Abstract
Intraneural perineurioma is a benign peripheral nerve sheath tumor of perineurial cell origin. We present the case of an intraneural perineurioma of the median nerve in a 23-year-old woman which posed a diagnostic challenge. Ultrasonography was found to be a quick, easy, and effective screening tool for identifying the source of the deficit followed by MRI to further elucidate the lesion. We discuss surgical management options for optimizing functional outcomes when addressing such lesions.
Keywords: Intraneural perineurioma, Nerve transfer, Ultrasound, MRI, Nerve tumor, Intraneural neurolysis
Introduction/Background
Intraneural perineurioma is a benign peripheral nerve sheath tumor of perineurial cell origin which was first histologically identified by Da Gama Imaginario [25] in 1964. This entity was distinguished from Schwannoma and neurofibroma by the ultrastructural work [32] of Lazarus and Trombetta in 1978 who found similarities between the cells of these tumors and the native perineurial cells of the peripheral nerve. Throughout the literature there have been many different names attributed to this clinical entity including localized hypertrophic (mono)neuropathy, interstitial hypertrophic neuropathy, pseudo-onion bulb (mono)neuropathy, hypertrophic neurofibroma, and intraneural neurofibroma [10]. The myriad of names of this lesion confound the literature and are confusing to the reader. These terms have historically been used to refer to both the intraneural perineurioma and reactive Schwann cell processes, thus the descriptions of the disease have been unclear [10, 47].
Perineurioma is divided into two variants: intraneural and extraneural (soft tissue) perineurioma. Soft tissue perineurioma is often found in a subcutaneous tissue and is generally unassociated with nerve, in contrast to intraneural perineurioma which are restricted to the substance of peripheral nerve. There has been some debate whether intraneural perineurioma is a reactive change within peripheral nerves as some authors have claimed [27, 35, 49, 52] or whether they are neoplastic [9, 51], in nature. There is increasing consensus in the literature [10] that these lesions are indeed clonal neoplasms featuring cytogenic abnormalities on chromosome 22 [15]. These chromosomal abnormalities are similar to those seen in meningioma [43], soft tissue perineurioma [16], and Schwannoma [41].
These lesions are frequently misdiagnosed and due to their rarity, management has not been clearly defined. We present a case of intraneural perineurioma of the median nerve which posed a diagnostic challenge.
Clinical Case
A 23-year-old woman presented to our center with long-standing history of spontaneous right median nerve mononeuropathy. Prior to presentation, her initial symptoms included difficulty keying with her thumb and index finger of her right hand. She gradually developed numbness in the right first and second fingers and associated weakness in the anterior interosseous nerve distribution. This weakness progressed until she was unable to flex the thumb at the interphalangeal joint or the index finger at its distal interphalangeal joint.
Initial management was undertaken at an outside institution. She had an electrodiagnostic study, the findings of which were felt to be consistent with anterior interosseous nerve (AIN) palsy with a superimposed mild to moderate carpal tunnel syndrome. At that time, approximately 1 year after the initial development of symptoms, she underwent a standard carpal tunnel release and median nerve release within the forearm. Despite this procedure, she did not regain function within the AIN distribution or sensation within the index finger. As a result, she then underwent cervical spine imaging which was non-diagnostic.
In the interval prior to her referral to our institution, her numbness progressed to involve the long finger with additional development of thenar eminence atrophy. She had a diffusely positive Tinel-like sign within the upper arm, which could not be well localized.
The patient was then referred to the senior author for further evaluation and management of a possible brachial plexus lesion. Based on the above findings she was presumed to be suffering from brachial neuritis (Parsonage–Turner Syndrome) with a superimposed carpal tunnel syndrome and she was taken for a re-release of the carpal tunnel with appropriate tendon transfers. This was performed 19 months after her first procedure.
