Abstract
Purpose of Review
The purpose of this review is to identify new advances in our understanding of skeletal muscle dysfunction in patients with COPD.
Recent findings
Recent studies have confirmed the relevance of muscle dysfunction as an independent prognosis factor in COPD. Animal studies have shed light on the molecular mechanisms governing skeletal muscle hypertrophy/atrophy. Recent evidence in patients with COPD highlighted the contribution of protein breakdown and mitochondrial dysfunction as pathogenic mechanisms leading to muscle dysfunction in these patients.
Summary
Chronic Obstructive Pulmonary Disease (COPD) is a debilitating disease impacting negatively on health status and the functional capacity of patients. COPD goes beyond the lungs and incurs significant systemic effects among which muscle dysfunction/wasting in one of the most important. Muscle dysfunction is a prominent contributor to exercise limitation, healthcare utilization and an independent predictor of morbidity and mortality. Gaining more insight into the molecular mechanisms leading to muscle dysfunction/wasting is key for the development of new and tailored therapeutic strategies to tackle skeletal muscle dysfunction/wasting in COPD patients.
Keywords: COPD, systemic effects, muscle dysfunction, muscle wasting
Introduction
Chronic Obstructive Pulmonary Disease (COPD) affects approximately 280 million people worldwide(1-3), is the forth leading cause of death, claiming 2.75 million lives annually(4). COPD is a debilitating disease impacting negatively on health status and limiting the functional capacity of patients.
The largely irreversible nature of the airway obstruction defines the disease. Nevertheless, the degree of airway obstruction measured as the forced expiratory volume in the first second (FEV1) correlates poorly with the severity of symptoms, health-related quality of life (HRQoL) and survival.
Exercise intolerance is one of the main complains of COPD patients and it has been classically attributed to respiratory system constrains. However, more than 15 years ago, Killian et al(5) demonstrated that, as well as age matched controls, a large proportion of COPD patients experience leg fatigue during exercise, implying that lower limb dysfunction may contribute to reduced exercise capacity. Years later other investigators confirmed leg fatigue as an objective contributor to exercise intolerance in COPD patients(6;7)* independent of the degree of airway obstruction(8). A true dissociation between airway obstruction and exercise tolerance can be defined. Lung function deterioration after lung volume reduction surgery (LVRS) occurs faster than deterioration in exercise capacity(9). Although improved after double lung transplant, exercise tolerance does not reach normal predicted values(10;11). Exercise training improves exercise tolerance without improving lung function(12). Moreover, pulmonary rehabilitation further improves exercise tolerance following lung transplantation(13)*. All this evidence suggests that, besides lung function, peripheral and circulatory factors are critical in limiting exercise capacity.
COPD is a preventable and treatable disease that goes beyond the lungs and incurs significant systemic effects with an impact on morbidity and mortality(14). Different phenotypes of the disease can be defined, particularly associated with systemic consequences of the disease. Moreover, multidimensional grading systems that take into account not only lung function, but also parameters reflecting the patient's perception and the systemic impact of the disease, show a greater ability to predict important outcomes such as mortality, compared to lung function assessment alone(15;16)*.
Among the systemic effects of the disease, peripheral muscle dysfunction is one of the most important and is a prominent contributor to exercise limitation(8), healthcare utilization(17) and an independent predictor of morbidity and mortality(18).
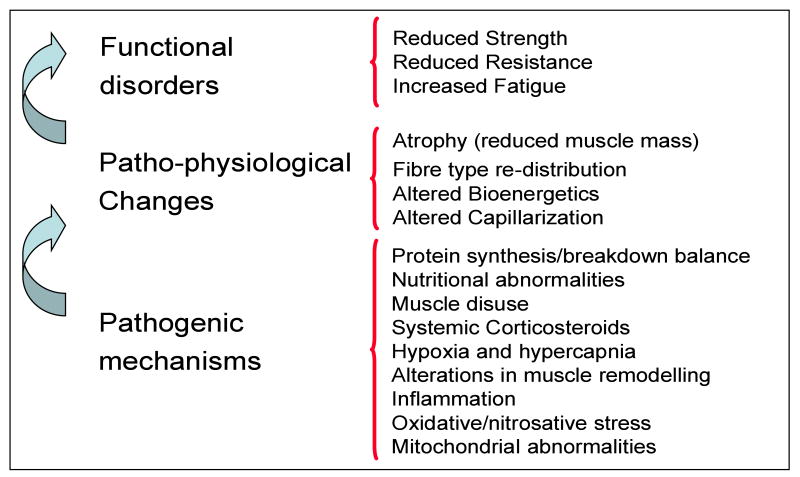
From a physiological point of view, muscle function can be defined as the ability to produce force (muscle strength), and sustain a muscle contraction for a time (muscle endurance). The latter is inversely related to muscle fatigue. Peripheral muscle dysfunction, particularly leg muscle dysfunction, has been largely demonstrated in COPD patients. Peripheral muscle strength(19-21), endurance(22-25), and fatigability(6;26) are impaired in COPD (Figure 1).
Figure 1.
Peripheral muscle dysfunction in COPD.
Skeletal muscle functional disorders and its relationship with the responsible patho-physiological changes and pathogenic mechanisms leading to muscle dysfunction/wasting in COPD patients.
Peripheral muscles patho-physiological findings
Muscle dysfunction is characterised by two related phenomena: a) malfunctioning of the muscle; and, b) net loss of muscle mass, which occurs in a subgroup of patients(18).
Skeletal muscle atrophy
Muscle mass loss is present in 18 to 36% of these patients(27;28) and is responsibly for weight loss(28) evident in 17 to 35% of COPD patients depending on the studied population(27-31). Indeed, muscle wasting is present in 6 to 21% of patients with normal weight(27-29). Moreover, muscle loss relates to muscle strength(19;32;33) and exercise tolerance(28;34-36) independent of the degree of airway obstruction(36). Hence, muscle wasting is a better predictor of health related quality of life(37) and survival(38;39) than body weight.
Net loss of muscle mass is responsible for the diminished muscle strength(40)* in these patients by a decrease in functional units available for muscle contraction.
Interestingly, when corrected by muscle mass, the differences in muscle strength between COPD patients and healthy controls vanishes(19;41). This shows that a reduced muscle mass relates to an impairment in muscle strength, but it does not account for abnormal muscle endurance(41)* which seems to be related to alterations in skeletal muscle bioenergetics. Moreover, muscle weakness can be present in early stages of the disease(42;43)* and has also been related to ACE(44) and Vitamin D(45)* genotype in these patients.
A number of patho-physiological findings responsibly for the malfunction of the muscle have been described in the peripheral muscles of patients with COPD. Most of these findings come from studies on thigh muscle biopsies from patients with COPD (Figure 1) and are described below:
Fibre type re-distribution
Peripheral skeletal muscle of patients with COPD present an increment of type II (less oxidative) fibre proportion to the detriment of type I (more oxidative) fibres(46-53). This increment of type II fibres is characterized by a rise of the number of type IIx fibres(47;49;54;55). The presence of hybrid fibres (I/IIa y IIa/IIx) has also been described, suggesting that the transformation from one fibre type to another could constitute a mechanism leading to the re-distribution of fibres seen in COPD(55).
Type IIx and hybrid fibres IIa/IIx present the highest level of atrophy(54). Since disuse-related atrophy affects mainly type I fibres(56), the prevalence of type IIx atrophy may suggest other causes of atrophy in COPD. Moreover, this kind of fibre type re-distribution has been described in association with hypoxia(57) and energy imbalance conditions such as anorexiaI(58).
