Humans have an irrational desire for medicines. The authors explain that this ‘pharmophilia' could have evolutionary roots and may have a profound impact for public health policies.
An evolutionary perspective on the human love of pills, potions and placebo
Humans love medicinal drugs; we cannot get enough. Worldwide, the amount of money spent on medicines annually is growing exponentially and is expected to reach around US$1 trillion in 2012. So far, there has been no satisfactory explanation for human ‘pharmophilia', our powerful tropism to medicines. Most studies that have attempted to provide an explanation have focused on classical supply–demand economics. Here, we suggest a different explanation: pharmophilia evolved as a means to cope with disease and sickness and is mediated through belief-induced neurological and immunological signalling pathways. Given that our love for drugs seems to be hard-wired into our biology, such an assertion has both social and economic repercussions. If public health policies do not take into account our strong pharmophilia, we will continue to overspend on and ‘over-value' drugs at the expense of non-medicinal treatments and prevention strategies. Human pharmophilia is also a threat to biodiversity; one that has already brought many animal and plant species to the brink of extinction.
If public health policies do not take into account our strong pharmophilia, we will continue to overspend on and ‘over-value' drugs…
The World Trade Organization estimates that global spending on pharmaceuticals reached US$427 billion in 2008 and, given an annual growth rate of 5.5%, projects a staggering US$929 billion in 2012. In many countries, expenditure on medicines now accounts for more than 1% of GDP (Hubbard & Love, 2004) and even were the human population to stabilize somewhere around 2050, we would still spend increasing amounts of money on medicines to improve and extend our lives. Today, most of the money spent comes from the 1.3 billion customers in the established market economies (EME), while most of the global population still rely on traditional medicines: 65% of the 6.5 billion humans on Earth depend on folk materia medica. This will change rapidly during the next 40 years. The populations of the EME—the current principal users of expensive pharmaceuticals—will ultimately account for only 11% of the global population by 2050 (UN Population Division, 2008), but the huge population expansion in middle-income countries—of up to 7.8–9 billion people—will massively increase demand for both pharmaceuticals and traditional folk medicine (Sivin, 1987). The ‘bottom billion' in low-income countries will swell to nearly 2 billion by 2050, but will continue to rely almost entirely on folk medicine.
…the huge population expansion in middle-income countries […] will massively increase demand for both pharmaceuticals and traditional folk medicine…
This extraordinary expansion has significant implications for national health-care systems, the pharmaceutical industry and billions of patients. Although the EME spend the most on pharmaceuticals in absolute terms—USA, 46.7% of total expenditure; Europe, 24.8%; and Japan, 11.3%—compared with low- to middle-income countries—Sub-Saharan Africa, 1.3%; India, 1.8%—there is a huge difference in terms of out-of-pocket expenditure on medicines. Sub-Saharan Africa and India are leading the way with 65% and 81%, respectively, compared with a maximum of 40% in some EME countries (Davis, 1997).
Rightly or wrongly, public health policies have focused on economic factors with little regard for the social, biological and cultural causes of pharmophilia—a phenomenon that has had a considerable impact beyond public health systems on both global biodiversity and traditional medicine. Intelligent public health policies and new policy frameworks that encompass traditional medicines, biodiversity and public health therefore need a better understanding of what drives our consumption of medicines. In this regard, our evolutionary past could provide an explanation to help understand our pharmophilia, as it combines an evolutionary perspective of health with the placebo effect and its underlying biology.
The earliest evidence that our ancient ancestors actively sought to improve their health dates to the Middle Palaeolithic period, some 60,000 years ago, and is based on pollen found at a Neanderthal burial site, suggesting the use of medicinal plants (Solecki & Shanidar, 1975; Lietava, 1992). We do not know whether this behaviour extends further into the past, but the finding indicates that our ancient forbears probably used natural remedies to treat injuries and disease.