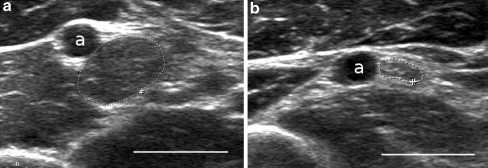
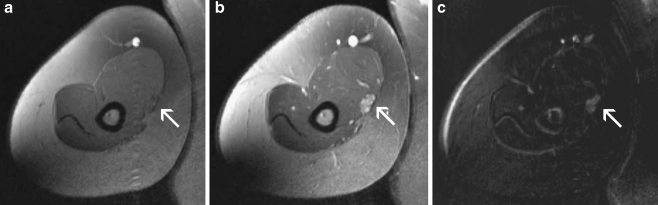
Ten months after her second surgery, there was no improvement of sensation from her pre-operative status. Electrodiagnostic studies were repeated at this juncture and demonstrated a median mononeuropathy at or proximal to the branch to the pronator teres. An ultrasound of the median nerve, performed at time of the electodiagnostic studies, demonstrated a fusiform dilatation of the median nerve at the mid-humeral level (Fig. 1). This investigation prompted further evaluation of the median nerve by MRI of the arm and proximal forearm. This study demonstrated a lobulated, hyperintense dilatation of the brachial median nerve measuring 2.8 cm in length and 1.1 × 0.7 mm in the axial dimension (Fig. 2). The image was initially interpreted as consistent with a traumatic neuroma or perineurial scarring.
Figure 1.
Ultrasound image depicting a the enlarged portion of the right median nerve with a cross-sectional area 46.7 mm2 and b normal left median nerve with a cross-sectional area of 8.1 mm2. Bar = 1 cm, a = brachial artery.
Figure 2.
MRI image of the right arm depicting the enlarged lobulated enhancing region of the median nerve. a Pre-contrast, b enhancing, and c subtraction image of pre and post contrast images.
Given our concern for malignancy, exploration was undertaken. The median nerve in the upper arm was found to be dilated with palpable firmness for a length of approximately 5 cm. Above and below this region the nerve was normal in appearance, texture, and consistency. Internal neurolysis revealed that each of the fascicles in this region was uniformly dilated in a fusiform fashion, without a single predominant fascicle. Individual fascicles were stimulated and only limited pronation was found in a single fascicle. This fascicle was therefore preserved as were the lateral presumably sensory fascicles due to the preserved sensation in her thumb. The remainder of the nerve at this level was then excised and sent for pathologic evaluation. Neither nerve grafting nor nerve transfer was performed at the time of biopsy since her deficits had now been long standing.
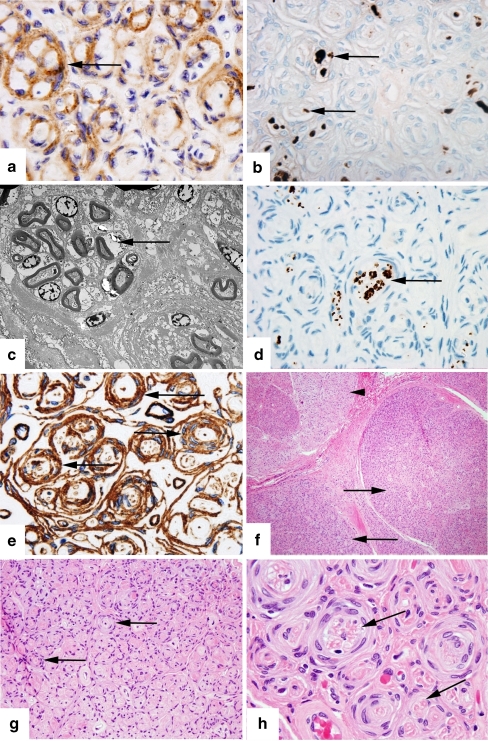
On pathologic examination, epithelial membrane antigen (EMA) staining of involved fascicles was positive (Fig. 3a) while the S-100 stain was negative (Fig. 3b), demonstrating that the whorls of cells seen were indeed composed of perineurial cells and not Schwann cells, thereby forming pseudo-onion bulbs. Electron microscopy showed perineurial cells circumferentially enwrapping collagen and nerve fibers (Fig. 3c). S-100 did highlight scattered Schwann cells within the fascicles (Fig. 3b) as would be expected, but note that these are not the predominant cell as would be in a Schwannoma. Neurofilament staining showed some residual axons in the involved fascicles (Fig. 3d) corresponding to the degree of functional loss in this nerve.
Figure 3.
