Abstract
Dupuytren’s disease (DD) is a familial, fibroproliferative, irreversible, and progressive disease of the palmar fascia, yet with unknown etiology. However, there is compelling evidence which has consistently suggested a genetic ethiopathogenesis given the high occurrence among the Northern European extraction, familial nature, and demonstration of concordance in twins. DD is an incurable, recurrent, and potentially debilitating disease with limited and ineffective treatments. Although a number of possible candidate genes have been investigated including matrix metalloproteinases (MMPs) and transforming growth factor-beta (TGF-β) genes, as yet, no consistent genetic biomarker has been identified for DD. The highly polymorphic human leukocyte antigen (HLA) region is an ideal biomarker target. There have been some coherent data within the literature to suggest a genotype to phenotype association between certain HLA loci and a number of fibrotic disorders such as keloid and scleroderma, markedly with class II molecules and disease pervasiveness and clinical progression. The aim of this review, therefore, was to investigate the evidence indicative of both positive and negative associations between particular HLA alleles and DD. There is a clear association with specific HLA alleles and predilection or protection to DD, though there is a pressing need for further supportive data. The most promising of links to the HLA region in terms of a definitive genetic biomarker is with the class II HLA-DR loci. This paper presents a detailed account of the immunogenetic component of DD and explores the possible mechanisms of association between specific HLA molecules and susceptibility to DD.
Keywords: Dupuytren's disease, Human leukocyte antigen (HLA), Dupuytren's contracture, Cord, Nodule, Fibrosis, Collagen, Myofibroblast
Introduction
Dupuytren’s disease (DD) is thought to be one of the most common connective tissue diseases in humans [30], characterized by thickening and shortening of the palmar fascia resulting in flexion deformities of the digits [29, 64]. Early studies have shown that the contracture contains raised deposition of type III collagen [13]. In addition, studies have confirmed a proliferation of contractile fibroblasts [27] and myofibroblasts within the palmar fascia [10, 19], which are thought to direct tissue contraction and formation of the characteristic fibrous bands and nodules [19, 37] (see Fig. 3). The major cellular element of the aponeurotic tissue in DD is thought to be the myofibroblast [27, 66], with resemblance to both the fibroblast and the smooth muscle cell [37]. In recent years, there has been increasing interest into the molecular factors involved in DD initiation and progression with recent advances in molecular and cellular biology [23]. Since the initial description by Baron Dupuytren in 1831, a countless number of studies have focused on the etiology and epidemiology of the disease. However, although the disease is well characterized in terms of its histology, the pathogenesis remains uncertain. Yet, there have been several studies which have focused on the genetic and immunological implications of DD [5–9, 22, 23, 29, 30, 34, 37, 38, 40, 52, 53], with much interest into the association between certain human leukocyte antigen (HLA) alleles and disease propensity considering the immunoregulatory role of the major histocompatibility complex (MHC) [8, 15, 25, 29, 32, 55, 62, 65], (see Table 1).
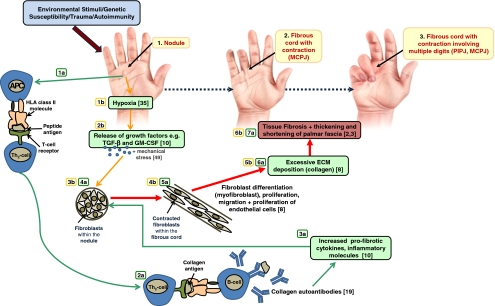
Figure 3.
Schematic diagram of Dupuytren’s disease stages. Evidence has shown that hypoxia due to environmental stimuli may lead to the characteristic increase in inflammatory mediators, including growth factors, following antigen presentation via HLA–T-cell interaction. In turn, fibroblasts differentiate and proliferate causing fibromatosis. This gives rise to fibrous cords and contracture. Autoantibodies have also been shown to be augmented in DD tissue. MCPJ metacarpophalangeal joint, PIPJ proximal interphalangeal joint. The two major pathways are autoimmunity (pathway a) and via hypoxia-mediated fibrosis (pathway b).
Table 1.
A table to show the reported HLA allelic associations with Dupuytren’s disease.
| HLA allele | Positive or negative association | Reference |
|---|---|---|
| HLA-DR3 | Positive | Neumuller et al. [29] |
| HLA-DR4 | Postive | Pereira et al. [51] |
| HLA-DR4 | Positive | Spencer and Walsh [53] |
| HLA-A1 | Positive (not statistically significant) | Spencer and Walsh [53] |
| HLA-B8 | Positive (not statistically significant) | Spencer and Walsh [53] |
| HLA-A1, B8, DR3 Haplotype | Positive | Spencer and Walsh [53] |
| HLA-DR3 (Devoid of A1 or B8) | Negative | Spencer and Walsh [53] |
| HLA-DRB1*15 | Positive | Brown et al. [54] |
DD is thought to be one of the most hereditary connective tissue disorder, with 4–6% of Caucasians susceptible to this disabling disorder [26], often causing severe disability [15]. The prevalence also greatly increases with age, with 28% of men affected being over the age of 60 [67]. Furthermore, corrective treatment or surgery is likely to only be sought from patients with significant functional impairment, which could result in an underestimation of disease prevalence. Furthermore, surgery remains the most widely used treatment strategy but with common complications in addition to a high recurrence rate [30, 39].
