Cables1 protects p63 from proteasomal degradation to ensure deletion of cells after genotoxic stress
Cables1 interacts with TAp63 to protect it from ubiquitin-mediated degradation after exposure to genotoxic stress. Oocytes lacking Cables1 accumulate less phosphorylated TAp63 in response to genotoxic stress, which enhances the survival of these cells.
Keywords: p63, Cables1, proteasome, oocyte, apoptosis
Abstract
The p63 gene product regulates epithelial morphogenesis and female germline integrity. In this study, we show that cyclin-dependent kinase 5 and Abl enzyme substrate 1 (Cables1) interacts with the trans-activating (TA) p63α isoform to protect it from proteasomal degradation. Using the female germline of Cables1-null mice as an in vivo model, we demonstrate further that oocytes lacking Cables1 exhibit lower basal levels of TAp63α and reduced accumulation of phosphorylated TAp63α in response to genotoxic stress. This in turn enhances the survival of these cells after ionizing radiation exposure. Thus, Cables1 modulates p63 protein stability and function during genotoxic stress.
Introduction
Members of the p53 family mediate genotoxic stress responses in cells (Vogelstein et al, 2000; Yang & McKeon, 2000; Moll & Slade, 2004; Westfall & Pietenpol, 2004). The identification of p63 and p73 greatly expanded the appreciation of the importance of p53 family members, not just for genome surveillance but also for coordinating normal development (Yang & McKeon, 2000; Moll & Slade, 2004; Westfall & Pietenpol, 2004). The p63 protein shares several conserved regions found in all p53 family members, including a trans-activating (TA) domain, a DNA-binding domain (DBD) and an oligomerization domain (Yang et al, 1998). The p63 gene is transcribed from two alternative transcription start sites that yield TA domain-containing isoforms and ΔN isoforms, the latter of which lack the TA domain and might act as dominant negatives of TAp63 isoforms (Yang et al, 1998; Petitjean et al, 2008).
Recent evidence indicates that regulation of protein stability is probably the principal mechanism by which the actions of p63 are controlled. For example, genotoxic stress-induced phosphorylation of p63 prevents its ubiquitination and degradation by the proteasome (Rossi et al, 2006; Li et al, 2008; MacPartlin et al, 2008). However, molecular modulators underlying these events remain poorly described. In addition, genetic models demonstrating the functional importance of p63 stability in vivo are lacking. In this study, we show that cyclin-dependent kinase (Cdk) 5 and Abl enzyme substrate 1 (Cables1), a Cdk-interacting protein (Zukerberg et al, 2000; Wu et al, 2001), protects p63 from ubiquitin-mediated proteasomal degradation through direct physical interaction. Using female germ cells lacking Cables1 as an in vivo model, we show that radiation-induced oocyte loss is partly rescued through reduced accumulation of phosphorylated TAp63α. These results identify a new regulatory step modulating p63 function in vivo and provide further insights into mechanisms that govern female germline integrity.
Results And Discussion
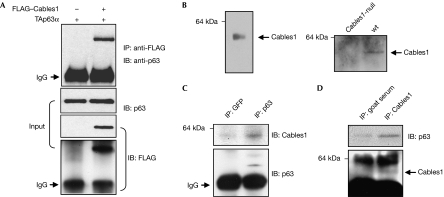
Cables1 interacts with both p53 and p73 (Tsuji et al, 2002) and modulates the activity of female germline stem cells and oocyte quality (Lee et al, 2007). We therefore felt it was reasonable to consider that p63, which is expressed predominantly in female germ cells, is regulated by direct interaction with Cables1. To test this, we first co-expressed TAp63α and FLAG-tagged Cables1 in COS7 cells, and lysates were immunoprecipitated. Cables1 was detected in immunocomplexes containing TAp63α, indicative of an interaction between the two proteins (Fig 1A). To determine whether endogenous TAp63α normally interacts with endogenous Cables1, we analysed lysates of neonatal (postnatal day 10; P10) mouse ovaries, which contain a large number of meiotically arrested oocytes that express both proteins (Fig 1B; Suh et al, 2006; Lee et al, 2007). The co-immunoprecipitation analysis revealed that Cables1 was present in the TAp63α immunocomplex and, reciprocally, TAp63α was present in the Cables1 immunocomplex (Fig 1C,D).