This set of images shows our pathologic specimen of intraneural perineurioma with appropriate staining methods. a Epithelial membrane antigen. Original magnification ×1,000. Epithelial membrane antigen (EMA) positive staining shows pseudo-onion bulbs consisting of perineurial cells (arrow). b S-100 stain. Original magnification ×1,000. S-100 staining highlights scattered Schwann cells within the fascicles (arrow). This again shows that the whorls are not composed of Schwann cells and therefore are pseudo-onion bulbs. c Electron microscopy. Direct magnification ×2,500. Electron microscopy shows perineurial cells circumferentially enwrapping multiple axons (arrow). d Neurofilament stain. Original magnification ×1,000. Neurofilament staining highlights scattered axons within the fascicles, some of which are in clusters (arrow) surrounded by perineurial cells. In chronic lesions such as this, axons are scant or absent. e Collagen IV stain. Original magnification ×1,000. Collagen IV highlights perineurial cells’ basement membranes (arrow). High quantities of collagen deposition as seen in this image occur with long-standing lesions. f Hematoxylin and eosin stain. Original magnification ×100. A low power cross-section view of the affected nerve showing involved fascicles (arrows) and a relatively uninvolved fascicle (arrowhead). Involved fascicles are enlarged and hyperchromatic. g Hematoxylin and eosin stain. Original magnification ×400. Pseudo-onion bulbs (arrows) consisting of multilayer cuffs of perineurial cells surrounding single or multiple nerve fibers, seen at higher magnification in c. h Hematoxylin and eosin stain. Original magnification ×1,000. High power view showing large pseudo-onion bulbs surrounding multiple axons (arrow).
As the disease progresses, the amount of myelin and axons seen in the fascicles diminish and the collagen content increases (as seen in Fig. 3e). Conspicuous pinocytotic vesicles were seen within thin cell processes, an ultrastructural finding typical for cells of perinuerial origin. The latter figures (Fig. 3f through h) show progressive magnification of the lesion with the hematoxylin and eosin stain with involved fascicles appearing hyperchromatic in their periphery due to the hypercellularity of the perineurium (Fig. 3f), and pseudo-onion bulbs seen in the perineurial lesions(Fig. 3h). Based on the features discussed, the diagnosis of intraneural perineurioma was made.
One month following this biopsy her pronation strength persisted and has not subsequently undergone any further decline in function.
Discussion
The perineurium is a layer composed of several concentric layers of flattened perineurial cells which form a laminar structure around single nerve fascicles [40]. Perineurial cells are mesenchyme-derived cells which maintain a constant intrafascicular pressure and guarantee a selective barrier for axons and Schwann cells [40]. Thus, the perineurium modulates traction forces, regulates the endoneurial pressure and milieu, and is a critical component of the blood–nerve barrier [40]. Intraneural perineurioma is a rare neoplasm of these perineurial cells and is associated with abnormalities of chromosome 22 [15]. According to recent reviews, there have been six definitive cases of intraneural perineurioma of the median nerve, though others have undoubtedly been encountered [1, 9, 10, 15, 18, 26, 35, 44].
The typical age of onset of intraneural perineurioma is either adolescence or young adulthood and no sexual predilection has been demonstrated. The symptomatic presentation is usually a painless mononeuropathy with progressive weakness in the affected muscles [12, 43]. In contrast to many instances of Parsonage–Turner Syndrome, there is no apparent inciting event, nor is there an association with antecedent trauma [12, 18, 43]. Electrodiagnostic studies typically demonstrate findings consistent with both demyelination and axonal degeneration [19, 39]. Most commonly muscles show denervation changes by the time the patient presents to the surgeon.
There are several entities which should be considered in the differential of a spontaneous mononeuropathy (Table 1). Compressive lesions are a common cause of mononeuropathies. In keeping with a common misdiagnosis of this lesion, this case was interpreted initially as an entrapment neuropathy—a combined anterior interosseous nerve (AIN) syndrome and carpal tunnel syndrome [44]. However, both conservative treatment and surgical release failed to improve the symptoms. Brachial neuritis (Parsonage–Turner syndrome or neuralgic amyotrophy) is another entity which can present in this manner [21, 36]. Brachial neuritis is usually characterized by sudden onset of pain in the shoulder region followed by weakness and atrophy in a number of possible nerve distributions, most commonly affecting multiple elements of the brachial plexus [36]. Spontaneous mononeuropathies can result from this entity and a painless subset is recognized [28, 29].
Table 1.
Differential diagnosis of spontaneous mononeuropathy.
| Compression neuropathy | Anterior interosseous nerve (AIN) syndrome |
| Inflammatory neuropathy | Parsonage–Turner syndrome (brachial neuritis or neuralgic amyotrophy) |
| Reactive process | Localized hypertrohic neuropathy |
| Benign peripheral nerve sheath tumors | Schwannoma |
| Neurofibroma | |
| Non-neural sheath tumors (compressive tumors) | Neuromuscular hamartomas |
| Ganglioneuromas | |
| Hemangiomas | |
| Hemangiopericytomas | |
| Hemangioblastomas | |
| Malignant peripheral nerve sheath tumors (neurogenic sarcomas) | Malignant schwannomas |
| Neurofibrosarcomas |
The differential diagnosis of spontaneous mononeuropathy divided by etiologic category. The main groups include compression, inflammation, benign peripheral nerve sheath tumors, non-neural sheath tumors, and malignant peripheral nerve sheath tumors.