There is a male/female ratio as high as 7:1 [57] and a significant racial difference in prevalence [1, 18, 30, 45, 50]. In terms of the genetic aspect of the disease, it is widely accepted that individuals of Caucasian descent have a higher tendency to DD development compared to those of African origin [29, 50]. Moreover, there is a racial difference in prevalence within the Caucasian population [50] with a higher susceptibility of individuals from Scandinavian, Celtic, and Scottish descent [1]. In contrast, people of Southern Italian and Greek descent have a reduced likelihood to develop DD [50]. One explanation for this geographic distribution is the Viking invasions of the British Isles which may have contributed to the genetic element towards DD etiology [50]. As such, the disease is often referred to as the “Viking disease” [45, 50]. However, sporadic forms of the disease are far from exceptional [18, 30]. It has long been noted that a high proportion of patients have a positive family history [40] and are often susceptible to other fibrotic disorders including Ledderhose’s disease and Garrod’s nodes [22]. Furthermore, disorders such as Ledderhose’s disease are often only discovered after they become significant enough to cause pain [22]. As such, the observed link between such disorders and DD may seem less significant than expected. The disease is widely accepted as an autosomal dominant trait [8, 30, 40]. Yet, the generally late onset of symptoms [30], incomplete/variable penetrance [30, 40], and variations in disease severity [30] lead to a high number of patients lacking a positive family history [30]. As such, the genetic link with DD may well be greater than observed. This also leads to difficulties in disease gene identification [10–12].
The MHC, or HLA system, is a tightly linked cluster of genes (see Fig. 1), which regulates intercellular recognition and discrimination between self and non-self with a major role in humoral and cell-mediated immune responses [28] (see Fig. 2). The MHC is the most polymorphic genetic system in all vertebrates [15]. Since MHC molecules act as antigen-presenting complexes, the set of MHC molecules which an individual possesses determines the range of antigens to which T lymphocytes can respond [28]. As such, the MHC complex has been a widely suggested factor in disease susceptibility [28]. As an ideal approach to decipher a hereditary component to DD, a number of studies have directed their efforts at identifying susceptibility loci within the HLA system [8, 15, 25, 29, 32, 55, 62, 65].
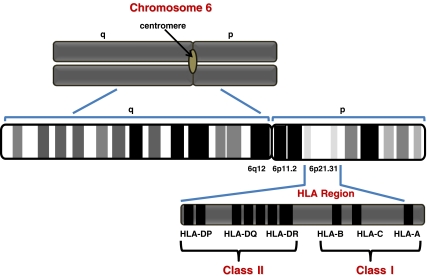
Figure 1.
Schematic diagram of the human leukocyte antigen (HLA) region on chromosome 6. The HLA region is located on the short arm of chromosome 6, at 6p21.31.
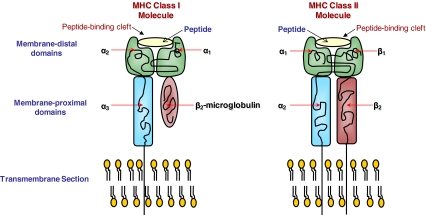
Figure 2.
Schematic diagram of classes I and II MHC structures. The peptide-binding cleft is formed by the membrane-distal domains in both MHC molecules. In class I molecules, the membrane-distal domain is composed of the α1 and α2 regions whereas in the class II molecules, the membrane-distal domain is composed of the α1 and β1 regions. In class II molecules, the β2 region also plays a part in the variation of the peptide-binding cleft regions.
The aim of this review, therefore, is to explore the current literature surrounding the area of genetic and immunological associations with DD, giving perspectives on the direction of future research in terms of HLA associations and the possible outcomes and clinical relevance of such research.
Method
Relevant research articles were identified via the systematic search of scientific search engines, specifically PubMed, Science Direct, and Scirus. A number of key search terms were used, including: Dupuytren’s disease (DD), fibrosis, human leukocyte antigen (HLA), and genetic linkage. These search terms yielded a considerable amount of literature surrounding the area of research explored in this review, which were then analyzed in terms of results, methodology, and study limitations.
Overview
Numerous genetic associations have been made between specific HLA alleles and various diseases with a suspected immunogenetic and/or a malignant etiology, including fibrotic cancers, keloid disease, sarcoidosis, scleroderma, and hypertrophic scarring. The HLA complex system is one of the most polymorphic systems in the human genome and is, therefore, an ideal target for identification of potential biomarkers of disease [28]. The source of HLA allele diversity differs from that of antibodies and T-cell receptors [28]. Diversity of antibodies and T-cell receptors is a continual process with random rearrangements and somatic mutations [28]. In contrast, MHC diversity does not change over time in an individual, but alleles may differ significantly between individuals [28], making the MHC complex an even more promising biomarker target.
Immunogenetic Basis of DD
Much focus has been placed upon the immunogenetic component of DD pathogenesis within the literature. Research has been aimed at unraveling the immunological associations with DD and identification of genetic biomarkers given the increasing plethora of evidence pointing towards a possible inherited aberrant immune response mechanism in DD pathogenesis.
DD has been linked to Peyronie’s disease (PD) [22], a similar pathological condition affecting the penile fascia [22]. The two diseases maybe seen together in the same patient [22, 25]. Connelly [22] presented a case report of a patient who presented with both PD and DD following separate respective episodes of trauma. The patient had a positive family history of DD, with a first-degree relative who developed DD at approximately the same age and in the same hand. In addition, the patient’s grandparents had immigrated from Sweden and Italy. Collaboratively, this evidence seems to suggest a genetic predisposition to DD with a common genesis of similar fibrotic disorders. It further suggests that trauma can be the initial causative event which allows for the unveiling of an inherited diathesis for both PD and DD [22].
Research has identified either positive or negative associations between DD and diabetes, epilepsy, and rheumatoid arthritis (RA) [50] along with environmental risk factors including high alcohol intake and cigarette smoking [53] (see Fig. 4). The observed link between DD and diabetes has been correlated to the microvascular related pathological basis of the disease [5], which in turn could link to the perceived immunological and possible autoimmune element of DD. With this in mind, there is a reported 16–30% increase in prevalence of DD in patients with diabetes [53, 63]. One other hypothesis which may link the suspected immunological and genetic associations with DD is that the inherited susceptibility may increase the tissue’s responsiveness to environmental stimuli [18].