Figure 1.
Interaction of Cables1 and p63. (A) Lysates of COS7 cells co-expressing FLAG-tagged Cables1 and TAp63α were immunoprecipitated with FLAG antibody, followed by immunoblotting with p63 antibody. For the input, lysate was probed independently with each antibody. (B) Immunoblot analysis of Cables1 in total protein extracts prepared from ten meiotically arrested oocytes collected from young adult mouse ovaries (left panel) and in wild-type and Cables1-null ovarian tissue lysates (right panel). (C,D) Cables1 interacts with p63 in lysates of neonatal mouse ovaries. A mouse monoclonal antibody against GFP was used as a negative control. Cables1, cyclin-dependent kinase 5 and Abl enzyme substrate 1; GFP, green fluorescent protein; IB, immunoblot; IgG, immunoglobulin G; IP, immunoprecipitation; TA, trans-activating; wt, wild type.
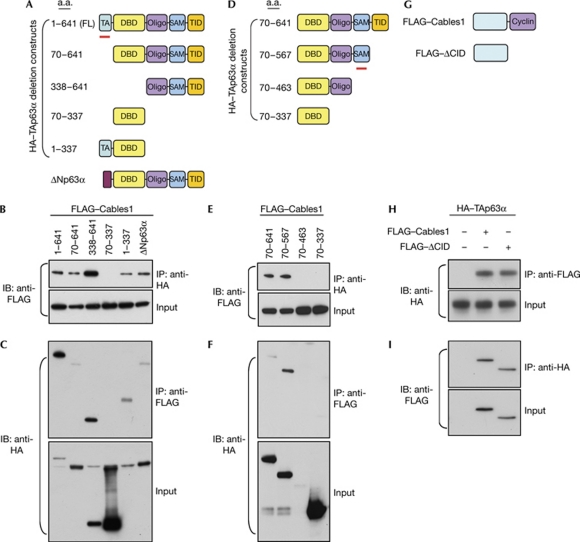
We next mapped the domain(s) of TAp63α that interact with Cables1 by constructing TAp63α deletions that removed various regions, including the TA domain, DBD and carboxyl terminus (Yang et al, 1998). We observed that Cables1 was lost in the immunocomplex only after both the TA domain and C terminus of TAp63α were deleted (Fig 2A,B). This result was confirmed by immunoprecipitation using a FLAG-specific antibody to detect FLAG-tagged Cables1, followed by immunoblotting with a haemagglutinin (HA)-specific antibody to detect HA-tagged TAp63α. The DBD of TAp63α alone did not interact with Cables1 (Fig 2C). The C terminus of TAp63α contains an oligomerization domain, a sterile alpha motif (SAM) and a TA-inhibitory domain (Yang et al, 1998). After constructing additional deletion mutants, we observed that interaction between TAp63α and Cables1 was lost when the SAM was removed (Fig 2E,F). Conversely, through expression of wild-type Cables1 and a Cables1 mutant lacking the cyclin-interaction domain (ΔCID), we observed that TAp63α immunoprecipitated with both wild-type and ΔCID Cables1 (Fig 2G–I). Together, these results indicate that the amino terminus of Cables1 interacts with the TA domain and SAM of TAp63α to promote physical association of the two proteins.
Figure 2.
Mapping of Cables1–p63 interaction domains. (A) Schematic of p63 deletion mutants. (B,C) Lysates of COS7 cells expressing FLAG-tagged Cables1 and the indicated HA-tagged p63 deletion mutants were immunoprecipitated with (B) HA or (C) FLAG antibody, followed by immunoblotting with (B) FLAG or (C) HA antibody. For the input, lysate was probed independently with each antibody. (D) Schematic of p63 carboxy-terminal deletion mutants. (E,F) Lysates of COS7 cells expressing FLAG-tagged Cables1 and the indicated HA-tagged p63 deletion mutants were immunoprecipitated with (E) HA or (F) FLAG antibody, followed by immunoblotting with (E) FLAG or (F) HA antibody. (G) Schematic of the wild-type and ΔCID Cables1 constructs. (H,I) Lysates of COS7 cells expressing HA-tagged TAp63α and FLAG-tagged wild-type or ΔCID Cables1 immunoprecipitated with (H) HA or (I) FLAG antibody, followed by immunoblotting with (H) FLAG or (I) HA antibody. ΔCID, Cables1 mutant lacking the cyclin-interaction domain; Cables1, cyclin-dependent kinase 5 and Abl enzyme substrate 1; CID, cyclin-interaction domain; DBD, DNA-binding domain; HA, haemagglutinin; Oligo, oligomerization domain; SAM, sterile alpha motif; TA, trans-activating; TID, transcriptional inhibitory domain; wt, wild type.