Nerve sheath tumors, such as Schwannomas and neurofibromas, can present with mononeuropathies as well, although they more commonly present with a much milder neurologic deficit if at all [8, 22, 47]. Perineurioma can be differentiated from these common lesions on the basis of several features. Schwannomas are firm, globular lesions derived from Schwann cells which are found within but are distinct from the healthy nerve in which they grow. The neurofibroma consists of Schwann cells, perinuerial-like cells, fibroblasts, and cells with intermediate features. Both of these lesions tend to be much larger by the time of presentation, usually multiples of the diameter of the parent nerve. In contrast, IPN forms a cylindrical often subtle enlargement of the nerve itself with coarsening of the fascicles [12]. Additionally, both Schwannomas and neurofibromas lack a significant onion bulb component. In difficult cases, S-100 staining can be used since neurofibromas and Schwannomas are S-100 positive while intraneural perineurioma is S-100 negative.
The whorls of cells that were found were EMA positive and S-100 negative, confirming that these whorls were of perineurial cell origin and thus pseudo-onion bulbs. In contrast, a true onion bulb is a whorl-shaped proliferation of Schwann cells, as can be found in Chacot Marie Tooth Disease and chronic inflammatory demyelinating polyneuropathy [10]. A wide gamut of other peripheral nerve lesions can present in a similar fashion to our case including those caused by several other benign tumors. Neuromuscular hamartomas can present as intraneural lesions and can produce similar symptoms [2]. Hemangiomas, hemangiopericytomas, and ganglioneuromas may also cause intraneural lesions [14, 30]. Lymphangiomas, myoblastomas, and desmoid tumors tend to cause extrinsic compression of peripheral nerves, but can invade the epineurium resulting in a similar presentation [14, 30].
Diagnostics: Imaging with US and MRI
In addition to the indispensible history, examination, and electrophysiological studies, imaging can be critical in the evaluation and treatment of peripheral nerve lesions [46]. Nerve lesions, from compression neuropathies to peripheral nerve sheath tumors, can often be readily identified by ultrasonography [5, 7, 48] and MRI [23, 46, 50]. We have found that ultrasound is an inexpensive and rapid means by which to screen for causes of neuropathy, particularly with an unusual history. In this case imaging was essential in localization and diagnosis. Ultrasound has been recommended as the first line imaging modality when an entrapment neuropathy or peripheral nerve tumor is suspected [34]. The main limitations of ultrasound and MRI are their lack of specificity in determining the precise lesion type [13, 46]. Nonetheless, in centers utilizing this on a routine basis, ultrasound has been shown to impact the therapeutic approach in up to a quarter of patients presenting with electrodiagnostic evidence of a mononeuropathy, as in this case [38].
Tumors of peripheral nerve origin can be reliably distinguished from other soft tissue masses on ultrasound by identifying continuity of the nerve with the tumor at its proximal and distal margins [8, 22]. Each of these lesions commonly appear as an echolucent fusiform dilation with well-defined margins on ultrasound [4, 5, 8, 22]. While the sonographic characteristics of an intraneural perineurioma are similar to the more common Schwannomma and neurofibroma, these tumors can often be distinguished from a perineurioma when a globoid lesion is demonstrated that displaces surrounding normal appearing fascicles, as is typical for these lesions [8, 17, 22, 42]. Malignant peripheral nerve sheath tumors are at times distinguishable from each of these benign entities due to indistinct margins or investment of the mass into the surrounding tissue [8].