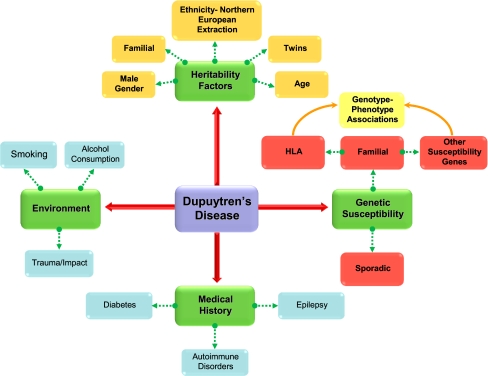
Figure 4.
Key factors contributing to Dupuytren’s disease. As well as genetic susceptibility, a number of other non-genetic factors seem to contribute to an individual’s risk of developing DD, including ethnic background, gender, age, and environmental stimuli. In terms of genetic susceptibility, both sporadic and familial cases of Dupuytren’s disease have been observed. A number of susceptibility genes have been suggested which confer genotype to phenotype associations.
In terms of the immunological features of DD, evidence has persistently suggested the involvement of both T and B lymphocytes in DD etiology [7, 38]. As such, the disease has frequently been termed a “T-cell-mediated autoimmune disorder” [6]. Baird et al [6] observed an increased HLA-DR-activated T-cell infiltrate in DD tissue compared to healthy control tissue. In addition, there are raised levels of IgM and IgA antibodies in DD tissues [34]. Furthermore, Neumuller et al. [52] noted a high number of collagen autoantibodies in DD tissue, further advocating an autoimmune mechanism.
Other genetic association studies with respect to DD susceptibility have provided varying results. Two studies by Bayat et al. [8, 9] investigated possible genetic markers for DD in the polymorphic transforming growth factor-beta 1 (TGF-β1) and transforming growth factor-beta 2 (TGF-β2) genes but found no significant associations. Other possible candidate genes have been suggested, including matrix metalloproteinases (MMPs) and nucleotide olligomerization domain/caspase recruitment domain (NOD/CARD) genes which are associated with tissue remodeling in response to trauma and inflammatory responses particularly following infection [30]. Although DD has been continually referred to as a familial trait, the late age of onset of the disease and reported non-genetic cases may impede such genetic studies [65].
Evidence that the hypodermis induces the fibrotic response [31], with observations that split skin grafts do not prevent recurrence of contractures in DD in comparison with the more successful full-thickness skin grafts [31], may suggest a trauma-induced contracture since the hypodermis fat is thought to be responsible for shock absorption [20] following skin impacts which could also influence the inflammatory response. DD has often been shown to be more common in individuals with chronic hand trauma [41, 48] which highlights the possible role of abnormal wound healing in the etiology of DD [30]. Furthermore, since surgical excision is the major method of treatment for DD, surgery itself may pose a risk for recurrence in terms of increased traumatic insult albeit on an elective basis. On the other hand, it was noted that there was a considerable time lapse between initial injuries to onset of symptoms [22], similar to that seen in keloid disease which may place some notion on the immune response and atypical inflammation theory of DD development. Additionally, the link between DD and lifestyle factors including alcohol consumption and smoking could be a result of decreased wound healing responses in such individuals [53].
A family-based linkage-mapping study conducted by Hu et al. [30] of a Swedish family with a five-generation history of DD demonstrated a link between certain wound healing genes and DD. Specifically, several MMPs and NOD/CARD genes, the latter of which are associated with inflammatory responses due to infection, have been found to be linked to an increased likelihood of DD [30].
Both topical and intra-lesional steroid treatment of DD is often successful in reducing symptoms [47, 54, 61]. Since steroid treatment can increase the rate of apoptosis of the affecting fibroblast [47] in addition to apoptosis of inflammatory cells [47], this seems to give partial evidence of an immune hallmark of DD. Additionally, the involvement of activated HLA-DR+ T cells has been linked to DD severity, since these immune cells have been shown to be increased in patients with both palmar and plantar involvement compared with patients with only palmar involvement [29]. In addition, CD-68+ cells, which are of macrophage lineage, have been identified in DD tissue [37]. Certain cytokines secreted by macrophages such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and TGF-β are known to have a role in tissue fibrosis [37]. Conversely, a significant decrease in CD5+ B cells was observed in DD tissue compared to control tissue [29]. Furthermore, autoimmune diseases are characterized by high levels of autoantibodies produced from an excess of CD5+ B cells [4]. As such, DD is rarely seen in patients suffering from RA [3]. However, the production of “natural” autoantibodies in both the case and control populations could alter the results, giving either a slightly reduced or heightened association. However, this study failed to take into account other fibrotic disorders and medical history, including additional treatment other than surgery, which may have affected the results.
Another biomolecular element associated with DD is the abnormal expression of certain growth factors which can cause abnormal autocrine control of fibroblast proliferation [37] (see Fig. 3). Such cell growth control factors could initiate the differentiation of fibroblasts to myofibroblasts and also stimulate the production of extracellular matrix (ECM) components [37], possibly including collagen. Furthermore, cytokines including transforming growth factor-beta (TGF-β), transforming growth factor-alpha (TGF-α), platelet derived growth factor (PDGF), and GM-CSF have all been linked to DD susceptibility given their role in cellular growth [23]. It seems likely that these raised levels of cytokines in DD tissue could stem from an initial immune response. Moreover, these cytokines influence proliferation of endothelial cells, an important process in tissue growth and repair [23]. Meek et al. [46] also noted that TGF-ß combined with mechanical stress (associated with DD prevalence) leads to differentiation of fibroblasts to myofibroblasts, ultimately resulting in Dupuytren’s contracture. It is also known that TGF-β is capable of inducing collagen production, as evident in DD tissue [23].