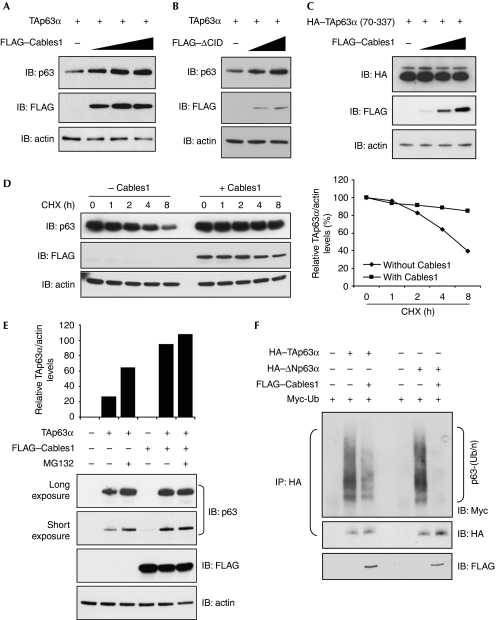
One consequence of Cables1–TAp63α interaction was the maintenance of TAp63α protein levels (Fig 3A,B). This interaction is required to stabilize TAp63α, as Cables1 has no effect on levels of the DBD of TAp63α (Fig 3C), which does not interact with Cables1 (Fig 2D–F). To better understand the mechanism underlying p63 stabilization by Cables1, we used Hep3B cells based on earlier studies that initially characterized p63 isoform stability in this cell type (Petitjean et al, 2008). The treatment of Hep3B cells with the protein synthesis inhibitor cycloheximide for up to 8 h decreased transfected TAp63α levels; this was not observed in the presence of Cables1 (Fig 3D), indicating that Cables1 stabilizes TAp63α protein. As Cables1 also interacts with the ΔNp63α isoform (Fig 2B,C), we also evaluated its protein stability. In accordance with previous observations (Petitjean et al, 2008), transfected ΔNp63α was less stable than TAp63α and showed degradation within 6 h after cycloheximide treatment. However, co-expression of Cables1 increased the half-life of ΔNp63α (supplementary Fig S1 online).
Figure 3.
Cables1 stabilizes p63 by preventing ubiquitination and proteasomal degradation. (A,B) FLAG-tagged (A) wild-type or (B) ΔCID Cables1 increases TAp63α protein levels in COS7 cells (10 μg of protein from each sample was analysed for TAp63α levels). (C) Cables1 does not stabilize the DNA-binding domain of TAp63α. (D) Hep3B cells expressing TAp63α without or with FLAG-tagged Cables1 were incubated with cycloheximide for up to 8 h, and analysed by immunoblotting for TAp63α and actin (loading control) levels. (E) Enhancement of TAp63α levels in COS7 cells after culture with MG132 for 16 h is lost in the presence of expressed Cables1. (F) Polyubiquitination (Ub/n) of p63α in COS7 cells is inhibited by expression of Cables1. Cables1, cyclin-dependent kinase 5 and Abl enzyme substrate 1; CHX, cycloheximide; CID, cyclin-interaction domain; HA, haemagglutinin; IB, immunoblot; IP, immunoprecipitation; TA, trans-activating; wt, wild type.