MRI findings of intraneural perineuriomas are similarly nonspecific, unlike the typical findings in nerve sheath tumors of isointensity to muscle on T1, hyperintensity on T2 (frequently with a “target sign” in which the central portion of the lesion is not quite as intense as the periphery) and bright homogenous enhancement with gadolinium. Several case reports of intraneural perineurioma have described nonspecific MRI imaging findings. T1 images may show a segmental thickening of nerve with or without post-gadolinium enhancement [1, 3, 20, 31]. The description usually includes T2-weighted hyperintensity [3, 20, 44, 45, 47]. As this is a rare entity with indistinct imaging features, it is not likely that diagnostic confidence can be arrived at based on imaging alone. Regardless, initial screening with ultrasound can both provide some initial indications of the source of the problem and can effectively localize the lesion enabling precise targeting of the lesion on MRI. In addition, when surgical exploration is indicated, ultrasound, MRI, and MR neurography can be of value for pre-operative planning [6, 24, 37, 46]
Management
The initial presentation and electrophysiological findings are similar for patients with mononeuropathy of any origin [19]. In evaluating mononeuropathies, determining the specific diagnosis and understanding the natural course and prognosis are key for appropriate surgical decision making. Surgery is recommended in patients with neurological deficit and a localizable source lesion, as in intraneural perineurioma. Based on one of the largest series of intraneural perineurioma with 14 patients, Gruen et al. recommended lesion resection with routine interposition nerve grafting [18]. Surgery is usually quite straightforward with this lesion because it commonly involves a well-circumscribed area of focal enlargement most often of a single nerve [19]. They recommended that if no action potential was recorded across the lesion or if one of poor amplitude was recorded, the lesion should be resected to normal appearing fascicles proximally and distally and a graft interposed. The surgeon must be careful to remove all of the lesion—that is to resect a bit further both proximally and distally than seems necessary. This is done because if the lesion is not completely resected, the graft repair may eventually deteriorate as well, precluding successful axonal extension to the target. This necessity contributes to longer grafts and therefore poorer results.
It has been suggested that a nerve with limited function could be further downgraded due to intraneural neurolysis. Additionally, that due to the progressive nature of these lesions, complete resection should be undertaken [33]. Intraneural neurolysis has been found to be a safe and reliable technique and was critical to preservation of residual function in our patient. Indeed, the residual pronation which we were able to preserve may be lost with time. Given the patient’s current level of deficit and its chronicity, we were not confident that any reconstructive approach would have effectively restored this already compromised pronation.
As an alternative to proximal grafting, our team has found that progressive neuropathies are often best addressed with distal nerve transfers. If a more timely diagnosis had occurred, nerve transfer would have been attempted, including transfer to the anterior interosseous nerve and the nerve to the pronator teres utilizing branches of the radial nerve. We prefer the use of nerve transfer as opposed to interposition grafting, for a number of reasons. First, utilizing a reconstruction that is distant from the site of the lesion allows the surgeon to resect widely without concern for the length of graft required. Secondly, chances of successful reinnervation of the target musculature are significantly improved due to decreased length of regeneration required, elimination of an additional repair site, and exclusion of grafts [11]. Finally, this eliminates a second surgery site and additional deficit as would be necessary in harvesting a nerve graft.
Unfortunately, this patient presented too late for these reconstructive interventions to be considered. That in mind, this case highlights the need for prompt diagnosis for possible salvage of function.
While spread to other nerves from a lesion confined to a single nerve is not known to occur, spread within contiguous elements of a plexus is possible. As a result, more aggressive resection of tumors in the plexus, rather than internal neurolysis alone, might be advocated, particularly if reliable nerve transfers are available to address the resulting deficits. Although some authors believe that this lesion progresses to complete motor loss and thus mandating complete resection, recent literature cites that the natural history of this lesion is still not known as of yet and attempts to predict the ultimate deficits remain speculative [18, 19, 47].
Conclusion
We have reported a case of intraneural perineurioma of the median nerve in which ultrasound was helpful in arriving at the diagnosis. When a patient has progressive loss of sensation with or without associated muscle atrophy, otherwise unexplained, one must rule out common extrinsic causes of nerve injury as well as intrinsic nerve diseases such as tumors. Ultrasound is a quick, safe, inexpensive, and effective test that can be invaluable in this setting. If such a lesion is found upon ultrasound, further evaluation with MRI is generally useful. Presumptive diagnosis is best arrived at by careful history, examination, electrodiagnostic evaluation, and imaging, but biopsy is required for definitive diagnosis. Intraneural neurolysis offers the possibility of preserving residual function but may compromise the elimination of this disease. We believe that resection should be coupled with early nerve transfers for the best chance of restoring function in these cases.