Observations of raised levels of oxygen free radicals in Dupuytren’s tissue [50] may also be suggestive of a local immune response (see Fig. 3). Hypoxia can be augmented by ischemia through the action of reactive oxygen species (ROS) [44, 58], a characteristic of Dupuytren’s tissue [50]. Furthermore, reactive oxygen intermediates can also indirectly stimulate the production of several mediators of inflammation [51]. It is speculated that the production of these free radicals causes proliferation of fibroblasts and collagen deposition which ultimately leads to tissue fibrosis [37], characteristic of DD. Furthermore, hypoxia is also related to progressive restriction of capillaries due to environmental factors such as smoking and age [23, 37], both of which are known risk factors for DD (Fig. 4). This evidence, therefore, links the free radical hypothesis of DD etiology with the immune concept.
There is also evidence that suggests a therapeutic role of agents which suppress prostaglandins in the inhibition of free radical-stimulated fibroblast proliferation [50]. This relates to the lack of DD precipitation in RA patients since these individuals often have a high intake of prostaglandin inhibitors [50] due to their role in immune regulation.
In light of such strong evidence suggestive of both a genetic and immunological mechanism involved in DD initiation and progression, a number of studies have focused upon the relationship between HLA alleles [13, 15, 25, 29, 32, 55, 62, 65], in particular the HLA class II loci and DD predisposition [15, 52, 55, 62] following early suggestions that DD may be a genetic disorder [43].
The HLA System and Fibrotic Cancers
A number of studies have identified specific HLA alleles as susceptibility markers for an increased risk of fibrotic cancers including sarcoma, breast cancer, and renal cell carcinoma. Given the immunoregulatory role of MHC molecules, it has been suggested that HLA alleles may act in the immuno-surveillance of tumors and may be related to oncogenicity [21]. One study by Mann et al. [42] focused upon sarcoma, a malignant tumor arising in supporting structures such as fibrous tissue. A positive genetic link was identified between Kaposi’s sarcoma, a type of sarcoma occurring in the skin which is associated with reduced immune function occurring in HIV-positive individuals, and HLA-B35, HLA-C4, HLA-DR1, and DQ-1 compared to a control population. Furthermore, both class I and II HLA alleles have been affiliated to prognosis and aggressiveness of breast cancer and laryngeal cancer [21]. Kaklamanis et al. [35] implicated the loss of HLA class I expression with malignant cell control and elimination [16, 35], with particular focus upon the prognosis of breast cancer, with expression of both class I and II MHC molecules related to a more advantageous differentiation and prognosis in breast tumors. Yet, Wintzer et al. [68] found no correlation between HLA class I antigenic expression and breast cancer survival. However, the study by Kaklamanis et al. [35] correlated the high incidence of HLA class I antigenic loss with lymph node metastasis in breast cancer patients when compared to primary tumors. Similarly, Cromme et al. [24] demonstrated the increased HLA antigenic loss in lymph node metastases from cervical carcinomas compared to the respective primary tumors. It has been suggested that HLA allele loss on mutated cells results in evasion of the immune system giving rise to immunoselection of such cells [14, 16]. Such findings may give rise to a possible role of the HLA system in immunomodulatory therapy for various cancers in the future in terms of anti-tumor cytotoxic lymphocyte stimulation and vaccination against cancer [16]. Schendel et al. [59] noted the promising effects of immunotherapy in metastatic renal cell carcinoma (RCC). The authors identified HLA-A2 as a MHC restriction molecule in the presentation of tumor-derived antigen to cytotoxic lymphocytes in RCC. The identification of HLA-A2 antigenic complexes which are displayed by various allogenic RCC may aid in the establishment of tumor cell lines which could be used as vaccines in the enhancement of immune responses [59].
DD and the HLA System—Possible Mechanisms of Association
Given the success of the genetic studies discussed, it seems plausible to investigate possible HLA associations with DD. Although some initial studies rejected the association between DD and class I HLA alleles [32], more recent studies have identified a positive relationship between class II alleles, in particular the HLA-DR loci [15, 52, 55, 62] (see Table 1). One study conducted by Pereira et al. [55] concluded that HLA genotype could influence collagen levels thus potentiating DD development. A high number of patients studied showed evidence of IgG antibody activity to a number of collagen sub-classes [55]. There was also an overall raised level of antibodies to denatured type II collagen; however, this observation was not significant in relation to the control population [55]. Yet, HLA-DR4 was shown to be significantly linked to antibody formation directed against collagen type II [55]. In contrast, this was not observed for patients with other combinations of HLA type and collagen antibodies [55]. Effort was made in order to reduce bias due to precipitation and nonspecific interaction of serum fibronectin (but not other possible nonspecific binding of proteins) with the ELISA assay plate used to determine the DD-associated collagen antibodies by measuring serum fibronectin levels independently for comparison [55]. In terms of the actual HLA typing method used, the method was poorly defined with no evidence of HLA allele sequencing to confirm the HLA status of each patient, a seemingly common downfall of early HLA studies. As in the study by Gudmunddson et al. [29], the results may have been affected by the presence of “natural antibodies” to collagen [29, 55] in the control group. The observed increase in antibodies to denatured type II collagen in DD have also been associated with other connective tissue disorders [55], in particular RA. However, in RA, the association is with HLA-DR7 and DR3 [36]. In contrast, Neumuller et al. [52] reported a relative risk of 2.94 of individuals with the HLA-DR3 antigen, the presence of which may confer autoimmune responses and the formation of autoantibodies against certain components of the ECM [23]. In turn, these autoantibodies may also induce the release of pro-fibrotic cytokines by immune cells, including macrophages [23]. These cytokines include TGF-β and GM-CSF which both play a role in fibromatosis [23]. However, the microlymphocytotoxicity test for HLA typing is restricted by a number of inherent limitations. This, along with the limited case numbers used, casts some doubt over the reliability of such early immunogenetic studies, validating the need for studies opting for more sophisticated allele typing techniques in terms of HLA associations with DD.