As p63 can be degraded by the proteasome (Rossi et al, 2006; Li et al, 2008; MacPartlin et al, 2008), we tested whether stabilization of TAp63α by Cables1 resulted from reduced ubiquitination and proteasomal degradation. The treatment of COS7 cells with the proteasome inhibitor MG132 resulted in a near-doubling of TAp63α levels (Fig 3E), consistent with p63 being turned over rapidly in cells under non-stress conditions. The accumulation of TAp63α was even greater in the presence of Cables1, and addition of MG132 to Cables1-expressing cells did not markedly alter the extent of TAp63α accumulation compared with that resulting from the presence of Cables1 alone (Fig 3E). In the absence of Cables1, both TAp63α and ΔNp63α were polyubiquitinated (Fig 3F). However, when Cables1 was expressed together with TAp63α or ΔNp63α, ubiquitination of both proteins was attenuated greatly (Fig 3F). These findings indicate that Cables1 stabilizes p63α by blocking ubiquitin-mediated proteasomal degradation of the protein.
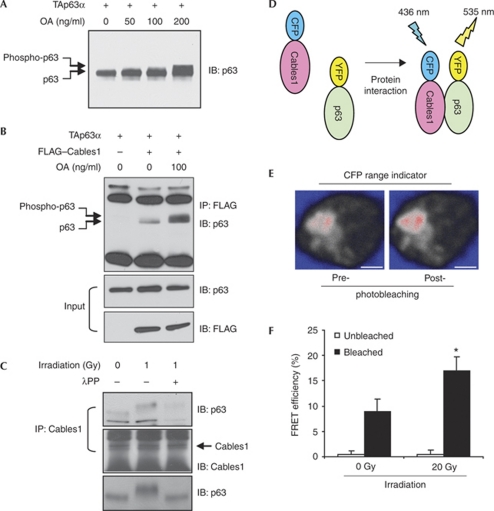
Genotoxic stress induces phosphorylation of TAp63 (Suh et al, 2006; MacPartlin et al, 2008), and a tight dose–response relationship exists between these events and cell death (Suh et al, 2006). We therefore tested whether radiation-induced DNA damage affects Cables1–TAp63 interaction by using H1299 cells so that our outcomes could be compared with those of earlier studies of TAp63γ phosphorylation in this cell type (MacPartlin et al, 2008). To aid in the detection of TAp63 phosphorylation, cells were treated with the serine and threonine phosphatase inhibitor okadaic acid (OA) for 4 h before collection (MacPartlin et al, 2008). Similar to the response reported for TAp63γ phosphorylation by IκB kinase-β (MacPartlin et al, 2008), OA caused a dose-dependent shift in TAp63α mobility (Fig 4A). Furthermore, the interaction of Cables1 with TAp63α was enhanced markedly by OA (Fig 4B), and the increased interaction between endogenous Cables1 and TAp63α in ovarian lysates, triggered by ionizing radiation exposure, was reduced significantly by pretreatment with λ-phosphatase (Fig 4C). Although more detailed information is needed to determine the significance of these findings, it seems that phosphorylation of TAp63α after a genotoxic insult might facilitate its interaction with Cables1. To test further whether DNA damage enhances this interaction, we used fluorescence resonance energy transfer (FRET) to visualize directly the interaction between Cables1 and TAp63 (Fig 4D–F). The exposure of H1299 cells to ionizing radiation increased FRET efficiency between Cables1–cyan fluorescent protein (CFP) and TAp63α–yellow fluorescent protein (YFP; Fig 4F). Thus, genotoxic stress-induced phosphorylation of TAp63α induces its interaction with Cables1, which in turn protects p63 protein from ubiquitination and proteasomal degradation.
Figure 4.
Ionizing radiation promotes TAp63α phosphorylation and its interaction with Cables1. (A) H1299 cells expressing TAp63α were treated with increasing amounts of OA for 4 h and analysed for a TAp63α mobility shift indicative of phosphorylation. (B) H1299 cells coexpressing TAp63α and FLAG-tagged Cables1 were treated without and with OA for 4 h and analysed for TAp63α levels in the Cables1 immunocomplex. (C) Ionizing radiation (1.0 Gy) enhances endogenous Cables1–TAp63α interaction, and pretreatment with λPP abolishes this effect. (D) Schematic representation of FRET, used to examine the interaction between Cables1 and p63 (monomeric CFP or YFP was fused with Cables1 or TAp63α, respectively). (E) Representative confocal imaging of CFP intensity pre- and post-photobleaching of acceptor (YFP) after coexpression of Cables1–CFP and TAp63α–YFP in H1299 cells. Scale bars, 5 μm. (F) Irradiation enhances FRET efficiency between Cables1–CFP and TAp63α–YFP in H1299 cells. *P<0.05 compared with photobleaching without irradiation; mean±s.d., n=6. λPP, λ-phosphatase; Cables1, cyclin-dependent kinase 5 and Abl enzyme substrate 1; CFP, cyan fluorescent protein; FRET, fluorescence resonance energy transfer; OA, okadaic acid; TA, trans-activating; YFP, yellow fluorescent protein.