References
- 1.Alfonso DT, Sotrel A, Grossman JA. Carpal tunnel syndrome due to an intraneural perineurioma in a 2-year-old child. J Hand Surg [Br] 2001;26:168–70. doi: 10.1054/jhsb.2000.0528. [DOI] [PubMed] [Google Scholar]
- 2.Bassett GS, Monforte-Munoz H, Mitchell WG, Rowland JM. Cavus deformity of the foot secondary to a neuromuscular choristoma (hamartoma) of the sciatic nerve. A case report. J Bone Joint Surg Am. 1997;79:1398–401. doi: 10.2106/00004623-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Beekman R, Slooff WB, Oosterhout MF, Lammens M, Berg LH. Bilateral intraneural perineurioma presenting as ulnar neuropathy at the elbow. Muscle Nerve. 2004;30:239–43. doi: 10.1002/mus.20052. [DOI] [PubMed] [Google Scholar]
- 4.Beggs I. Pictorial review: imaging of peripheral nerve tumours. Clin Radiol. 1997;52:8–17. doi: 10.1016/S0009-9260(97)80299-1. [DOI] [PubMed] [Google Scholar]
- 5.Beggs I. Sonographic appearances of nerve tumors. J Clin Ultrasound. 1999;27:363–8. doi: 10.1002/(SICI)1097-0096(199909)27:7<363::AID-JCU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335–49. doi: 10.1023/B:NEON.0000041891.39474.cb. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi S. Ultrasound of the peripheral nerves. Joint Bone Spine. 2008;75:643–9. doi: 10.1016/j.jbspin.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. New York: Springer; 2007. [Google Scholar]
- 9.Bilbao JM, Khoury NJ, Hudson AR, Briggs SJ. Perineurioma (localized hypertrophic neuropathy) Arch Pathol Lab Med. 1984;108:557–60. [PubMed] [Google Scholar]
- 10.Boyanton BL, Jr, Jones JK, Shenaq SM, Hicks MJ, Bhattacharjee MB. Intraneural perineurioma: a systematic review with illustrative cases. Arch Pathol Lab Med. 2007;131:1382–92. doi: 10.5858/2007-131-1382-IPASRW. [DOI] [PubMed] [Google Scholar]
- 11.Brown JM, Shah MN, Mackinnon SE. Distal nerve transfers: a biology-based rationale. Neurosurg Focus. 2009;26:E12. doi: 10.3171/FOC.2009.26.2.E12. [DOI] [PubMed] [Google Scholar]
- 12.Burger PC, Scheithauer BW, Vogel FS. The peripheral nervous system: surgical pathology of the nervous system and its coverings. Philadelphia: Churchill Livingstone; 2002. pp. 579–648. [Google Scholar]
- 13.Chiou HJ, Chou YH, Chiou SY, Liu JB, Chang CY. Peripheral nerve lesions: role of high-resolution US. Radiographics. 2003;23:e15. doi: 10.1148/rg.e15. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Ganju A, Tiel RL, Kline DG. Tumors of the brachial plexus. Neurosurg Focus. 2007;22:E26. doi: 10.3171/foc.2007.22.6.27. [DOI] [PubMed] [Google Scholar]
- 15.Emory TS, Scheithauer BW, Hirose T, Wood M, Onofrio BM, Jenkins RB. Intraneural perineurioma. A clonal neoplasm associated with abnormalities of chromosome 22. Am J Clin Pathol. 1995;103:696–704. doi: 10.1093/ajcp/103.6.696. [DOI] [PubMed] [Google Scholar]
- 16.Giannini C, Scheithauer BW, Jenkins RB, Erlandson RA, Perry A, Borell TJ, Hoda RS, Woodruff JM. Soft-tissue perineurioma. Evidence for an abnormality of chromosome 22, criteria for diagnosis, and review of the literature. AmJ SurgPathol. 1997;21:164–173. doi: 10.1097/00000478-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Gruber H, Glodny B, Bendix N, Tzankov A, Peer S. High-resolution ultrasound of peripheral neurogenic tumors. Eur Radiol. 2007;17:2880–8. doi: 10.1007/s00330-007-0645-7. [DOI] [PubMed] [Google Scholar]
- 18.Gruen JP, Mitchell W, Kline DG. Resection and graft repair for localized hypertrophic neuropathy. Neurosurgery. 1998;43:78–83. doi: 10.1097/00006123-199807000-00051. [DOI] [PubMed] [Google Scholar]
- 19.DG GJPK. Hypertrophic mononeuropathy. Neurosurgical Focus. 2007;22:E23. doi: 10.3171/foc.2007.22.6.24. [DOI] [PubMed] [Google Scholar]