In addition to the historical evidence of the use of medicinal plants, we have growing molecular and clinical knowledge of the placebo effect, which is key for explaining human pharmophilia. The word placebo actually comes from a mis-translation of the Bible by St Jerome, an Illyrian priest (Fig 1) who incorrectly translated the ninth line of Psalm 116, which should be “I will walk”, into “I will please”—placebo in Latin. The first use of placebo as a ‘dummy intervention' has been credited to the efforts of progressive Catholics in the sixteenth century attempting to discredit right-wing exorcisms (Kaptchuk et al, 2009). The modern confusion and controversy about the placebo effect in modern medicine results from the use of the term ‘placebo' to refer to an inert dummy medicine, whereas the placebo effect itself is now widely recognized as a real biological mechanism.
Health-seeking behaviour is still found across the animal kingdom and ranges from hard-wired, genetically determined behaviours to learned strategies
Figure 1.
St Jerome writing. Circa 1604 (oil on canvas), Michelangelo Merisi da Caravaggio (1571–1610). Galleria Borghese, Rome, Italy / Bridgeman, Berlin.
Notwithstanding the confusion, cultural anthropology has recognized the positive effects of placebo for more than 70 years. It was first described by the anthropologist Melville Herskovits (1948), and latter codified into a seminal article, The Powerful Placebo, by Henry Beecher (1955). Further research by anthropologists and molecular biologists revealed a unique neuroimmunological signalling and regulatory pathway, which is activated by a belief in the healing power of treatment and depends on the interaction of the patient, the medicine man and the medicine—nearly all of which include verbal communication. As Ankrah Twumasi described the Ashanti traditional system, “the positive psychological value of the medicine man as a medicine, which makes it possible for the patient to believe that he has established rapport with the “god” that controls him and contributes to his feeling of health has long been recognized” (Twumasi, 1987).
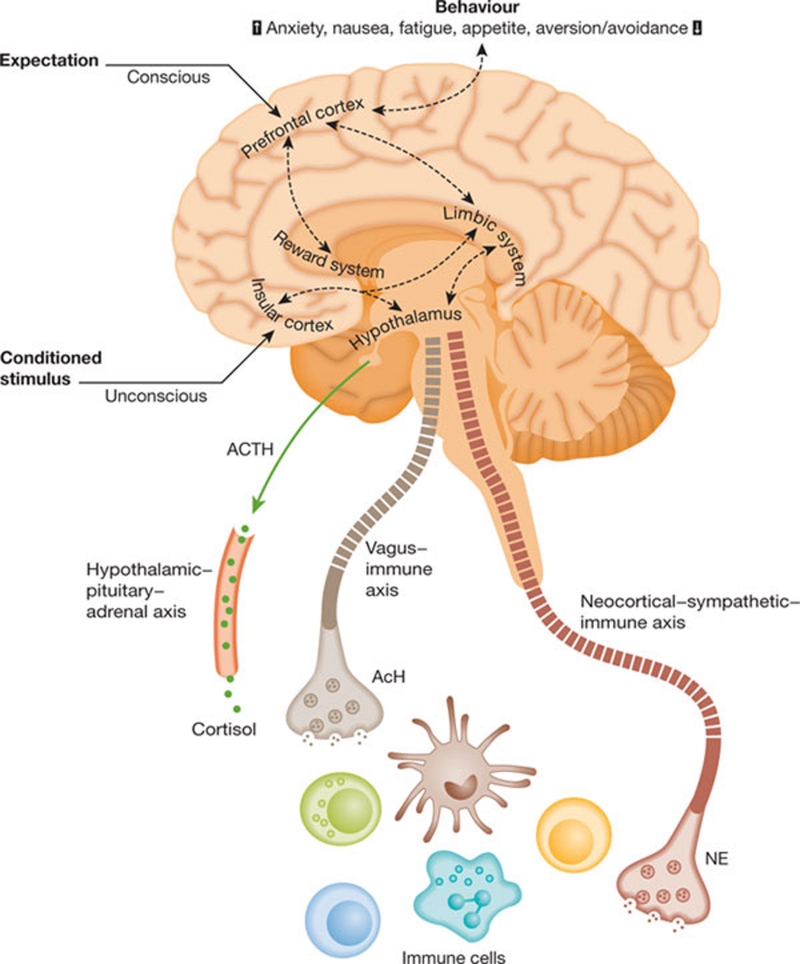
From a neurocognitive and psychophysiological stimulation perspective, the placebo effect is poorly understood. There have also been numerous claims about the efficiency of the placebo effect that have fallen foul of methodological issues and/or have wildly overestimated its potency (Hrobjartsson, 2002). However, recent evidence shows that placebo does produce changes in brain activity similar to agents that act directly on neurological pathways—such as fluoxetine to treat depression—and subsequent immunological pathways (Mayberg et al, 2002). The placebo effect thus seems to operate both by classical conditioning and through thought-induced mechanisms (Lieberman et al, 2004) in cortical areas that generate and maintain cognitive experience through dopaminergic reward pathways. Indeed, pharmacological and psychostimulation are both able to yield similar neuroimmune changes (Fig 2; Faria et al, 2008). This evidence from the neurological and cognitive sciences provides a plausible mechanism for our tropism towards medicines. Irrespective of the real potency of any ingested medicines, a sufficient ‘thought-induced' belief in their efficiency activates pathways that, in turn, generate a demonstrable biological effect.