A study by Spencer and Walsh [62] presented data which suggested a relationship between HLA-DR4, A1, and B8 with DD along with possible haplotype associations. However, these findings did not achieve statistical significance. Results showed that DD patients with the HLA-DR4 antigen often had an associated B12 antigen. Furthermore, the majority of DD patients with the HLA-DR3 antigen were also positive for both the A1 and the B8 antigens. However, the incidence of A1 B8 DR3 haplotype was raised though the incidence of DR3 occurring without A1 and B8 was lowered. As such, this haplotype association with DD could confirm the link to the autoimmune aspect of DD since this haplotype is associated with a number of autoimmune disorders including scleroderma, myasthenia gravis, and chronic active hepatitis [62]. In contrast, the HLA-DR3 positive cases were A1 or B8 negative and were shown to have a possible protective role against DD. On the contrary, this evidence is somewhat doubtful given the low patient number (37) and lack of patient medical history in terms of other autoimmune/inflammatory disorders, occupational background, reliability of patient data, and the recruitment place of the control group (which may not represent the true population at risk of DD), all of which have the potential to act as significant confounding variables. Additionally, the HLA typing method was unclear with no suggestion of allele sequencing.
The recent study by Brown et al. [15] revealed a significant genetic link between HLA-DRB1*15 with a 2.3 times increased risk of DD development. This HLA locus has also been linked to susceptibility to Goodpasteur’s disease, an immunological disorder with characteristic autoantibody generation with an odds ratio (OR) of 8.5 [56]. In addition, this particular allele has been linked to an increased susceptibility to Mycobacterium leprae infection [33], an infection which causes deformity of the digits [15]. Since leprosy is characterized by a T-cell-mediated immune response [2], it seems plausible to assume both a genetic and immunological aspect in DD development. However, the study by Brown et al. [15] utilized a commercially available semi-automated typing system which, although allowed for standardization of results, does not permit the analysis of allele subgroups which may have revealed more significant associations to disease prevalence. Furthermore, although the case and control groups were larger than many other comparable studies, the case number (67) still seemed too small to substantiate an unquestionable link between DD and specific HLA alleles, especially given the linkage disequilibrium present within the HLA system.
Discussion
It is apparent from the current literature that there is a likely immune component in the pathophysiology of DD. This is possible given that progressive fibrosis is a complex interaction between molecular and cellular components [37]. In a number of similar fibrotic disorders, inflammatory mediators including macrophages are related to the production of cytokines including TGF-β and GM-CSF in the affected tissues, the presence of which results in cell proliferation, collagen production, and cell transformation, which are all characteristics of DD [37]. Furthermore, downregulation of TGF-β has proven to aid in prognosis of dermal healing given the resulting decrease in macrophages, collagen, fibronectin, and blood vessels in the affected tissues [60]. The exact ethiopathogenesis, however, remains to be fully elucidated with a general lack of conclusive evidence in terms of mechanisms of association with identified candidate genes and the immunological component of DD. This, therefore, justifies the continuing need for research in this area given the physical restrictions this disease has on the patient. The identification of definitive biomarkers could lead to a potential diagnostic, therapeutic and prognostic promise.
The considerable amount of evidence linked to the involvement of immunogenetic predisposing factors, with specific interest into candidate genes of the highly polymorphic HLA loci, in particular the HLA class II loci, seems promising in terms of therapeutic advances given the immunoregulatory role and antigen presentation capacity of these molecules.
In terms of the reported immunological component of DD, an autoimmune response to collagen seems a plausible option as explored by Neumuller et al. [51]. Also, immune cell infiltrates have been observed in the nodules of DD [34], the early stage of DD development which strongly suggests that an unregulated immune response is, in part, responsible for the initiation of the disease. The identification of the components which act to inhibit the activity of normal wound healing [49] could be of considerable value in the development of novel therapies for fibrotic disorders. With one third of Dupuytren’s contractures associated with a positive family history and a clear racial difference in prevalence [62], it is a conceivable rationale to consider an immunogenetic etiology.
There are a number of limitations which have proven to hinder immunogenetic studies in terms of disease pathogenesis. One major barrier is the lack of an animal model for DD [17]. There remain a number of problems and limitations which need to be taken into consideration during the design and conduct of research projects and in the interpretation of results to avoid the risk of missing subtle but significant clues to disease pathogenesis and to enable the appreciation of the significance of observations thus avoiding the production of spurious associations. Also, it is important to recognize the impact of linkage disequilibrium which can limit the authenticity of allelic associations. It also seems to prove more difficult to define negative allelic associations with protective effects.
In terms of future research, it seems logical to study both the genetic and immunological basis of DD to enable the development of diagnostic strategies and more appropriate intervention methods, since the majority of studies have focused on clinical trials and effectiveness of current treatments. It could also prove beneficial to utilize multicenter collaborations as most studies are limited to one clinical setting.
Justification for such future studies stems from the number of case studies showing evidence that DD often results in significant psychological and physical morbidity. Furthermore, there is a paucity of effective therapeutic options and evidence-based strategies on individual treatments. The capacity for early diagnosis through clearly defined HLA biomarkers could lead to the development of immunomodulatory therapies and would enable clinicians to generate individual treatment plans in susceptible individuals, especially those with a positive family history of DD.
Conclusion
The pathogenesis of DD is not fully understood, though clear evidence has suggested some of the key molecules involved in this complex process. Significant limitations in study design seems to have hampered the progress in this area of research and given the physical burden of DD further research into early diagnosis and prevention is surely justified. There is a clear association with specific HLA alleles and predilection or protection to DD. In particular, the class II HLA-DR loci is the most promising region for a biomarker of DD. Of importance when designing HLA typing studies is sample number, patient confounding variables, and sequencing methods to confirm allelic variants. The ability to accurately perform an assessment of an individual’s potential immunogenetic susceptibility to DD may potentially lead to a more personalized approach to their diagnostic, therapeutic and prognostic management in the future.