- 20.Heilbrun ME, Tsuruda JS, Townsend JJ, Heilbrun MP. Intraneural perineurioma of the common peroneal nerve. Case report and review of the literature. J Neurosurg. 2001;94:811–5. doi: 10.3171/jns.2001.94.5.0811. [DOI] [PubMed] [Google Scholar]
- 21.Hess JR, Rocque BG, Mackinnon SE, Hunter DA. Ulnar nerve deficit after catfish sting. South Med J. 2005;98:750–1. doi: 10.1097/01.smj.0000168836.55216.05. [DOI] [PubMed] [Google Scholar]
- 22.Hoddick WK, Callen PW, Filly RA, Mahony BS, Edwards MB. Ultrasound evaluation of benign sciatic nerve sheath tumors. J Ultrasound Med. 1984;3:505–7. doi: 10.7863/jum.1984.3.11.505. [DOI] [PubMed] [Google Scholar]
- 23.Hof JJ, Kliot M, Slimp J, Haynor DR. What’s new in MRI of peripheral nerve entrapment? Neurosurg Clin N Am. 2008;19:583–595. doi: 10.1016/j.nec.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Huang JH, Zhang J, Zager EL. Diagnosis and treatment options for nerve sheath tumors. Expert Rev Neurother. 2005;5:515–23. doi: 10.1586/14737175.5.4.515. [DOI] [PubMed] [Google Scholar]
- 25.Imaginariojda G, Coelho B, Tome F, Luis ML. Monosymptomatic interstitial hypertrophic neuritis. J Neurol Sci. 1964;64:340–7. [PubMed] [Google Scholar]
- 26.Jazayeri MA, Robinson JH, Legolvan DP. Intraneural perineurioma involving the median nerve. Plast Reconstr Surg. 2000;105:2089–91. doi: 10.1097/00006534-200005000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PC, Kline DG. Localized hypertrophic neuropathy: possible focal perineurial barrier defect. Acta Neuropathol. 1989;77:514–8. doi: 10.1007/BF00687253. [DOI] [PubMed] [Google Scholar]
- 28.Kaeser HE, Leimgruber HR. Painless brachial plexus neuritis (author’s transl) Schweiz Rundsch Med Prax. 1979;68:731–5. [PubMed] [Google Scholar]
- 29.Kaeser HE, Nigst H. Brachial plexus neuritis may be painless and a good indication for surgery (authors transl) Schweiz Rundsch Med Prax. 1977;66:189–91. [PubMed] [Google Scholar]
- 30.Kim DH, Murovic JA, Tiel RL, Kline DG. Operative outcomes of 546 Louisiana State University Health Sciences Center peripheral nerve tumors. Neurosurg Clin N Am. 2004;15:177–92. doi: 10.1016/j.nec.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Lacour-Petit MC, Lozeron P, Ducreux D. MRI of peripheral nerve lesions of the lower limbs. Neuroradiology. 2003;45:166–70. doi: 10.1007/s00234-002-0932-6. [DOI] [PubMed] [Google Scholar]
- 32.Lazarus SS, Trombetta LD. Ultrastructural identification of a benign perineurial cell tumor. Cancer. 1978;41:1823–9. doi: 10.1002/1097-0142(197805)41:5<1823::AID-CNCR2820410525>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Mackinnon SE, O’Brien JP, Dellon AL, McLean AR, Hudson AR, Hunter DA. An assessment of the effects of internal neurolysis on a chronically compressed rat sciatic nerve. Plast Reconstr Surg. 1988;81:251–8. doi: 10.1097/00006534-198802000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Martinoli C, Bianchi S, Dahmane M, Pugliese F, Bianchi-Zamorani MP, Valle M. Ultrasound of tendons and nerves. Eur Radiol. 2002;12:44–55. doi: 10.1007/s00330-001-1161-9. [DOI] [PubMed] [Google Scholar]
- 35.Mitsumoto H, Estes ML, Wilbourn AJ, Culver JE., Jr Perineurial cell hypertrophic mononeuropathy manifesting as carpal tunnel syndrome. Muscle Nerve. 1992;15:1364–8. doi: 10.1002/mus.880151212. [DOI] [PubMed] [Google Scholar]
- 36.Moghekar AR, Moghekar AR, Karli N, Chaudhry V. Brachial plexopathies: etiology, frequency, and electrodiagnostic localization. J Clin Neuromuscul Dis. 2007;9:243–7. doi: 10.1097/CND.0b013e3181450f7a. [DOI] [PubMed] [Google Scholar]
