.1055/s-2007-974654. [DOI] [PubMed] [Google Scholar]
- 32.Venail F, et al. Outcomes and complications of direct end-to-side facial-hypoglossal nerve anastomosis according to the modified May technique. J Neurosurg. 2009;110:786–791. doi: 10.3171/2008.9.JNS08769. [DOI] [PubMed] [Google Scholar]
- 33.Viterbo F, Trindade JC, Hoshino K, Mazzoni A. Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath: experimental study in rats. Br J Plast Surg. 1994;47:75–80. doi: 10.1016/0007-1226(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 34.Voche P, Ouattara D. End-to-side neurorrhaphy for defects of palmar sensory digital nerves. Br J Plast Surg. 2005;58:239–244. doi: 10.1016/j.bjps.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Fischer KA. End-to-side neurorrhaphy. Microsurgery. 2002;22:122–127. doi: 10.1002/micr.21736. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, et al. Repair of the main nerve trunk of the upper limb with end-to-side neurorrhaphy: an experimental study in rabbits. Microsurgery. 2006;26:245–252. doi: 10.1002/micr.20235. [DOI] [PubMed] [Google Scholar]
- 37.Zhu QT, Zhu JK, Chen GY. Location of collateral sprouting of donor nerve following end-to-side neurorrhaphy. Muscle Nerve. 2008;38:1506–1509. doi: 10.1002/mus.21116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(WMV 2355 kb)
(WMV 10354 kb)