Acknowledgements
We would like to acknowledge the support of the following organisations for this study: NIHR (UK). In addition, we would like to specially thank the GAT family Foundation, and Steve and Kathy Fitzpatrick for generous funding and support.
Conflict of interest The authors declare no conflict of interest.
References
- 1.Aladin A, Oni JA. Bilateral Dupuytren's contracture in a black patient. Int J Clin Pract. 2001;55(9):641. [PubMed] [Google Scholar]
- 2.Anderson GA. The surgical management of deformities of the hand in leprosy. J Bone Jt Surg, Br Vol. 2006;88(3):290–294. doi: 10.1302/0301-620X.88B3.17100. [DOI] [PubMed] [Google Scholar]
- 3.Arafa M, Steingold RF, Noble J. The incidence of Dupuytren's disease in patients with rheumatoid arthritis. J Hand Surg, British and European Volume. 1984;9(2):165. doi: 10.1016/S0266-7681(84)80020-0. [DOI] [PubMed] [Google Scholar]
- 4.Arinbjarnarson S, Jonsson T, Steinsson K, et al. IgA rheumatoid factor correlates with changes in B and T lymphocyte subsets and disease manifestations in rheumatoid arthritis. J Rheumatol. 1997;24(2):269–274. [PubMed] [Google Scholar]
- 5.Arkkila PE, Kantola IM, Viikari JS. Dupuytren's disease: association with chronic diabetic complications. J Rheumatol. 1997;24(1):153. [PubMed] [Google Scholar]
- 6.Baird KS, Alwan WH, Crossan JF, Wojciak B. T-cell-mediated response in Dupuytren's disease. Lancet (British ed) 1993;341(8861):1622–1623. doi: 10.1016/0140-6736(93)90760-e. [DOI] [PubMed] [Google Scholar]
- 7.Baird KS, Crossan JF, Ralston SH. Abnormal growth factor and cytokine expression in Dupuytren's contracture. BMJ. 1993;46(5):425–428. doi: 10.1136/jcp.46.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayat A, Alansar A, Hajeer AH, et al. Genetic Susceptibility in Dupuytren's disease: lack of association of a novel transforming growth factor beta 2 polymorphism in Dupuytren's disease. J Hand Surg, Eur Vol. 2002;27(1):47. doi: 10.1054/jhsb.2001.0689. [DOI] [PubMed] [Google Scholar]
- 9.Bayat A, Watson JS, Stanley JK. Genetic susceptibility in Dupuytren’s disease: TGF-1 polymorphisms and Dupuytren’s disease. J Bone Joint Surg Br. 2002;84:211–215. doi: 10.1302/0301-620X.84B2.12083. [DOI] [PubMed] [Google Scholar]
- 10.Bayat A, Watson JS, Stanley JK, et al. Genetic susceptibility to Dupuytren disease: association of Zf9 transcription factor gene. Plast Reconstr Surg. 2003;111(7):2133. doi: 10.1097/01.PRS.0000060531.98180.32. [DOI] [PubMed] [Google Scholar]
- 11.Bayat A, Stanley JK, Watson JS, Ferguson MWJ, Ollier WER. Genetic susceptibility to Dupuytren's disease: transforming growth factor beta receptor (TGF R) gene polymorphisms and Dupuytren's disease. Br J Plast Surg. 2003;56(4):328–333. doi: 10.1016/S0007-1226(03)00176-0. [DOI] [PubMed] [Google Scholar]
- 12.Bayat A, Walter J, Lambe H, et al. Identification of a novel mitochondrial mutation in Dupuytren's disease using multiplex DHPLC. Plast Reconstr Surg. 2005;115(1):134. [PubMed] [Google Scholar]
- 13.Bazin S, Lous MLE, Duance VC, et al. Biochemistry and histology of the connective tissue of Dupuytren's disease lesions. Eur J Clin Investig. 1980;10(S1):9–16. doi: 10.1111/j.1365-2362.1980.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 14.Bodmer WF, Browning MJ, Krausa P, et al. Tumor escape from immune response by variation in HLA expression and other mechanisms. Ann NY Acad Sci. 1993;690:42–49. doi: 10.1111/j.1749-6632.1993.tb43994.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown JJ, Ollier W, Thomson W, Bayat A. Positive association of HLA-DRB1* 15 with Dupuytren's disease in Caucasians. Tissue Antigens. 2008;72(2):166. doi: 10.1111/j.1399-0039.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- 16.Browning MJ, Krausa P, Rowan A, et al. Loss of human leukocyte antigen expression on colorectal tumor cell lines: implications for anti-tumor immunity and immunotherapy. J Immunother Emphasis Tumor Immunol. 1993;14(3):163. doi: 10.1097/00002371-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bulstrode NW, Bisson M, Jemec B, et al. A Prospective randomised clinical trial of the intra-operative use of 5-fluorouracil on the outcome of Dupuytren's disease. J Hand Surg. 2004;29(1):18–21. doi: 10.1016/j.jhsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Burge P. Genetics of Dupuytren's disease. Hand Clin. 1999;15(1):63–71. [PubMed] [Google Scholar]
- 19.Childs SG. CE Dupuytren's disease. Orthop Nurs. 2005;24(2):160. doi: 10.1097/00006416-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Chouvardas VG, Miliou AN, and Hatalis MK. Tactile displays: a short overview and recent developments. In Proceedings of the ICTA. 2005. p. 246-251.