…evidence from field and laboratory studies demonstrate that human tropism towards medicines is not a recent social phenomenon, but has old evolutionary roots
Figure 2.
Neurobiology and immunobiology of the placebo effect. Adapted from Pacheco-Lopez et al (2006).
From a systems perspective, the placebo effect is a highly flexible neuroimmunological system. Multiple integrating pathways coalesce into a common ‘mission-critical' system—in this case the neuroimmunological axis, an evolutionarily conserved pathway that is essential for the functioning of the organism—that can be fired up in response to a noxious insult. This biological system fits with the anthropologists' view that, “the value of medicines seems to be based on a perception of them as having an inherent power to heal” (van der Geest & Whyte, 1989).
Indeed, if we look at the placebo effect from an evolutionary perspective, its evolution and impact on our species makes even more sense. First, humans did not evolve in the presence of highly efficient medicines—most were developed only within the past few decades. Second, our ancestors obviously ingested pharmacological agents, mostly from plants, which are much less potent than today's drugs, but might still have had a mild effect. Finally, we know from a systems perspective that diversity builds resilience. Therefore, we suggest that evolution would have favoured a web of diverse signalling pathways, such as the neuroimmunological axis, to increase resilience and adaptability and help us to heal ourselves.
Put another way, as Gustavo Pacheco-Lopez and colleagues eloquently summarize in their extensive review of the neurobiology of immunomodulatory placebo effects: “Placebo effects can, therefore, benefit end organ functioning and the overall health of the individual through the healing power of belief, positive expectations and conditioning processes” (Pacheco-Lopez et al, 2006). But how then has the placebo effect arisen? Or put another way, what is the ultimate causation of this proximate mechanism? (Tinbergen, 1972).
To address this question we need to look at evidence from comparative biology to ascertain the evolutionary origins of pharmophilia. In fact, our species is not the only one that uses proto-medicines. Health-seeking behaviour is still found across the animal kingdom and ranges from hard-wired, genetically determined behaviours to learned strategies. At one end of the spectrum, eusocial organisms, such as wood ants, incorporate conifer resin into their nests, which inhibits the growth of a wide range of pathogenic organisms. Medicinal strategies such as geophagy—the consumption of soil and charcoal to detoxify poisonous substances (Struhsaker et al, 1997)—also appear in a wide range of species, from parrots and new-world monkeys to apes such as gorillas and humans. Some of these behaviours might actually be feeding strategies to eat plants with high levels of phenols, which would otherwise be poisonous, or they might be learned strategies to cope with gastric problems after the accidental ingestion of a toxin. Either way, geophagy has been observed across a broad range of taxa, including species that we do not usually consider highly ‘intelligent'. Indeed, there is now good experimental evidence that sheep actively medicate themselves with tannins to control parasites (Lisonbee et al, 2009). The point is that proto-medicine-seeking behaviour appears in two species—sheep and man—that shared a common ancestor around 100 million years ago.