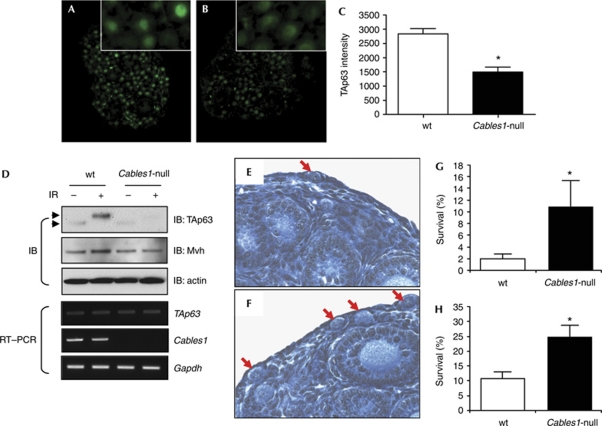
In a final set of experiments, we used Cables1-null mice to evaluate the consequences of an absence of Cables1 on TAp63α levels and function in vivo. The female germline of these mice was selected for analysis because: (i) TAp63α is expressed at high levels in female germ cells during meiotic arrest (Suh et al, 2006); (ii) Cables1 and its components are expressed in female germ cells and regulate their function (Fig 1B; Iwaoki et al, 1993; Lee et al, 2004, 2007; Park et al, 2004); (iii) endogenous Cables1 interacts with endogenous p63 in germ cells of mouse ovaries (Fig 1C); and (iv) TAp63α has an indispensable role in mediating DNA damage-induced oocyte loss (Suh et al, 2006; Livera et al, 2008). Under physiological conditions, TAp63 levels in oocytes were lower in ovaries of Cables1-null mice compared with wild-type females (Fig 5A–D). Phosphorylated TAp63α did not accumulate in oocytes of Cables1-null ovaries after exposure to ionizing radiation, in contrast to the pronounced accumulation of phosphorylated TAp63α observed in oocytes of irradiated wild-type ovaries (Fig 5D; supplementary Fig S2 online). The levels of TAp63 mRNA showed no significant differences in wild-type and Cables1-null ovaries, confirming that changes in TAp63 levels were due to changes in protein stability rather than transcriptional regulation of the p63 gene. To assess the consequence of this partial loss of p63 stability in Cables1-null mice, female mice were irradiated at P5 and ovaries were collected 5 days later (P10). Non-irradiated wild-type and Cables1-null mice had a comparable number of oocytes at P10 (data not shown; Lee et al, 2007). However, the near-complete depletion of oocytes from wild-type ovaries after exposure to ionizing radiation was attenuated significantly in ovaries of Cables1-null mice (Fig 5E–H).
Figure 5.
Female germ cells lacking Cables1 exhibit reduced TAp63 content and are more resistant to DNA-damage-induced loss in vivo. (A,B) Immunofluorescence staining of TAp63 in (A) wild-type and (B) Cables1-null ovaries at postnatal day 5 (P5). (C) Quantitative analysis of TAp63 immunofluorescence staining intensity from randomly selected germ cells of each genotype. *P<0.05 compared with wild type, n=15. (D) Immunoblot and RT–PCR analysis of TAp63 protein (arrowheads indicate TAp63α mobility shift associated with phosphorylation) and messenger RNA levels in P5 wild-type and Cables1-null ovaries under physiological conditions or 4 h after ionizing irradiation (1.0 Gy). (E,F) Representative ovarian histology (arrows demarcate primordial oocytes) and comparison of survival rates of (G) primordial or (H) total immature oocytes in (E) wild-type and (F) Cables1-null mice 5 days after exposure to ionizing radiation at P5. *P<0.05 compared with wild type. Mean±s.d., n=5 mice per group. Cables1, cyclin-dependent kinase 5 and Abl enzyme substrate 1; IB, immunoblot; IR, ionizing irradiation; RT, reverse transcriptase; TA, trans-activating; wt, wild type.