- 21.Concha A, Esteban F, Cabrera T, Ruiz-Cabello F, Garrido F. Tumor aggressiveness and MHC class I and II antigens in laryngeal and breast cancer. Semin Cancer Biol. 1991;2:47–54. [PubMed] [Google Scholar]
- 22.Connelly TJ. Development of Peyronie's and Dupuytren's diseases in an individual after single episodes of trauma: a case report and review of the literature. J Am Acad Dermatol. 1999;41(1):106. doi: 10.1016/S0190-9622(99)70415-9. [DOI] [PubMed] [Google Scholar]
- 23.Cordova A, Tripoli M, Corradino B, Napoli P, Moschella F. Dupuytren's contracture: an update of biomolecular aspects and therapeutic perspectives. J Hand Surg. 2005;30(6):557–562. doi: 10.1016/j.jhsb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Cromme FV, Bommel PF, Walboomers JM, et al. Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumours. Br J Cancer. 1994;69(6):1176. doi: 10.1038/bjc.1994.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devine CJJ, Somers KD, Jordan GH, Schlossberg SM. Proposal: trauma as the cause of the Peyronie's lesion. J Urol. 1997;157(1):285–290. doi: 10.1016/S0022-5347(01)65361-8. [DOI] [PubMed] [Google Scholar]
- 26.Early PF. Population studies in Dupuytren's contracture. J Bone Jt Surg, Br Vol. 1962;44(3):602–613. [Google Scholar]
- 27.Gabbiani G, Majno G. Dupuytren's contracture: fibroblast contraction?: An ultrastructural study. Am J Pathol. 1972;66(1):131. [PMC free article] [PubMed] [Google Scholar]
- 28.Goldsby RA, Kindt TJ, Osborne BA. Kuby immunology. New York: Freeman; 2000. p. 186. [Google Scholar]
- 29.Gudmundsson KG, Arngrimsson R, Arinbjarnarson S, Olafsson A, Jonsson T. T-and B-lymphocyte subsets in patients with Dupuytren's disease Correlations with disease severity. J Hand Surg. 1998;23(6):724–727. doi: 10.1016/s0266-7681(98)80083-1. [DOI] [PubMed] [Google Scholar]
- 30.Hu FZ, Nystrom A, Ahmed A, et al. Mapping of an autosomal dominant gene for Dupuytren's contracture to chromosome 16q in a Swedish family. Clin Genet. 2005;68(5):424. doi: 10.1111/j.1399-0004.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 31.Hueston J. The role of the skin in Dupuytren's disease. Ann R Coll Surg Engl. 1985;67(6):372. [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter T, Shanahan WR, Jr, Robertson GA, Stranc MF, Schroeder ML. The distribution of histocompatibility antigens in patients with Dupuytren's contracture. Arthritis Rheum. 1981;24(9):1218–1219. doi: 10.1002/art.1780240926. [DOI] [PubMed] [Google Scholar]
- 33.Joko S, Numaga J, Maeda H. Immunogenetics of uveitis in leprosy. Jpn J Ophthalmol. 1999;43:97–102. doi: 10.1016/S0021-5155(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 34.Jozsa L, Demel S, Pinter T, et al. Immunopathological study on palmar aponeurosis in Dupuytren's disease. Acta Histochem. 1988;83(2):153. doi: 10.1016/S0065-1281(88)80049-7. [DOI] [PubMed] [Google Scholar]
- 35.Kaklamanis L, Leek R, Koukourakis M, Gatter KC, Harris AL. Loss of transporter in antigen processing 1 transport protein and major histocompatibility complex class I molecules in metastatic versus primary breast cancer. Cancer Res. 1995;55(22):5191–5194. [PubMed] [Google Scholar]
- 36.Klimiuk PS, Clague RB, Grennan DM, et al. Autoimmunity to native type II collagen–a distinct genetic subset of rheumatoid arthritis. J Rheumatol. 1985;12(5):865. [PubMed] [Google Scholar]
- 37.Kloen P. New insights in the development of Dupuytren’s contracture: A review. Br J Plast Surg. 1999;52(8):629–635. doi: 10.1054/bjps.1999.3187. [DOI] [PubMed] [Google Scholar]
- 38.Kloen P, Jennings CL, Gebhardt MC, Springfield DS, Mankin HJ. Transforming growth factor- : possible roles in Dupuytren's contracture. J Hand Surg. 1995;20(1):101–108. doi: 10.1016/S0363-5023(05)80067-X. [DOI] [PubMed] [Google Scholar]
- 39.Krüger-Sayn M, Porzberg G, and Paschmeyer HD. Does the open palm technique for surgery of Dupuytren's contracture extend treatment and disability duration? A retrospective study. Handchirurgie, Mikrochirurgie, plastische Chirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft für Handchirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft für Mikrochirurgie der Peripheren Nerven und Gefässe: Organ der Vereinigung der Deutschen Plastischen Chirurgen. 1998;30(4):269. [PubMed]
- 40.Ling RSM. The genetic factor in Dupuytren's disease. J Bone Jt Surg, Br Vol. 1963;45(4):709–718. [PubMed] [Google Scholar]
- 41.Liss GM, Stock SR. Can Dupuytren's contracture be work-related?: review of the evidence. Am J Ind Med. 1996;29(5):521–532. doi: 10.1002/(SICI)1097-0274(199605)29:5<521::AID-AJIM12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Mann DL, Murray C, O'Donnell M, Blattner WA, Goedert JJ. HLA antigen frequencies in HIV-1-related Kaposi's sarcoma. J Acquir Immune Defic Syndr. 1990;3:S51. [PubMed] [Google Scholar]