The supposed schism between prevention and treatment might simply be a reflection of our deep-seated pharmophilia
However, it is species with higher intelligence that provide the most compelling evidence for the evolutionary roots of pharmophilia. Over the past two decades, Michael Huffman and colleagues have investigated the use of plants with medicinal properties by other species, in particular non-human primates. Through field studies and the observation of captive primates, they found that bonobos and chimpanzees—our closest living relatives with whom we shared a common ancestor around 6–7 million years ago—use herbaceous leaves such as Desmodium gangeticum for their phytochemical properties, or rough hispid leaves as a mechanical device by which to rid themselves of parasitic infections such as the worm Oesophagostomum stephanostomum (Huffman & Hirata, 2004; Fowler et al, 2007; Dupain et al, 2002). A recent field study of bonobos in Wamba, Congo, observed febrile, clearly sick adults eating an unidentified species of Manniophyton, known locally as Lukosa (Fig 3); it is a plant that is used in many traditional medicines to control fever.
Figure 3.
Bonobo (Pan paniscus) in the wild. The inset shows Manniophyton fulvum.
These learned medicinal behaviours are not unique to higher primates. In South Africa, sick Knysa elephants seek out and eat specific types of medicinal mushroom known for their immunostimulatory effects. The fact that these bracket tree fungi are extremely bitter and are not part of the elephants' normal diet suggests strongly that this is medicine-seeking behaviour (Patterson, 2004). Together, this evidence from field and laboratory studies demonstrates that human tropism towards medicines is not a recent social phenomenon, but has old evolutionary roots.
Pharmophilia has profound implications for public policy. In fact, the understanding that the placebo effect probably developed from proto-medicine-seeking behaviour millions of years ago among a range of animal species provides a novel framework to understand why medicines are globally ‘over-valued'. So far, the medicalization of health has been seen almost exclusively as an issue of supply—that is, the promotion of medicines and the medicalization of disease by society and the pharmaceutical industry. Yet, ‘value' is a complex multidimensional concept that incorporates sociocultural, political and economic parameters. From an evolutionary and psychological perspective, pharmophilia is therefore likely to contribute substantially to increased expenditures across most therapeutic categories of pharmaceutical products.
Public health policy and the economic analysis of pharmaceuticals has largely explained our use of medicines to treat illness in terms of rational factors, such as medical needs, patterns of care, access to technology, marketing forces, pricing and costs. However, neither of the two standard views of rational behaviour—‘consistent choice' or ‘self-interest maximization'—has been able to provide an adequate representation of rationality or of the actual situation, according to the Indian economist Amartya Sen (Sen, 2009). Perhaps the answer lies in pharmophilia, which operates through both the supply and demand side of medicines and creates the uncertainty that current rational behaviour models find so difficult to predict.
The ongoing demand for TM […] is accelerating the loss of biodiversity and pushes many plant and animal species close to extinction
If we include pharmophilia into the analysis, neither the consumer nor the supplier acts rationally—both are driven by our evolutionary desire to seek medicines. If this is really the case, unregulated supply and demand will continue to feed on each other to create an ever-increasing spiral of consumption and costs.
Current public policy approaches should take pharmophilia into account. Regulation is therefore the only efficient method of controlling the use of medicines by attempting to reduce demand; perhaps by controlling direct-to-consumer advertising and accepting that people will not act rationally within the context of health and medication. The assumption that a rational, logical argument can lead to a down-valuation of medicines is, according to this view, wrong. The supposed schism between prevention and treatment might simply be a reflection of our deep-seated pharmophilia. As such, extensive public debate about the need to shift public health policies from treatment to prevention will change little.