In summary, physical association of Cables1 with p63 inhibits its ubiquitination and degradation through the proteasome. This process is required for maximal stabilization of TAp63 in female germ cells, and the subsequent death of these cells, after exposure to a genotoxic stress in vivo. However, Cables1-null oocytes retain approximately 50% of the TAp63α protein content observed in wild-type oocytes, and the radioprotection conveyed in oocytes by Cables1 deficiency is less than that observed in p63-null mice (Suh et al, 2006). Thus, although the loss of Cables1 reduces TAp63α levels through increased degradation, TAp63α that remains is sufficient in some, but not all, Cables1-null oocytes for triggering apoptosis after exposure to ionizing radiation. Similar to other reports (Suh et al, 2006; MacPartlin et al, 2008), our findings also indicate that TAp63 is phosphorylated rapidly in response to genotoxic stress, and this might facilitate its interaction with Cables1. Previous studies with doxorubicin have reported that this phosphorylation event involves serine residues on TAp63, which then stabilizes the protein (Petitjean et al, 2008). However, cisplatin triggers the phosphorylation of TAp63 on tyrosine residues through induction of the c-Abl kinase, which also results in the stabilization of p63 (Gonfloni et al, 2009). It was further reported that inhibition of the tyrosine kinase activity of c-Abl by imatinib partly prevents cisplatin-induced oocyte loss in mice through destabilization of TAp63α (Gonfloni et al, 2009). Interestingly, Cables1 promotes tyrosine phosphorylation of c-Abl substrates (Zukerberg et al, 2000). Although the mechanisms responsible for differential phosphorylation of p63 by various genotoxic stresses remain to be elucidated, Cables1 might participate as a common upstream integrator of these events.
Methods
Methods are described in more detail in the supplementary information online.
Animals. Mice harbouring a targeted inactivation of the Cables1 gene have been described earlier by our laboratories (Zukerberg et al, 2004). All animal protocols were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Plasmids. Full-length human Cables1 complementary DNA has been reported previously (Sakamoto et al, 2008), whereas TAp63α complementary DNA was provided by G. Wu (Wayne State University). The HA- and FLAG-tagged constructs, and all deletion mutants, were generated by PCR. Identity of all plasmids was confirmed by sequence analysis.
Antibodies, immunoblotting and immunoprecipitation. See the supplementary information online.
Oocyte counts. Non-atretic oocyte-containing primordial and total immature (primordial, primary and preantral) follicle numbers were determined by histomorphometric procedures detailed previously (Jones & Krohn, 1961; Skaznik-Wikiel et al, 2007).
FRET analysis. Photobleaching-based FRET analysis was conducted essentially as described previously (Wan et al, 2008). The fusion of CFP or YFP to Cables1 or TAp63α, respectively, does not alter protein function (supplementary Fig S3 online).
Immunofluorescence. Immunofluorescence was performed, as described previously (Matikainen et al, 2001; Suh et al, 2006), using TAp63 antibody provided by F. McKeon (Harvard Medical School). Sections from ovarian tissues of wild-type and Cables1-null mice were always mounted adjacent to each other on same slide to ensure identical exposure to all treatments and sample processing.
Ubiquitination assay. Ubiquitination analysis was conducted as described previously (Ohta & Xiong, 2001) with minor modifications.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank F. McKeon for TAp63 antibody and T. Ohta for helpful discussions regarding the ubiquitination assay. N.W. is a recipient of a Massachusetts General Hospital Fund for Medical Discovery award. This study was supported by National Institute on Aging MERIT Award R37-AG012279, National Institutes of Health R01-CA098333, the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation and Vincent Memorial Research Funds.
Footnotes
The authors declare that they have no conflict of interest.