Alteration in muscle bioenergetics
Several studies have demonstrated a deficit in peripheral muscle oxidative capacity in COPD patients(51;59;60) which correlates with exercise tolerance(61). Furthermore, an early lactate release during exercise has been described in these patients(62-64). This phenomenon is explained by lactate production by leg muscles and not by the respiratory muscles(65) and contributes to explain, at least in part, the exercise intolerance of COPD patients(62). The early lactate release described during exercise in COPD patients can be explained by different phenomena such as the impaired O2 delivery to the muscle, the recruitment of fibre type II, with a predominant lactate metabolism, or the diminished oxidative capacity of the muscle cell.
Alteration of the O2delivery/O2utilization relationship is associated with a lower efficiency of the muscle in these patients. Also, the relationship phoshate/phospho creatine during sub-maximal exercise is increased in the skeletal muscle of COPD patients(60). Moreover, these patients have a higher leg VO2 at comparable sub-maximal exercise loads in comparison with healthy controls(53;60), which might be explained by the higher percentage of fibre type II. There is convincing evidence that the energetic cost is elevated in the peripheral muscle of these patients(66). An increment of cytochrome oxidase activity has been described, which may contribute to the incremented VO2 described for iso-load(67).
Interestingly, it has recently been shown that skeletal muscle of patients with COPD exhibit lower Na/K ATPase activity compared to healthy controls. This may have major effects on membrane excitability and fatigability(68)*.
Alteration in muscle capillarization
Electro(69) and optic(50) microscopy studies have shown that there is a reduced capillary density in peripheral muscles of patients with COPD. Moreover, the number of contacts between capillaries and fibres it is also reduced(47;50). However, one study(53) did not show these abnormalities in the muscle capillarization, but interestingly, a large proportion of the patients included in this study had followed a pulmonary rehabilitation program. Pulmonary rehabilitation has been associated with an increase in the number of capillary-fibre contacts in patients with COPD(47). This alteration in the micro vascular bed may have an impact on the tissue oxygenation particularly in those patients presenting with continuous or intermittent hypoxemia or in situations of increased skeletal muscle oxygen demand such as during exercise.
Pathogenic mechanisms of Muscle dysfunction
Despite the relevance of skeletal muscle dysfunction in COPD, the pathogenic mechanisms of this phenomenon remain unclear. Several potential mechanisms have been related to peripheral muscle dysfunction/wasting in patients with COPD: a) protein synthesis/breakdown balance, b) nutritional abnormalities, c) muscle disuse, d) systemic corticosteroids, e) tissue hypoxia and hypercapnia, f) alterations in muscle remodelling, g) inflammation, h) oxidative/nitrosative stress; and, i) mitochondrial abnormalities. (Figure 1)
Protein synthesis/breakdown balance
Skeletal muscle mass is maintained by a delicate balance between protein synthesis and protein breakdown and experiences hypertrophy and atrophy in response to altered functional demands by adjusting either side of this equilibrium. Several studies showed an abnormal protein turnover in patients with COPD(70-72).
The signalling pathways that govern muscle hypertrophy and/or atrophy have yet to be fully defined. However, several key actors have been identified so far (Figure 2). Akt (protein kinase B), an intracellular serine/threonine protein kinase, is a central regulator of involved in the regulation of both hypertrophy and atrophy signalling pathways(73;74). Akt is activated by insulin like growth factor-1 (IGF-1) through the phosphorylation of Akt by phosphoinositide 3-kinase (PI3k) and, by inactivation of the forkhead box class O (FoxO) of transcription factors, is able to block muscle protein breakdown by down regulation of the muscle-specific E3-ligases atrogin-1 and muscle-specific RING finger protein 1 (MuRF1). Phosphorylated AKT also stimulates a variety of hypertrophic pathways, including mammalian target of rapamycin (mTOR) and glycogen synthase kinase-3beta (GSK3β). mTOR can promote protein synthesis through the activation of 70-kD ribosomal S6 protein kinase (p70S6K) and by the inhibition of eukaryotic translation initiation factor 4E binding protein-1 (4E-BP1).
Figure 2.
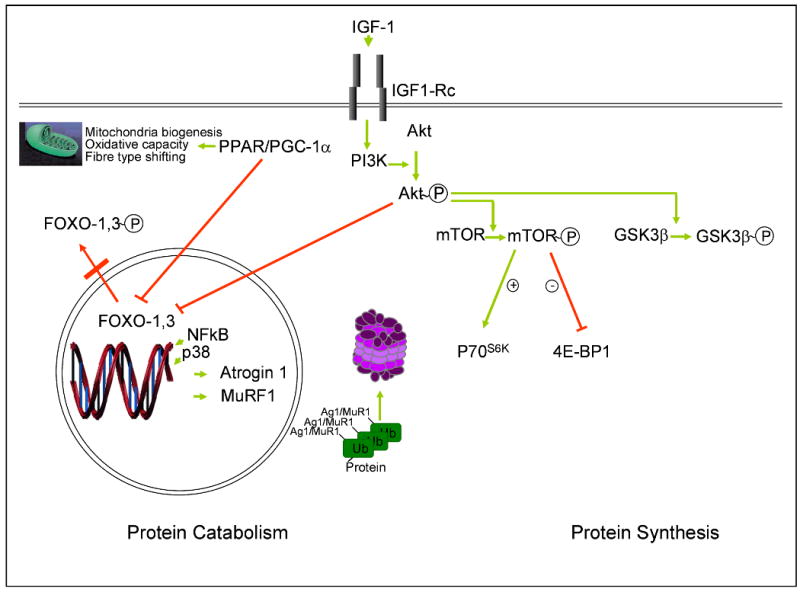
Signalling pathways that govern muscle hypertrophy and/or atrophy.
Complexity of pathways governing skeletal muscle hypertrophy and atrophy. See main text for explanation and abbreviations.
Several studies have focused on the balance between catabolic and anabolic hormones in COPD(75-78). Ubiquitin-mediated protein degradation seems to have a role in skeletal muscle protein breakdown in COPD patients. Doucet et al(79) showed increased levels of atrogin-1 and MuRF1 mRNA, and of phosphorylated AKT and 4E-BP1 and FoxO-1 proteins in skeletal muscle of patients with COPD with muscle atrophy compared with healthy control subjects, whereas atrogin-1, p70S6K, GSK3β, and FoxO-3 protein levels were similar. Patients with COPD with muscle atrophy showed an increased expression of p70S6K, GSK3β, and 4EBP1 compared with patients with COPD with preserved muscle mass. They conclude that the increase in the expression of the ligases may occur via FoxO-1 while the over expression of the muscle hypertrophic signalling pathways could represent an attempt to restore muscle mass.
Plant et al(80)* showed increased levels of atrogin-1 and Nedd4, two ligases regulating ubiquitin-mediated protein degradation, in the muscle of COPD patients compared to healthy controls. They did not find differences in the level of phosphorilation of Akt, GSK3β or p70S6K.
Nutritional depletion
Muscle wasting is the main mechanism leading to weight loss observed in patients with COPD(28). It is important to differentiate “malnutrition” from “cachexia” being the first one associated with a diminished calorie intake and a reduced basal metabolism with a good response to nutritional support and a relatively preserved muscle mass. The latter better reflects the situation of some COPD patients. A third condition, sarcopenia, has also been described in patients with COPD and consist of a the loss of muscle mass without an overall loss of weight(28;29;81).
In contrast to acute exacerbations of COPD (ECOPD), a reduction in calorie intake does not seem to be relevant in stable patients(82). However, basal metabolism is increase in patients with COPD(83), particularly in those with weight loss(84). Traditionally this increment was explained by the increased oxygen utilization by the respiratory muscles(85). Nevertheless, the increased oxygen uptake for an established workload(53;60) and the increased energy expenditure during activities of daily living(86;87) may contribute to the increase in the energy consumption.