- 43.Manson JS. Heredity and dupuytren's contraction. Br Med J. 1931;2(3678):11. doi: 10.1136/bmj.2.3678.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32(2):157–170. doi: 10.1016/S1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 45.McFarlane RM. On the origin and spread of Dupuytren's disease. J Hand Surg. 2002;27(3):385–390. doi: 10.1053/jhsu.2002.32334. [DOI] [PubMed] [Google Scholar]
- 46.Meek RMD, McLellan S, Crossan JF. Dupuytren's disease A MODEL FOR THE MECHANISM OF FIBROSIS AND ITS MODULATION BY STEROIDS. J Bone Jnt Surg. Br Vol. 1999;81(4):732–738. doi: 10.1302/0301-620X.81B4.9163. [DOI] [PubMed] [Google Scholar]
- 47.Meek RMD, McLellan S, Reilly J, Crossan JF. The effect of steroids on Dupuytren’s disease: role of programmed cell death. J Hand Surg, British and European Volume. 2002;27(3):270–273. doi: 10.1054/jhsb.2001.0742. [DOI] [PubMed] [Google Scholar]
- 48.Mikkelsen OA. Dupuytren’s disease the influence of occupation and previous hand injuries. JHand Surg, Eur Vol. 1978;10(1):1–8. doi: 10.1016/s0072-968x(78)80019-9. [DOI] [PubMed] [Google Scholar]
- 49.Muir IFK. On the nature of keloid and hypertrophic scars. Br J Plast Surg. 1990;43(1):61–69. doi: 10.1016/0007-1226(90)90046-3. [DOI] [PubMed] [Google Scholar]
- 50.Murrell GAC, Hueston JT. Aetiology of Dupuytren's contracture. ANZ J Surg. 1990;60(4):247–252. doi: 10.1111/j.1445-2197.1990.tb07362.x. [DOI] [PubMed] [Google Scholar]
- 51.Nau R, Brück W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 2002;25(1):38–45. doi: 10.1016/S0166-2236(00)02024-5. [DOI] [PubMed] [Google Scholar]
- 52.Neumüller J, Menzel J, Millesi H. Prevalence of HLA-DR3 and autoantibodies to connective tissue components in Dupuytren's contracture. Clin Immunol Immunopathol. 1994;71(2):142–148. doi: 10.1006/clin.1994.1064. [DOI] [PubMed] [Google Scholar]
- 53.Noble J, Arafa M, Royle SG, McGeorge G, Crank S. The association between alcohol, hepatic pathology and Dupuytren's disease. J Hand Surg. 1992;17(1):71. doi: 10.1016/0363-5023(92)90116-7. [DOI] [PubMed] [Google Scholar]
- 54.Pentland AP, Anderson TF. Plantar fibromatosis responds to intralesional steroids. J Am Acad Dermatol. 1985;12(1):212–214. doi: 10.1016/S0190-9622(85)80020-7. [DOI] [PubMed] [Google Scholar]
- 55.Pereira RS, Black CM, Turner SM, Spencer JD. Antibodies to collagen types I-VI in Dupuytren's contracture. J Hand Surg, Eur Vol. 1986;11(1):58. doi: 10.1016/0266-7681(86)90014-8. [DOI] [PubMed] [Google Scholar]
- 56.Phelps RG, Jones V, Turner AN, Rees AJ. Properties of HLA class II molecules divergently associated with Goodpasture's disease. Int Immunol. 2000;12(8):1135–1143. doi: 10.1093/intimm/12.8.1135. [DOI] [PubMed] [Google Scholar]
- 57.Riolo J, Young VL, Ueda K, Pidgeon L. Dupuytren's Contracture. South Med J. 1991;84(8):983. doi: 10.1097/00007611-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Sawyer RG, Spengler MD, Adams RB, Pruett TL. The peritoneal environment during infection. Ann Surg. 1991;2(1):3. doi: 10.1097/00000658-199103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schendel DJ, Gansbacher B, Oberneder R, et al. Tumor-specific lysis of human renal cell carcinomas by tumor-infiltrating lymphocytes. I. HLA-A2-restricted recognition of autologous and allogeneic tumor lines. J Immunol. 1993;151(8):4209–4220. [PubMed] [Google Scholar]
- 60.Shah M, Foreman DM, Ferguson MWJ. Control of scarring in adult wounds by neutralising antibody to transforming growth factor [beta] Lancet. 1992;339(8787):213–214. doi: 10.1016/0140-6736(92)90009-R. [DOI] [PubMed] [Google Scholar]
- 61.Shelley ED. Response of Dupuytren's contracture to high-potency topical steroid. Lancet (British ed) 1993;342(8867):366–366. doi: 10.1016/0140-6736(93)91507-i. [DOI] [PubMed] [Google Scholar]
- 62.Spencer JD, Walsh KI. Histocompatibility antigen patterns in Dupuytren's contracture. J Hand Surg, Eur Vol. 1984;9(3):276. doi: 10.1016/0266-7681(84)90041-X. [DOI] [PubMed] [Google Scholar]
- 63.Spring M, Fleck H, Cohen BD. Dupuytren's contracture. Warning of diabetes? NY State J Med. 1970;70(9):1037. [PubMed] [Google Scholar]
- 64.Tait BD, Mackay IR. HLA phenotypes in Dupuytren's contracture. Tissue Antigens. 1982;19(3):240–241. doi: 10.1111/j.1399-0039.1982.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 65.Thurston AJ. Dupuytren's disease. J Bone Jnt Surg Br. 2003;85(4):469–477. doi: 10.1302/0301-620X.85B4.14215. [DOI] [PubMed] [Google Scholar]
- 66.Tomasek JJ, Vaughan MB, Haaksma CJ. Cellular structure and biology of Dupuytren's disease. Hand Clin. 1999;15(1):21–34. [PubMed] [Google Scholar]
- 67.Williams PL, Dann J, James DCO, Timlin D. Histocompatibility antigens in subgroups of Dupuytren's contracture. Br Soc Rheum. 1983;22:60–61. doi: 10.1093/rheumatology/22.1.60-a. [DOI] [PubMed] [Google Scholar]
- 68.Wintzer HO, Benzing M, Kleist S. Lacking prognostic significance of beta 2-microglobulin. MHC class I and class II antigen expression in breast carcinomas. Br J Cancer. 1990;62(2):289. doi: 10.1038/bjc.1990.280. [DOI] [PMC free article] [PubMed] [Google Scholar]