Pharmaceutical public policy should turn this view around and regard the placebo effect as an ally of the medicalization of health. As Peter Davis, a medical sociologist at the University of Auckland in New Zealand, has argued, the use of medicines is a “visible expression of concern”; it is the ‘total drug effect' that helps to increase the well-being of the patient (Davis, 1997). Although this seems initially to be a rather weak argument, closer inspection reveals that interaction with a doctor and the giving and receiving of medicines clearly does increase well-being. The unfettered popularity of complementary and alternative medicine (CAM)—or rather integrative medicine, as it is now called—is a case in point. While orthodox medicine has been constantly rallying against CAM, all evidence suggests that this has been a Canute-like reaction, a tide we cannot hold back. Despite the pronouncements of eminent scientists and many clinical trials, most of which show modest or no effect, the uptake of such practices is increasing. Cultural arguments that this is filling a holistic lacunae might be partly true, but it does not explain why so many patients believe in the benefits of CAM. The concept of pharmophilia would comfortably explain this apparent mismatch between CAM and patients' beliefs.
However, in both cases—pharmaceuticals and CAM—the problem is not so much the concept as the cost and the potential for harm, both of which need to be managed from a public policy perspective. Surveys in developing and middle-income countries by the World Health Organization and Health Action International have shown that 90% of the population in these countries purchase drugs through out-of-pocket payments, which makes medicines the biggest family expenditure after food (Cameron et al, 2009). The mantra of prevention, public health, non-pharmaceutical interventions, and the doctrine of ‘global public good'—that is, health policy responding to the objectively greatest need—might be intellectually satisfying, but it clearly does not reflect reality and the future trajectory of the continuing ‘pharmaceuticalization' of disease in these countries (Smith & Mackellar, 2007).
The great gap between prevention and cure is not simply a matter of history but a fundamental aspect of our evolution. Public policy cannot expect a rational choice based on utility when our evolved psychologies have such a strong tropism for medicines (Sen, 2009). Recognition of this sheds new light on the issue of how we promote medicines and in particular how we regulate or accept direct-to-consumer advertising, one of the most contentious battlegrounds in market economies. By ‘over-valuing' medicines, unconstrained public policies in favour of drugs and medicines will have two effects: first, they will further drive up expenditure beyond rational-use limits; second, they will under-value the contribution towards health and disease management of prevention and non-medicinal modalities, such as surgery. The nature of human pharmophilia suggests that continued stringent controls on advertising and more thoughtful rational approaches to cost-effectiveness analyses need to come from public policy as they are unlikely to arise through market forces.