The increase in the energy expenditure together with an unmatched calorie intake may contribute to explain the waste of muscle mass in the cachectic COPD patients(83).
Muscle disuse
Dyspnoea associated with exercise is the main complaint of patients with COPD and contributes to the sedentary habit of these patients. Activities of daily living are reduced in COPD(88-92)*. Changes in the work load of the muscles have a dramatic effect in the muscle size and metabolic capacity of the fibres(93-96). Skeletal muscle plasticity is remarkable. The fact that exercise training contributes to improved muscle function in patients with COPD, reinforces the role of muscle disuse in occurrence of skeletal muscle dysfunction in these patients(60;97;98). Moreover, several of the skeletal muscle abnormalities found in COPD patients are similar to other populations of deconditioned patients(99).
Systemic corticosteroids
Corticosteroids associated myopathy is the most common pharmacological adverse event in the muscle associated with COPD. It has been described as an acute and chronic steroid myopathy, being the first rare condition not described in patients with COPD. The chronic corticosteroid myopathy constitutes the classical condition associated to the chronic use of systemic corticosteroids. It is characterized for diffuse muscle atrophy with a prominent effect on fibre type IIx(100). There is a close relationship between the duration and doses of the treatment and the functional and structural changes(101). The use of systemic corticosteroids for relatively short periods of time does not seems to have a deleterious effect on the muscle(102), while long-term use of corticosteroids, even in low doses had significant effects in muscle strength and bulk(19).
Tissue hypoxia and hypercapnia
A chronic or intermittent alteration in arterial blood gas composition is a common feature in COPD. The deleterious effect of tissue hypoxia on the muscle is supported by several publications on healthy humans exposed to high altitude hypobaric hypoxia and animal models. Tissue hypoxia limits the production of energy and affects the protein synthesis(103) leading to muscle loss(104;105), increasees in glycolytic enzyme activity and a fall in oxidative enzymes activity(106;107). Hypoxia inhibits mitochondrial protein synthesis(108) and muscle protein synthesis reducing myosin contents(109;110) and oxidative capacity(51). Hypoxic patients have a lower proportion of type I fibres(55). Hypoxemia can also trigger other of the mentioned pathogenic mechanisms related to muscle dysfunction such as increase the levels of cytokines(111), oxidative stress(112), or reduction of anabolic hormones(113). Hypoxemia has been related to mitochondrial uncoupling and early lactate release during exercise in COPD patients (114). Hypercapnia increments the intracellular acidosis in the skeletal muscle(115) which inhibit the activity of oxidative enzymes(116) and accelerate protein degradation(117). Elevated levels of CO2 reduce the deposits of Pcr and ATP(118) in experimental models and COPD patients(119).
Alterations in muscle remodelling
Adult skeletal muscle fibres are terminally differentiated, their nuclei are post mitotic and are thus not able to replicate. Muscle injury repairs and growth (hypertrophy and hyperplasia) are accomplished by satellite cells. Satellite cells, the stem cells of adult skeletal muscle first described by Mauro in 1961(120), reside beneath the basal lamina closely juxtaposed to the muscle fibres. Satellite cells constitute around 30 % of the total population of skeletal muscle cells in newborn and 5 % in adult life. Although mitotically quiescent, they are activated and re-enter the cell cycle in response to different stimulus like stress induced by weight-bearing exercise and, trauma including injury. In recent years, the importance of satellite cells has been emphasised by the discovery that their proliferation is evoked not only by acute muscle injury but also by muscle overuse and increased muscle tension. Myogenic regulatory factors (MRFs) are part of a super family of basic helix-loop-helix (bHLH) transcription factors involved in the satellite cell differentiation process(121)*. The primary MRFs, MyoD and Myf-5, appear to be required for myogenic determination, whereas the secondary MRFs, myogenin and MRF4, are required downstream of MyoD and Myf-5 as differentiation factors (Figure 3)(122). Several animal models and cell culture studies have helped to progress the understanding of muscle repair mechanisms. Few studies assessed the molecular aspects of muscle remodelling in COPD. Plant et al(80) showed no differences in skeletal muscle expression of Myf5, MyoD or myogenin. Crul et al showed no differences in MyoD in stable COPD patients. However, patients undergoing an ECOPD present with reduced levels of MyoD compared to healthy controls(78). Vogiatzis et al(123) showed that exercise training increased the expression of MyoD in peripheral muscle of patents with COPD. Lewis et al(124) showed an increment in IGF-I protein with exercise training and a combination of exercise training and testosterone together with an increment in myogenin mRNA expression. More studies in this field are needed to clarify whether abnormalities in muscle differentiation may play a role in the muscle dysfunction/wasting occurring in these patients.
Figure 3.
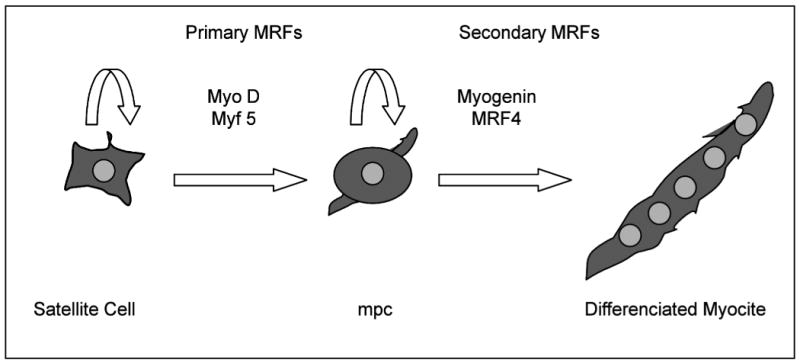
Skeletal muscle differentiation regulatory factors
Satellite cells re-enter the cell cycle in response to acute muscle injury and muscle overuse and tension. Primary (MyoD and Myf5) and secondary (Myogenin and MRF4) myogenic regulatory factors (MRFs) are required for myogenic determination (myogenic precursor cell [mpc]) and differentiation (differenciated myocite).
Inflammation
COPD is recognized as an inflammatory disease(14). Whether or not originating in the lungs, evidence of systemic inflammation in COPD has been previously shown in several studies(29;125-127). Elevated pro-inflammatory cytokines(128)* have been associated with reduced lean mass(29), muscle wasting(77), and increased rest energy expenditure(127;129). Moreover, patients who fail to gain weight in response to nutritional support present high circulating levels of TNFα(130).
The presence of local inflammation in the skeletal muscle of patients with COPD is still a controversial issue. Some studies have shown increased levels of TNFα expression in the peripheral muscle of COPD patients(131;132). Other investigators could not reproduce these findings(78;133)*. Cell culture models showed that pro-inflammatory cytokines such as TNFα induced protein breakdown and interfere with muscle differentiation process through the activation of NFkB via increased production of mitochondrial ROS(134-136). Whether this can be extrapolated to COPD patients remains to be elucidated. Interestingly, Agusti et al demonstrated an increased in NFkB-DNA binding activity in the peripheral muscle of COPD patients compared to healthy controls(137).
Oxidative/Nitrosative stress
An imbalance between oxidants and antioxidant capacity of the cells can lead to oxidative damage of protein, lipids and nucleic acids, a process known as oxidative stress. Several studies have shown increased levels of systemic(133;138-144) and local oxidative/nitrosative stress(144-148)*.
Oxidative stress can alter muscle contractility(149) potentially affecting muscle strength and contribute to muscle fatigue. The administration of antioxidants improve exercise tolerance in COPD patients(150), showing a direct effect of ROS on exercise capacity in these patients.