- 37.Mrugala MM, Batchelor TT, Plotkin SR. Peripheral and cranial nerve sheath tumors. Curr Opin Neurol. 2005;18:604–10. doi: 10.1097/01.wco.0000179507.51647.02. [DOI] [PubMed] [Google Scholar]
- 38.Padua L, Aprile I, Pazzaglia C, Frasca G, Caliandro P, Tonali P, Martinoli C. Contribution of ultrasound in a neurophysiological lab in diagnosing nerve impairment: a one-year systematic assessment. Clin Neurophysiol. 2007;118:1410–6. doi: 10.1016/j.clinph.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Phillips LH, 2nd, Persing JA, Vandenberg SR. Electrophysiological findings in localized hypertrophic mononeuropathy. Muscle Nerve. 1991;14:335–41. doi: 10.1002/mus.880140408. [DOI] [PubMed] [Google Scholar]
- 40.Pina-Oviedo S, Ortiz-Hidalgo C. The normal and neoplastic perineurium: a review. Adv Anat Pathol. 2008;15:147–64. doi: 10.1097/PAP.0b013e31816f8519. [DOI] [PubMed] [Google Scholar]
- 41.Rey JA, Bello MJ, Campos JM, Vaquero J, Kusak ME, Sarasa JL, Pestana A. Abnormalities of chromosome 22 in human brain tumors determined by combined cytogenetic and molecular genetic approaches. Cancer Genet Cytogenet. 1993;66:1–10. doi: 10.1016/0165-4608(93)90140-H. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds DL, Jr, Jacobson JA, Inampudi P, Jamadar DA, Ebrahim FS, Hayes CW. Sonographic characteristics of peripheral nerve sheath tumors. AJR Am J Roentgenol. 2004;182:741–4. doi: 10.2214/ajr.182.3.1820741. [DOI] [PubMed] [Google Scholar]
- 43.Scheithauer BW, Woodruff JM, Erlandson RA. Miscellaneous benign neurogenic tumors: tumors of the peripheral nervous system. Washington DC: Armed Forces Institute of Pathology; 1999. pp. 219–82. [Google Scholar]
- 44.Scheller C, Richter HP, Scheuerle A, Kretschmer T, Konig RW, Antoniadis G. Intraneural perineuriomas; a rare entity. Clinical, surgical and neuropathological details in the management of these lesions. Zentralbl Neurochir. 2008;69:134–8. doi: 10.1055/s-2008-1077081. [DOI] [PubMed] [Google Scholar]
- 45.Simmons Z, Mahadeen ZI, Kothari MJ, Powers S, Wise S, Towfighi J. Localized hypertrophic neuropathy: magnetic resonance imaging findings and long-term follow-up. Muscle Nerve. 1999;22:28–36. doi: 10.1002/(SICI)1097-4598(199901)22:1<28::AID-MUS6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 46.Singh T, Kliot M. Imaging of peripheral nerve tumors. Neurosurg Focus. 2007;22:E6. doi: 10.3171/foc.2007.22.6.7. [DOI] [PubMed] [Google Scholar]
- 47.Spinner RJ, Amrami KK. What’s new in the management of benign peripheral nerve lesions? Neurosurg Clin N Am. 2008;19:517–31. doi: 10.1016/j.nec.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Spratt JD, Stanley AJ, Grainger AJ, Hide IG, Campbell RS. The role of diagnostic radiology in compressive and entrapment neuropathies. Eur Radiol. 2002;12:2352–64. doi: 10.1007/s00330-001-1256-3. [DOI] [PubMed] [Google Scholar]
- 49.Stanton C, Perentes E, Phillips L, VandenBerg SR. The immunohistochemical demonstration of early perineurial change in the development of localized hypertrophic neuropathy. Hum Pathol. 1988;19:1455–7. doi: 10.1016/S0046-8177(88)80239-9. [DOI] [PubMed] [Google Scholar]
- 50.Toussaint CP, Zager EL. What’s new in common upper extremity entrapment neuropathies. Neurosurg Clin N Am. 2008;19:573–81. doi: 10.1016/j.nec.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 51.Tranmer BI, Bilbao JM, Hudson AR. Perineurioma: a benign peripheral nerve tumor. Neurosurgery. 1986;19:134–8. doi: 10.1227/00006123-198607000-00024. [DOI] [PubMed] [Google Scholar]
- 52.Tsang WY, Chan JK, Chow LT, Tse CC. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756–63. doi: 10.1097/00000478-199208000-00003. [DOI] [PubMed] [Google Scholar]