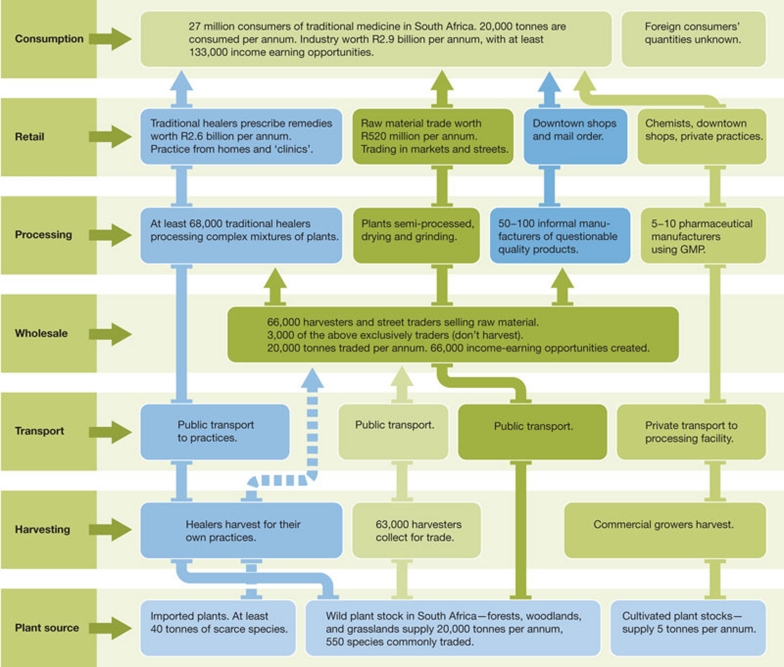
Pharmaceuticals represent one end of the spectrum in terms of human medicines. However, the most abundant usage of medicines by far, now and in the future, is traditional medicine (TM). This pharmacopoeia of folk medicine, as well as organized TM systems such as Ayurvedic and Chinese medicine, contains hundreds of thousands of plants, animal, mineral and other substances (Alves & Rosa, 2007). TM dominates health care outside high-income countries and has an increasing role in complementary and/or integrative systems in developed countries (Fig 4). The World Bank estimates that the ratio of those trained in Western medicine to TM practitioners in various African countries is between 1:1,639 in urban South Africa to 1:50,000 in Malawi and Mozambique (Cunningham, 1993). Higher resolution studies, for example in South Africa, estimate that about 5.6% of the national health budget is spent on TM; much more, however, comes from out-of-pocket payments.
…any public policies to address the health situation in both affluent and developing countries can only be successful if they take into account the human factor
Figure 4.
The South African medical plants industry. Adapted from Mander et al (2007). GMP, good manufacturing process.
It is not only the cost that is at issue here. The ongoing demand for TM, a product of both population growth and increasing per capita purchasing power, coupled with a loss of habitats through climate change, over-usage, deforestation and other factors, is accelerating the loss of biodiversity and pushes many plant and animal species close to extinction. For example, some 200 animal and 550 plant species are actively traded in KwaZulu-Natal (South Africa); 60% of these are now reported as scarce (Mander et al, 2007). Population increases in Asia and Africa with unconstrained demand for TM coupled to non-sustainable habitat loss is a massive threat to biodiversity. The focus of the Convention on International Trade in Endangered Species and other bodies on critical species represents only the tip of the iceberg and public policy has only recently realized the extent of the problem. While problems such as deforestation and habitat loss have attracted public notice and led to public policies to alleviate these, the issue of how to provide sustainable TM for populations in much of Africa and Asia has received scant attention. Integrating TM into public health systems with policy approaches centred on conservation is a huge challenge, in particular because TM remains a totally unregulated arena. However, it is essential that countries that are dependent on TM as a source of health care urgently address the problem. It is only within these nations that effective measures can be taken.
More generally, though, any public policies to address the health situation in both affluent and developing countries can only be successful if they take into account the human factor. Our pharmophilia is a deeply engrained behaviour and an important aspect of our health and well-being. It needs to be better understood and incorporated into global health policy frameworks.