Oxidative stress can also contribute to accelerate protein breakdown(151-154) as a potential mechanism leading to muscle wasting(145;155). It is worthwhile to mention levels of uncoupling protein 3 in the skeletal muscle (UCP3) are reduced(156), particularly in the subgroup of patients with low BMI(114) and in the more oxidative fibres(157). UCP3 is a protein that may protect mitochondria against lipotoxicity preventing fatty acid from ROS-induced oxidative damage in cases where fatty acid influx exceeds the capacity to oxidise them(97). Moreover, UCP3 levels correlates with fat free mass (FFM) index in skeletal muscle of COPD patients(114) and may account for a reduced ability to prevent fatty acids oxidation favouring lipid peroxidation, particularly at mitochondria level.
Mitochondrial abnormalities
When compared with healthy controls, mitochondrial density is reduced in the skeletal muscle of patients with COPD(158). One study has shown that the acceptor control ratio (ACR), an index of mitochondrial complexes coupling in ex vivo mitochondria, is reduced in patients with COPD and low body mass index (BMI) compared with COPD patients with normal BMI and healthy controls(114). Moreover, the levels of ACR correlated significantly with PaO2 and with early lactate release during exercise in this population of patients(114). Interestingly, Picard et al showed a reduced mitochondrial oxygen uptake in COPD patients, however, when normalized by mitochondrial density this difference vanished(159)*. Puente-Maestu et al also showed reduced mitochondrial oxygen uptake in COPD patients with normal BMI with a reduced acceptor control ratio(160)*. These authors also showed a higher cytochrome c release in ex vivo mitochondria stimulated with H2O2(161)*. Release of cytochrome c constitutes an early event in the signalling of apoptosis. Agusti et al showed increased skeletal muscle apoptosis in patients with COPD and low BMI compared with COPD with normal BMI and healthy controls(162).
Peroxisome-proliferator-activated receptors (PPARs) and PPAR-γ co-activator 1α (PGC-1α) have been shown to be key regulators of skeletal muscle oxidative capacity(163), mitochondrial biogenesis(164) and fibre-type shifting towards more oxidative fibre(165;166). Remels et al showed reduced PPARδ protein levels and PGC1α mRNA expression in the skeletal muscle of patients with COPD(167). Moreover, cachectic COPD patients showed lower levels of PPARα mRNA expression compared to non-cachectic patients(167).
Interventions directed to improve peripheral muscle dysfunction
Without any doubt, exercise training is the most successful strategy to treat muscle dysfunction/wasting in patients with COPD(168;169). Exercise training improves exercise tolerance(170;171)* through improving muscle strength, endurance and reducing fatigue(168;169). Exercise training improves body weight through improving FFM(172), skeletal muscle oxidative capacity and fibre type distribution(60;97;173); and is clearly recommended for COPD patients with exercise intolerance independent of the degree of severity of airway obstruction(174).
Nutritional support has proven not to be effective in improving weight in patients with COPD as a group(175). However, it is worthwhile mentioning that the absence of response to nutritional support has been associated with higher levels of markers of systemic inflammation(130). When subgroups of COPD patients have been analyzed, it has been shown that nutritional support improves survival in those patients that gain more than 2 kg of weight(176) or 1 Kg.m-2 of BMI(177). In contrast to nutritional support, most trials of pharmacological anabolic replacement have documented significant improvements in muscle mass and strength(178-180). However, the absence of an impact of increased muscle mass on physiological effects such as exercise tolerance(178-182) and the fact that the use of anabolic replacement is not exempt from adverse reactions such as benign prostatism, prostate cancer, erythrocytosis and oedema (183), do not encourage the use of this therapy.
Conclusion
The systemic nature of COPD is recognized. Different phenotypes of the disease can be defined, and are particularly associated with systemic consequences of the disease. Peripheral muscle dysfunction/wasting, one of the most important systemic effects, relates to several patho-physiological findings caused by multiple pathogenic mechanisms. Recent studies in COPD highlighted the role of the ubiquitine proteasome system in the skeletal muscle protein breakdown in COPD patients. A malfunctioning of the mitochondria has also recently been identified in these patients. Exercise training constitutes the most successful strategy orientated to reverse peripheral muscle dysfunction/wasting.
Acknowledgments
The Spanish Ministry of Industry. ActivA Project: TSI-020110-2009-431. Chief Scientist Office, Scotland 06/S1103/5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 2.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 3.de Marco R, Accordini S, Cerveri I, et al. An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stages. Thorax. 2004;59(2):120–125. doi: 10.1136/thorax.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Annex Table 2 Deaths by cause, sex and mortality stratum in WHO regions, a estimates for 2002. World Health Report Statistical Annex. 2004:120–124. [Google Scholar]
- 5.Killian KJ, Summers E, Jones NL, et al. Dyspnea and leg efforts during incremental cycle ergometry. Am Rev Respir Dis. 1992;145:1339–1345. doi: 10.1164/ajrccm/145.6.1339. [DOI] [PubMed] [Google Scholar]
- 6.Mador MJ, Kufel TJ, Pineda L. Quadriceps fatigue after cycle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:447–453. doi: 10.1164/ajrccm.161.2.9904092. [DOI] [PubMed] [Google Scholar]
- *7.Gagnon P, Saey D, Vivodtzev I, et al. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol. 2009;107(3):832–840. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]; This article illustrates the importance of fatigue on exercise tolerance
- 8.Saey D, Debigare R, Leblanc P, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(4):425–430. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KR, Kazerooni EA, Curtis JL, et al. Short-term and long-term outcomes after bilateral lung volume reduction surgery : prediction by quantitative CT. Chest. 2001;119(5):1337–1346. doi: 10.1378/chest.119.5.1337. [DOI] [PubMed] [Google Scholar]
- 10.Theodore J, Robin ED, Morris AJ, et al. Augmented ventilatory response to exercise in pulmonary hypertension. Chest. 1986;89(1):39–44. doi: 10.1378/chest.89.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Williams TJ, Patterson GA, Mcclean PA, et al. Maximal exercise testing in single and double lung transplant recipients. Am Rev Respir Dis. 1992;145:101–105. doi: 10.1164/ajrccm/145.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Lacasse Y, Wong E, Guyatt GH, et al. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet. 1996;348:1115–1119. doi: 10.1016/S0140-6736(96)04201-8. [DOI] [PubMed] [Google Scholar]
- *13.Munro PE, Holland AE, Bailey M, et al. Pulmonary rehabilitation following lung transplantation. Transplant Proc. 2009;41(1):292–295. doi: 10.1016/j.transproceed.2008.10.043. [DOI] [PubMed] [Google Scholar]; This article shows the effect of pulmonary rehabilitation on exercise tolerance in lung transplant patients
- 14.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 15.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- **16.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374(9691):704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]; This article revisits the importance of a multidimensional grading system on the prognosis of COPD
- 17.Decramer M, Gosselink LE, Troosters T, et al. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J. 1997;10:417–423. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 18.Agusti AG, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 19.Bernard S, Leblanc P, Whittom F, et al. Peripheral Muscle Weakness in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 20.Man WD, Soliman MG, Nikoletou D, et al. Non-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax. 2003;58(8):665–669. doi: 10.1136/thorax.58.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Man WD, Hopkinson NS, Harraf F, et al. Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax. 2005;60(9):718–722. doi: 10.1136/thx.2005.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronell C, Orozco-Levi M, Mendez R, et al. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J. 2004;24(1):129–136. doi: 10.1183/09031936.04.00079603. [DOI] [PubMed] [Google Scholar]
- 23.Serres I, Gautier V, Varray A, et al. Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest. 1998;113(4):900–905. doi: 10.1378/chest.113.4.900. [DOI] [PubMed] [Google Scholar]
- 24.Allaire J, Maltais F, Doyon JF, et al. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax. 2004;59(8):673–678. doi: 10.1136/thx.2003.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swallow EB, Gosker HR, Ward KA, et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol. 2007;103(3):739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- 26.Mador MJ, Bozkanat E, Kufel TJ. Quadriceps fatigue after cycle exercise in patients with COPD compared with healthy control subjects. Chest. 2003;123(4):1104–1111. doi: 10.1378/chest.123.4.1104. [DOI] [PubMed] [Google Scholar]
- 27.Engelen MPKJ, Schols AMWJ, Baken WC, et al. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J. 1994;7:1793–1797. doi: 10.1183/09031936.94.07101793. [DOI] [PubMed] [Google Scholar]
- 28.Schols AMWJ, Soeters PB, Dingemans AMC, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–1156. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 29.Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(8):1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 30.Braun SR, Keim NL, Dixon RM, et al. The prevalence and determinants of nutritional changes in chronic obstructive pulmonary disease. Chest. 1984;86(4):558–563. doi: 10.1378/chest.86.4.558. [DOI] [PubMed] [Google Scholar]
- 31.Gray-Donald K, Gibbons L, Shapiro SH, et al. Effect of nutritional status on exercise performance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140(6):1544–1548. doi: 10.1164/ajrccm/140.6.1544. [DOI] [PubMed] [Google Scholar]
- 32.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–980. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 33.Engelen MPKJ, Schols AMWJ, Does JD, et al. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–738. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 34.Baarends EM, Schols AM, Mostert R, et al. Peak exercise response in relation to tissue depletion in patients with chronic obstructive pulmonary disease. Eur Respir J. 1997;10(12):2807–2813. doi: 10.1183/09031936.97.10122807. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Yoneda T, Yoshikawa M, et al. The relation of fat-free mass to maximum exercise performance in patients with chronic obstructive pulmonary disease. Lung. 2000;178(2):119–127. doi: 10.1007/s004080000014. [DOI] [PubMed] [Google Scholar]
- 36.Schols AM, Mostert R, Soeters PB, et al. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax. 1991;46(10):695–699. doi: 10.1136/thx.46.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mostert R, Goris A, Weling-Scheepers C, et al. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):859–867. doi: 10.1053/rmed.2000.0829. [DOI] [PubMed] [Google Scholar]
- 38.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 39.Mador MJ. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):787–789. doi: 10.1164/rccm.2206003. [DOI] [PubMed] [Google Scholar]
- *40.Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64(5):418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]; This article highlight the importance of muscle mass on muscle strength using a non invasive, free of radiation approach
- *41.Vilaro J, Rabinovich R, Gonzalez-deSuso JM, et al. Clinical assessment of peripheral muscle function in patients with chronic obstructive pulmonary disease. Am J Phys Med Rehabil. 2009;88(1):39–46. doi: 10.1097/PHM.0b013e31818dff86. [DOI] [PubMed] [Google Scholar]; This article shows the importance of muscle mass in strength and the independence of mass and endurance
- 42.Hopkinson NS, Tennant RC, Dayer MJ, et al. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res. 2007;8:25. doi: 10.1186/1465-9921-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Shrikrishna D, Albert P, Calverley P, et al. Quadriceps weakness in gold stage II COPD: data from the eclipse study. 2009 [Google Scholar]; This study illustrates the presence of muscle weakness in early stages of the disease
- 44.Hopkinson NS, Nickol AH, Payne J, et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):395–399. doi: 10.1164/rccm.200304-578OC. [DOI] [PubMed] [Google Scholar]
- *45.Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87(2):385–390. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]; This shows an association of common variations in the gene of Vitamin D receptor with skeletal muscle strength in COPD and healthy controls
- 46.Hughes RL, Katz H, Sahgal V, et al. Fiber size and energy metabolites in five separate muscles from patients with chronic obstructive lung diseases. Respiration. 1983;44(5):321–328. doi: 10.1159/000194564. [DOI] [PubMed] [Google Scholar]
- 47.Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 1998;30(10):1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Maltais F, Sullivan MJ, Leblanc P, et al. Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur Respir J. 1999;13(4):850–854. doi: 10.1034/j.1399-3003.1999.13d26.x. [DOI] [PubMed] [Google Scholar]
- 49.Satta A, Migliori GB, Spanevello A, et al. Fibre types in skeletal muscles of chronic obstructive pulmonary disease patients related to respiratory function and exercise tolerance. Eur Respir J. 1997;10(12):2853–2860. doi: 10.1183/09031936.97.10122853. [DOI] [PubMed] [Google Scholar]
- 50.Jobin J, Maltais F, Doyon JF, et al. Chronic obstructive pulmonary disease: capillarity and fiber characteristics of skeletal muscle. J Cardiopulm Rehabil. 1998;18:432–437. doi: 10.1097/00008483-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Jakobsson P, Jorfeldt L, Brundin A. Skeletal muscle metabolits and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J. 1990;3:192–196. [PubMed] [Google Scholar]
- 52.Hildebrand IL, Sylven C, Esbjornsson M, et al. Does chronic hypoxaemia induce transformations of fibre types? Acta Physiol Scand. 1991;141(3):435–439. doi: 10.1111/j.1748-1716.1991.tb09102.x. [DOI] [PubMed] [Google Scholar]
- 53.Richardson RS, Leek BT, Gavin TP, et al. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med. 2004;169(1):89–96. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 54.Gosker HR, Engelen MP, van Mameren H, et al. Muscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr. 2002;76(1):113–119. doi: 10.1093/ajcn/76.1.113. [DOI] [PubMed] [Google Scholar]
- 55.Gosker HR, van Mameren H, van Dijk PJ, et al. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;19(4):617–625. doi: 10.1183/09031936.02.00762001. [DOI] [PubMed] [Google Scholar]
- 56.Booth FW, Gollnick PD. Effects of disuse on the structure and function of skeletal muscle. Med Sci Sports Exerc. 1983;15(5):415–420. [PubMed] [Google Scholar]
- 57.Itoh K, Moritani T, Ishida K, et al. Hypoxia-induced fibre type transformation in rat hindlimb muscles. Histochemical and electro-mechanical changes. Eur J Appl Physiol Occup Physiol. 1990;60(5):331–336. doi: 10.1007/BF00713495. [DOI] [PubMed] [Google Scholar]
- 58.Lindboe CF, Askevold F, Slettebo M. Changes in skeletal muscles of young women with anorexia nervosa. An enzyme histochemical study. Acta Neuropathol (Berl) 1982;56(4):299–302. doi: 10.1007/BF00691262. [DOI] [PubMed] [Google Scholar]
- 59.Fiaccadori E, Del Canale S, Vitali P, et al. Skeletal muscle energetics, acid-base equilibrium and lactate metabolism in patients with severe hypercapnia and hypoxemia. Chest. 1987;92:883–887. doi: 10.1378/chest.92.5.883. [DOI] [PubMed] [Google Scholar]