Isabel Behncke
Richard Sullivan
Arnie Purushotham
Acknowledgments
We thank R. Dunbar and M. Ridely for kindly reviewing earlier proofs; Bank Bice and Planet Heritage (I.B.); European Cancer Research Managers Foundation (R.S.); and the Department of Health owing to the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust (A.D.P.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Alves RRN, Rosa IML (2007) Biodiversity, traditional medicine and public health: where do they meet? J Ethnobiol Ethnomed 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher HK (1955) The powerful placebo. JAMA 159: 1602–1606 [DOI] [PubMed] [Google Scholar]
- Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R (2009) Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet 373: 240–249 [DOI] [PubMed] [Google Scholar]
- Cunningham AB (1993) African Medicinal Plants: Setting Priorities at the Interface Between Conservation and Primary Health Care. Working Paper 1. Paris, France: UNESCO [Google Scholar]
- Davis P (1997) Managing Medicines: Public Policy and Therapeutic Drugs. Buckingham, UK: Open University Press [Google Scholar]
- Dupain J, Elsacker LV, Nell C, Garcia P, Ponce F, Huffman MA (2002) New evidence for leaf swallowing and Oesophagostomum infection in bonobos (Pan paniscus). Int J Primatol 23: 1053–1062 [Google Scholar]
- Faria V, Fredrikson M, Furmark T (2008) Imaging the placebo response: a neurofunctional review. Eur Neuropsychopharmacol 18: 473–485 [DOI] [PubMed] [Google Scholar]
- Fowler A, Koutsioni Y, Siommer V (2007) Leaf-swallowing in Nigerian chimpanzees: evidence for assumed self-medication. Primates 48: 73–76 [DOI] [PubMed] [Google Scholar]
- Herskovits MJ (1948) Man and His Works. New York, NY, USA: Alfred A Knopf [Google Scholar]
- Hrobjartsson A (2002) What are the main methodological problems in the estimation of the placebo effects? J Clin Epidemiol 55: 430–435 [DOI] [PubMed] [Google Scholar]
- Hubbard T, Love J (2004) A new trade framework for global healthcare R&D. PLoS Biol 2: E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman MA, Hirata S (2004) An experimental study of leaf swallowing in captive chimpanzees: insights into the origin of a self-medicative behavior and the role of social learning. Primates 45: 113–118 [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kerr CE, Zanger A (2009) Placebo controls, exorcisms, and the devil. Lancet 374: 1234–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA (2004) The neural correlates of placebo effects: a disruption account. Neuroimage 22: 447–455 [DOI] [PubMed] [Google Scholar]
- Lietava J (1992) Medicinal plants in a Middle Paleolithic grave Shanidar IV? J Ethnopharmacology 35: 263–266 [DOI] [PubMed] [Google Scholar]
- Lisonbee LD, Villalba JJ, Provenza FD, Hall JO (2009) Tannins and self-medication: implications for sustainable parasite control in herbivores. Behav Processes 82: 184–189 [DOI] [PubMed] [Google Scholar]
- Mander M, Ntuli L, Diedericks N, Mavundla K (2007) Economies of the traditional medicine trade in South Africa. In South African Health Review 2007, pp 189–200. Durban, South Africa: Health Systems Trust [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA (2002) The functional neuroanatomy of the placebo effect. Am J Psychiatry 159: 728–737 [DOI] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Engler H, Niemi M-B, Schedlowski M (2006) Expectations and associations that heal: immunomodulatory placebo effects and its neurobiology. Brain Behav Immun 20: 430–446 [DOI] [PubMed] [Google Scholar]
- Patterson G (2004) Knysa elephants and the medicinal mushrooms: a case of self-mediciation that has contributed to the survival of a relic elephant population. Discov Innov 16: 1–4 [Google Scholar]
- Sen A (2009) The Idea of Justice. London, UK: Allen Lane [Google Scholar]
- Sivin N (1987) Traditional Medicine in Contemporary China. Ann Arbor, MI, USA: University of Michigan Center for Chinese Studies [Google Scholar]
- Smith RD, Mackellar L (2007) Global public goods and the global health agenda: problems, priorities and potential. Global Health 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki R, Shanidar IV (1975) Neanderthal flower burial in northern Iraq. Science 190: 880–881 [Google Scholar]
- Struhsaker TT, Cooney DO, Siex K (1997) Charcoal consumption by Zanzibar red colobus monkeys: its function and its ecological and demographic consequences. Int J Primatol 18: 61–72 [Google Scholar]
- Tinbergen N (1972) The Croonian lecture, 1972: functional ethology and the human sciences. Proc R Soc Lond B Biol Sci 182: 385–410 [DOI] [PubMed] [Google Scholar]
- Twumasi PA (1987) Ashanti traditional medicine. Transition 41: 50–63 [Google Scholar]
- UN Population Division (2008) World Population Prospectus. New York, NY, USA: United Nations, Department of Economic and Social Affairs [Google Scholar]
- van der Geest S, Whyte SR (1989) The charm of medicines: metaphors and metonyms. Med Anthropol Q 3: 345–367 [Google Scholar]