- 60.Sala E, Roca J, Marrades RM, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1726–1734. doi: 10.1164/ajrccm.159.6.9804136. [DOI] [PubMed] [Google Scholar]
- 61.Maltais F, Leblanc P, Whittom F, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax. 2000;55(10):848–853. doi: 10.1136/thorax.55.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maltais F, Jobin J, Sullivan MJ, et al. Lower limb metabolic and hemodynamic responses during exercise in normal subjects and in COPD. J Appl Physiol. 1998;84:1573–1580. doi: 10.1152/jappl.1998.84.5.1573. [DOI] [PubMed] [Google Scholar]
- 63.Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 64.Patessio A, Casaburi R, Carone M, et al. Comparison of gas exchange, lactate, and lactic acidosis thresholds in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148:622–626. doi: 10.1164/ajrccm/148.3.622. [DOI] [PubMed] [Google Scholar]
- 65.Engelen MP, Casaburi R, Rucker R, et al. Contribution of the respiratory muscles to the lactic acidosis of heavy exercise in COPD. Chest. 1995;108(5):1246–1251. doi: 10.1378/chest.108.5.1246. [DOI] [PubMed] [Google Scholar]
- 66.Hunter GR, Newcomer BR, Larson-Meyer DE, et al. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. 2001;24(5):654–661. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- 67.Sauleda J, García-Palmer F, Wiesner RJ, et al. Cytochrome oxidase activity and mitochondrial gene expression in skeletal muscle of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1413–1417. doi: 10.1164/ajrccm.157.5.9710039. [DOI] [PubMed] [Google Scholar]
- *68.Green HJ, Burnett ME, D'Arsigny CL, et al. Vastus lateralis Na(+)-K(+)-ATPase activity, protein, and isoform distribution in chronic obstructive pulmonary disease. Muscle Nerve. 2009;40(1):62–68. doi: 10.1002/mus.21296. [DOI] [PubMed] [Google Scholar]; This shows changes in the catalytic activity of the Na/K pump in the vastus lateralis of patients with COPD
- 69.Simard C, Maltais F, Leblanc P, et al. Mitochondrial and Capillarity Changes in Vastus Lateralis Muscle of COPD Patients: Electron Microscopy Study. 1996:S95. [Google Scholar]
- 70.Engelen MPKJ, Deutz NEP, Wouters EFM, et al. Enhanced levels of whole-body protein turnover in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1488–1492. doi: 10.1164/ajrccm.162.4.2002045. [DOI] [PubMed] [Google Scholar]
- 71.Morrison WL, Gibson J, Scrimegeour C, et al. Muscle wasting in emphysema. Clin Sci. 1988;75:415–420. doi: 10.1042/cs0750415. [DOI] [PubMed] [Google Scholar]
- 72.Aguilaniu B, Goldstein-Shapses S, Pajon A, et al. Muscle protein degradation in severely malnourished patients with chronic obstructive pulmonary disease subject to short-term total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1992;16(3):248–254. doi: 10.1177/0148607192016003248. [DOI] [PubMed] [Google Scholar]
- 73.Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol. 2005;37(10):1985–1996. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 74.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103(1):378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 75.Creutzberg EC, Casaburi R. Endocrinological disturbances in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:76s–80s. doi: 10.1183/09031936.03.00004610. [DOI] [PubMed] [Google Scholar]
- 76.Kamischke A, Kemper DE, Castel MA, et al. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998;11(1):41–45. doi: 10.1183/09031936.98.11010041. [DOI] [PubMed] [Google Scholar]
- 77.Debigare R, Marquis K, Cote CH, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003;124(1):83–89. doi: 10.1378/chest.124.1.83. [DOI] [PubMed] [Google Scholar]
- 78.Crul T, Spruit MA, Gayan-Ramirez G, et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest. 2007;37(11):897–904. doi: 10.1111/j.1365-2362.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 79.Doucet M, Russell AP, Leger B, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(3):261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- *80.Plant PJ, Brooks D, Faughnan M, et al. Cellular Markers of Muscle Atrophy in Chronic Obstructive Pulmonary Disease (COPD) Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0382OC. [DOI] [PubMed] [Google Scholar]; This study shows the relevance of molecular pathways linked to ubiquitin proteasome in the process of muscle atrophy
- 81.Engelen MP, Wouters EF, Deutz NE, et al. Effects of exercise on amino acid metabolism in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(4):859–864. doi: 10.1164/ajrccm.163.4.2006137. [DOI] [PubMed] [Google Scholar]
- 82.Schols AM, Wouters EF. Nutritional abnormalities and supplementation in chronic obstructive pulmonary disease. Clin Chest Med. 2000;21(4):753–762. doi: 10.1016/s0272-5231(05)70182-9. [DOI] [PubMed] [Google Scholar]
- 83.Schols AMWJ, Soeters PB, Mostert R, et al. Energy balance in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143:1248–1252. doi: 10.1164/ajrccm/143.6.1248. [DOI] [PubMed] [Google Scholar]
- 84.Wilson DO, Donahoe M, Rogers RM, et al. Metabolic rate and weight loss in chronic obstructive lung disease. J Parenter Enteral Nutr. 1990;14(1):7–11. doi: 10.1177/014860719001400107. [DOI] [PubMed] [Google Scholar]
- 85.Baarends EM, Schols AMWJ, Slebos DJ, et al. Metabolic and ventilatory response pattern to arm elevation in patients with COPD and helathy age-matched subjects. Eur Respir J. 1995;8:1345–1351. doi: 10.1183/09031936.95.08081345. [DOI] [PubMed] [Google Scholar]
- 86.Baarends EM, Schols AMWJ, Pannemans DLE, et al. Total free living energy expenditure in patients with severe COPD. Eur Respir J. 1997;155:549–554. doi: 10.1164/ajrccm.155.2.9032193. [DOI] [PubMed] [Google Scholar]
- 87.Hugli O, Schutz Y, Fitting JW. The daily energy expenditure in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:294–300. doi: 10.1164/ajrccm.153.1.8542132. [DOI] [PubMed] [Google Scholar]
- 88.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 89.Singh S, Morgan MD. Activity monitors can detect brisk walking in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2001;21(3):143–148. doi: 10.1097/00008483-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Schonhofer B, Ardes P, Geibel M, et al. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10(12):2814–2819. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- *91.Walker PP, Burnett A, Flavahan PW, et al. Lower limb activity and its determinants in COPD. Thorax. 2008;63(8):683–689. doi: 10.1136/thx.2007.087130. [DOI] [PubMed] [Google Scholar]; This study confirms that activities of daily living are reduced in COPD patients and that this is related to leg activity
- 92.Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 93.Roca J, Weisman IM, Palange P, et al. In: Clinical Exercise Testing. Roca J, Whipp B, editors. 1997. pp. 88–114. [Google Scholar]
- 94.Jaspers SR, Tischler ME. Atrophy and growth failure of rat hindlimb muscles in tail-cast suspension. J Appl Physiol. 1984;57(5):1472–1479. doi: 10.1152/jappl.1984.57.5.1472. [DOI] [PubMed] [Google Scholar]
- 95.Ferrando AA, Lane HW, Stuart CA, et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270(4 Pt 1):E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 96.Gibson JN, Halliday D, Morrison WL, et al. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72(4):503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- 97.Maltais F, Leblanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1996;154:442–447. doi: 10.1164/ajrccm.154.2.8756820. [DOI] [PubMed] [Google Scholar]
- 98.Maltais F, Leblanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):555–561. doi: 10.1164/ajrccm.155.2.9032194. [DOI] [PubMed] [Google Scholar]
- 99.Franssen FM, Wouters EF, Schols AM. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clin Nutr. 2002;21(1):1–14. doi: 10.1054/clnu.2001.0485. [DOI] [PubMed] [Google Scholar]
- 100.Decramer M, De Bock V, Dom R. Functional and histologic picture of steroid-induced myopathy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:1958–1964. doi: 10.1164/ajrccm.153.6.8665061. [DOI] [PubMed] [Google Scholar]
- 101.Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11–16. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 102.Hopkinson NS, Man WD, Dayer MJ, et al. Acute effect of oral steroids on muscle function in chronic obstructive pulmonary disease. Eur Respir J. 2004;24(1):137–142. doi: 10.1183/09031936.04.00139003. [DOI] [PubMed] [Google Scholar]
- 103.Preedy VR, Smith DM, Sugden PH. The effects of 6 hours of hypoxia on protein synthesis in rat tissues in vivo and in vitro. Biochem J. 1985;228(1):179–185. doi: 10.1042/bj2280179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Green HJ, Sutton JR, Cymerman A, et al. Operation Everest II: adaptations in human skeletal muscle. J Appl Physiol. 1989;66(5):2454–2461. doi: 10.1152/jappl.1989.66.5.2454. [DOI] [PubMed] [Google Scholar]
- 105.Bigard AX, Brunet A, Guezennec CY, et al. Skeletal muscle changes after endurance training at high altitude. J Appl Physiol. 1991;71:2114–2121. doi: 10.1152/jappl.1991.71.6.2114. [DOI] [PubMed] [Google Scholar]
- 106.Howald H, Pette D, Simoneau JA, et al. Effects of chronic hypoxia on muscle enzyme activities. Int J Sports Med. 1990;11:S10–S14. doi: 10.1055/s-2007-1024847. [DOI] [PubMed] [Google Scholar]
- 107.Pastoris O, Dossena M, Foppa P, et al. Modifications by chronic intermittent hypoxia and drug treatment on skeletal muscle metabolism. Neurochem Res. 1995;20(2):143–150. doi: 10.1007/BF00970538. [DOI] [PubMed] [Google Scholar]
- 108.Kwast KE, Hand SC. Acute depression of mitochondrial protein synthesis during anoxia: contributions of oxygen sensing, matrix acidification, and redox state. J Biol Chem. 1996;271(13):7313–7319. doi: 10.1074/jbc.271.13.7313. [DOI] [PubMed] [Google Scholar]
- 109.Bigard AX, Sanchez H, Birot O, et al. Myosin heavy chain composition of skeletal muscles in young rats growing under hypobaric hypoxia conditions. J Appl Physiol. 2000;88(2):479–486. doi: 10.1152/jappl.2000.88.2.479. [DOI] [PubMed] [Google Scholar]
- 110.Rennie MJ, Edwards RH, Emery PW, et al. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clin Physiol. 1983;3(5):387–398. doi: 10.1111/j.1475-097x.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 111.Takabatake M, Nakamura H, Abe H, et al. The Relationship between Chronic Hypoxemia and Activation of the Tumor Necrosis Factor-a System in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 112.Heunks LM, Viña J, van Herwaarden CL, et al. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277(6 Pt 2):R1697–R1704. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- 113.Semple PD, Beastall GH, Watson WS, et al. Serum testosterone depression associated with hypoxia in respiratory failure. Clin Sci (Lond) 1980;58(1):105–106. doi: 10.1042/cs0580105. [DOI] [PubMed] [Google Scholar]
- 114.Rabinovich RA, Bastos R, Ardite E, et al. Mitochondrial Dysfunction in COPD Patients with low Body Mass Index. Eur Respir J. 2007;29(4):643–650. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- 115.Cechetto D, Mainwood GW. Carbon dioxide and acid base balance in the isolated rat diaphragm. Pflugers Arch. 1978;376(3):251–258. doi: 10.1007/BF00584959. [DOI] [PubMed] [Google Scholar]
- 116.Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966;241(17):4110–4112. [PubMed] [Google Scholar]
- 117.Mitch WE. Robert H Herman Memorial Award in Clinical Nutrition Lecture, 1997. Mechanisms causing loss of lean body mass in kidney disease. Am J Clin Nutr. 1998;67(3):359–366. doi: 10.1093/ajcn/67.3.359. [DOI] [PubMed] [Google Scholar]
- 118.Sahlin K, Edstrom L, Sjoholm H. Fatigue and phosphocreatine depletion during carbon dioxide-induced acidosis in rat muscle. Am J Physiol. 1983;245(1):C15–C20. doi: 10.1152/ajpcell.1983.245.1.C15. [DOI] [PubMed] [Google Scholar]
- 119.Gertz I, Hedenstierna G, Hellers G, et al. Muscle metabolism in patients with chronic obstructive lung disease and acute respiratory failure. Clin Sci. 1977;52:395–403. [PubMed] [Google Scholar]
- 120.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *121.Rudnicki MA, Le GF, McKinnell I, et al. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol. 2008;73:323–331. doi: 10.1101/sqb.2008.73.064. [DOI] [PubMed] [Google Scholar]; This study revisits the molecular pathways involved in skeletal muscle differentiation
- 122.Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57(1):16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 123.Vogiatzis I, Stratakos G, Simoes DC, et al. Effects of rehabilitative exercise on peripheral muscle TNFalpha, IL-6, IGF-I and MyoD expression in patients with COPD. Thorax. 2007;62(11):950–956. doi: 10.1136/thx.2006.069310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lewis MI, Fournier M, Storer TW, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol. 2007;103(4):1299–1310. doi: 10.1152/japplphysiol.00150.2007. [DOI] [PubMed] [Google Scholar]
- 125.Burnett D, Chamba A, Hill SL, et al. Neutrophils from subjects with chronic obstructive lung disease show enhanced chemotaxis and extracellular proteolysis. Lancet. 1987;2(8567):1043–1046. doi: 10.1016/s0140-6736(87)91476-0. [DOI] [PubMed] [Google Scholar]
- 126.Noguera A, Busquets X, Sauleda J, et al. Expression of adhesion molecules and G proteins in circulating neutrophils in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1664–1668. doi: 10.1164/ajrccm.158.5.9712092. [DOI] [PubMed] [Google Scholar]
- 127.Di Francia M, Barbier D, Mege J, et al. Tumor necrosis factor-alpha and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- *128.Piehl-Aulin K, Jones I, Lindvall B, et al. Increased serum inflammatory markers in the absence of clinical and skeletal muscle inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2009;78(2):191–196. doi: 10.1159/000207793. [DOI] [PubMed] [Google Scholar]; This study confirm the presence of elevated circulating inflammatory markers in patients with COPD
- 129.Schols AMWJ, Buurman WA, Staal van den Brekel AJDMWE. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–824. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Creutzberg EC, Schols AMWJ, Weling-Scheepers CAPM, et al. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:745–752. doi: 10.1164/ajrccm.161.3.9808075. [DOI] [PubMed] [Google Scholar]
- 131.Rabinovich RA, Figueras M, Ardite E, et al. Increased tumour necrosis factor-alpha plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J. 2003;21(5):789–794. doi: 10.1183/09031936.03.00042702. [DOI] [PubMed] [Google Scholar]
- 132.Montes de OM, Torres SH, De SJ, et al. Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J. 2005;26(3):390–397. doi: 10.1183/09031936.05.00107404. [DOI] [PubMed] [Google Scholar]
- *133.Barreiro E, Schols AM, Polkey MI, et al. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax. 2008;63(2):100–107. doi: 10.1136/thx.2007.078030. [DOI] [PubMed] [Google Scholar]; In contrast to previous publications, this study shows no differences in cytokine profile in the skeletal muscle of COPD patients compared to healthy controls
- 134.Li YP, Schwartz RJ, Waddell IA, et al. Skeletal muscle myocites undergo ptotein loss and reactive oxygen-mediated NF-kB activation in response to TNF-α. FASEB J. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 135.Li YP, Atkins CM, Sweatt JD, et al. Mitochondria mediate tumor necrosis factor-alpha/NF-kappaB signaling in skeletal muscle myotubes. Antioxid Redox Signal. 1999;1(1):97–104. doi: 10.1089/ars.1999.1.1-97. [DOI] [PubMed] [Google Scholar]
- 136.Chandel NS, Trzyna WX, McClintock DS, et al. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165(2):1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 137.Agusti A, Morla M, Sauleda J, et al. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004;59(6):483–487. doi: 10.1136/thx.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nowak D, Antczak A, Krol M, et al. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. Eur Respir J. 1996;9(4):652–657. doi: 10.1183/09031936.96.09040652. [DOI] [PubMed] [Google Scholar]
- 139.MacNee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S58–S65. doi: 10.1164/ajrccm.160.supplement_1.15. [DOI] [PubMed] [Google Scholar]
- 140.Dekhuijzen PN, Aben KK, Dekker I, et al. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154(3 Pt 1):813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 141.Thompson AB, Bohling T, Heires A, et al. Lower respiratory tract iron burden is increased in association with cigarette smoking. J Lab Clin Med. 1991;117(6):493–499. [PubMed] [Google Scholar]
- 142.Pratico D, Basili S, Vieri M, et al. Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane F2alpha-III, an index of oxidant stress. Am J Respir Crit Care Med. 1998;158(6):1709–1714. doi: 10.1164/ajrccm.158.6.9709066. [DOI] [PubMed] [Google Scholar]
- 143.Rahman I, Skwarska E, MacNee W. Attenuation of oxidant/antioxidant imbalance during treatment of exacerbations of chronic obstructive pulmonary disease. Thorax. 1997;52(6):565–568. doi: 10.1136/thx.52.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in COPD patients. Am J Respir Crit Care Med. 2003;167(12):1664–1669. doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- 145.Allaire J, Maltais F, Leblanc P, et al. Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Muscle Nerve. 2002;25(3):383–389. doi: 10.1002/mus.10039. [DOI] [PubMed] [Google Scholar]
- 146.Couillard A, Koechlin C, Cristol JP, et al. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(5):1123–1129. doi: 10.1183/09031936.02.00014302. [DOI] [PubMed] [Google Scholar]
- 147.van Helvoort HA, Heijdra YF, Heunks LM, et al. Supplemental oxygen prevents exercise-induced oxidative stress in muscle-wasted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(10):1122–1129. doi: 10.1164/rccm.200512-1957OC. [DOI] [PubMed] [Google Scholar]
- *148.Barreiro E, Rabinovich R, Marin-Corral J, et al. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax. 2009;64(1):13–19. doi: 10.1136/thx.2008.105163. [DOI] [PubMed] [Google Scholar]; This study shows enhanced oxidative stress markers in the vastus lateralis of patients with COPD
- 149.Reid MB. Plasticity in skeletal, cardiac, and smooth muscle. Invited review: Redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 150.Koechlin C, Couillard A, Simar D, et al. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2004;169(9):1022–1027. doi: 10.1164/rccm.200310-1465OC. [DOI] [PubMed] [Google Scholar]
- 151.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335(25):1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 152.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15(8):1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 153.Stadtman ER. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- 154.Barreiro E, Gea J, Corominas JM, et al. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2003;29(6):771–778. doi: 10.1165/rcmb.2003-0138OC. [DOI] [PubMed] [Google Scholar]
- 155.Barreiro E, Gea J, Matar G, et al. Expression and carbonylation of creatine kinase in the quadriceps femoris muscles of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2005;33(6):636–642. doi: 10.1165/rcmb.2005-0114OC. [DOI] [PubMed] [Google Scholar]
- 156.Gosker HR, Schrauwen P, Hesselink MK, et al. Uncoupling protein-3 content is decreased in peripheral skeletal muscle of patients with COPD. Eur Respir J. 2003;22(1):88–93. doi: 10.1183/09031936.03.00089802. [DOI] [PubMed] [Google Scholar]
- 157.Russell AP, Somm E, Debigare R, et al. COPD results in a reduction in UCP3 long mRNA and UCP3 protein content in types I and IIa skeletal muscle fibers. J Cardiopulm Rehabil. 2004;24(5):332–339. doi: 10.1097/00008483-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 158.Gosker HR, Hesselink MK, Duimel H, et al. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J. 2007;30(1):73–79. doi: 10.1183/09031936.00146906. [DOI] [PubMed] [Google Scholar]
- *159.Picard M, Godin R, Sinnreich M, et al. The mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease: disuse or dysfunction? Am J Respir Crit Care Med. 2008;178(10):1040–1047. doi: 10.1164/rccm.200807-1005OC. [DOI] [PubMed] [Google Scholar]; This study shows a reduced mitochondrial oxygen uptake in COPD patients which vanishes when normalized by mitochondrial density
- *160.Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J. 2009;33(5):1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]; This article illustrates the relevance of mitochondrial dysfunction on the skeletal muscle of COPD
- *161.Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal transition pore kinetics and cytochrome C release in muscle mitochondria of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;40(6):746–750. doi: 10.1165/rcmb.2008-0289OC. [DOI] [PubMed] [Google Scholar]; This article shows an abnormal mitochondrial pore sensitivity to hydrogen peroxide which may account for the induction of apoptosis
- 162.Agusti AG, Sauleda J, Miralles C, et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(4):485–489. doi: 10.1164/rccm.2108013. [DOI] [PubMed] [Google Scholar]
- 163.Luquet S, Lopez-Soriano J, Holst D, et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17(15):2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 164.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280(39):33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 165.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 166.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 167.Remels AH, Schrauwen P, Broekhuizen R, et al. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J. 2007;30(2):245–252. doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- 168.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 169.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 170.Lacasse Y, Brosseau L, Milne S, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(3):CD003793. doi: 10.1002/14651858.CD003793. [DOI] [PubMed] [Google Scholar]
- *171.Mador MJ, Krawza M, Alhajhusian A, et al. Interval training versus continuous training in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2009;29(2):126–132. doi: 10.1097/HCR.0b013e31819a024f. [DOI] [PubMed] [Google Scholar]; This article confirms the positive effect of exercise training on exercise tolerance
- 172.Franssen FM, Broekhuizen R, Janssen PP, et al. Effects of Whole-Body Exercise Training on Body Composition and Functional Capacity in Normal-Weight Patients With COPD. Chest. 2004;125(6):2021–2028. doi: 10.1378/chest.125.6.2021. [DOI] [PubMed] [Google Scholar]
- 173.Vogiatzis I, Terzis G, Nanas S, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128(6):3838–3845. doi: 10.1378/chest.128.6.3838. [DOI] [PubMed] [Google Scholar]
- 174.Global Initiative for Chronic Obstructive Lung Disease - Updated 2004. 2007
- 175.Ferreira IM, Brooks D, Lacasse Y, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(2):CD000998. doi: 10.1002/14651858.CD000998.pub2. [DOI] [PubMed] [Google Scholar]
- 176.Schols AMWJ. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 177.Prescott E, Almdal T, Mikkelsen KL, et al. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20(3):539–544. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 178.Burdet L, de Muralt B, Schutz Y, et al. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156(6):1800–1806. doi: 10.1164/ajrccm.156.6.9704142. [DOI] [PubMed] [Google Scholar]
- 179.Schols AM, Soeters PB, Mostert R, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–1274. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 180.Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(8):870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 181.Creutzberg EC, Wouters EF, Mostert R, et al. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124(5):1733–1742. doi: 10.1378/chest.124.5.1733. [DOI] [PubMed] [Google Scholar]
- 182.Pape GS, Friedman M, Underwood LE, et al. The effect of growth hormone on weight gain and pulmonary function in patients with chronic obstructive lung disease. Chest. 1991;99(6):1495–1500. doi: 10.1378/chest.99.6.1495. [DOI] [PubMed] [Google Scholar]
- 183.Shapiro J, Christiana J, Frishman WH. Testosterone and other anabolic steroids as cardiovascular drugs. Am J Ther. 1999;6(3):167–174. doi: 10.1097/00045391-199905000-00008. [DOI] [PubMed] [Google Scholar]