References
- Gonfloni S et al. (2009) Inhibition of the c-Abl–TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 15: 1179–1185 [DOI] [PubMed] [Google Scholar]
- Iwaoki Y, Matsuda H, Mutter GL, Watrin F, Wolgemuth DJ (1993) Differential expression of the proto-oncogenes c-abl and c-mos in developing mouse germ cells. Exp Cell Res 206: 212–219 [DOI] [PubMed] [Google Scholar]
- Jones PB, Krohn PL (1961) The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J Endocrinol 21: 469–495 [DOI] [PubMed] [Google Scholar]
- Lee KY, Rosales JL, Lee BC, Chung SH, Fukui Y, Lee NS, Lee KY, Jeong YG (2004) Cdk5/p35 expression in the mouse ovary. Mol Cell 17: 17–22 [PubMed] [Google Scholar]
- Lee HJ et al. (2007) Loss of CABLES1, a cyclin-dependent kinase-interacting protein that inhibits cell cycle progression, results in germline expansion at the expense of oocyte quality in adult female mice. Cell Cycle 6: 2678–2684 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou Z, Chen C (2008) WW domain-containing E3 ubiquitin protein ligase 1 targets p63 transcription factor for ubiquitin-mediated proteasomal degradation and regulates apoptosis. Cell Death Differ 12: 1941–1951 [DOI] [PubMed] [Google Scholar]
- Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R (2008) p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 135: 3–12 [DOI] [PubMed] [Google Scholar]
- MacPartlin M, Zeng SX, Lu H (2008) Phosphorylation and stabilization of TAp63γ by IκB kinase-β. J Biol Chem 283: 15754–15761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen T et al. (2001) Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet 28: 355–360 [DOI] [PubMed] [Google Scholar]
- Moll UM, Slade N (2004) p63 and p73: roles in development and tumor formation. Mol Cancer Res 2: 371–386 [PubMed] [Google Scholar]
- Ohta T, Xiong Y (2001) Phosphorylation- and SKP1-independent in vitro ubiquitination of E2F1 by multiple ROC-Cullin ligases. Cancer Res 61: 1347–1353 [PubMed] [Google Scholar]
- Park CE, Kim YH, Jeon EH, Cha KY, Lee SH, Lee KA (2004) Expression of wee1 and its related cell cycle components in mouse early stage follicles. Cells Tissues Organs 177: 221–228 [DOI] [PubMed] [Google Scholar]
- Petitjean A, Ruptier C, Tribollet V, Hautefeuille A, Chardon F, Cavard C, Puisieux A, Hainaut P, Caron de Fromentel C (2008) Properties of the six isoforms of p63: p53-like regulation in response to genotoxic stress and cross talk with ΔNp73. Carcinogenesis 29: 273–281 [DOI] [PubMed] [Google Scholar]
- Rossi M, Aqeilan RI, Neale M, Candi E, Salomoni P, Knight RA, Croce CM, Melino G (2006) The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc Natl Acad Sci USA 103: 12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H et al. (2008) Mechanisms of Cables1 gene inactivation in human ovarian cancer development. Cancer Biol Ther 7: 180–188 [DOI] [PubMed] [Google Scholar]
- Skaznik-Wikiel M, Tilly JC, Lee H-J, Niikura Y, Kaneko-Tarui T, Johnson J, Tilly JL (2007) Serious doubts over “Eggs Forever?” Differentiation 75: 93–99 [DOI] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F (2006) p63 protects the female germ line during meiotic arrest. Nature 444: 624–628 [DOI] [PubMed] [Google Scholar]
- Tsuji K, Mizumoto K, Yamochi T, Nishimoto I, Matsuoka M (2002) Differential effect of ik3-1/cables on p53- and p73-induced cell death. J Biol Chem 277: 2951–2957 [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408: 307–310 [DOI] [PubMed] [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X (2008) Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev 22: 2968–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall MD, Pietenpol JA (2004) p63: Molecular complexity in development and cancer. Carcinogenesis 6: 857–864 [DOI] [PubMed] [Google Scholar]
- Wu CL, Kirley SD, Xiao H, Chuang Y, Chung DC, Zukerberg LR (2001) Cables enhances cdk2 tyrosine 15 phosphorylation by Wee1, inhibits cell growth, and is lost in many human colon and squamous cancers. Cancer Res 61: 7325–7332 [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillette E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F (1998) p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2: 305–316 [DOI] [PubMed] [Google Scholar]
- Yang A, McKeon F (2000) p63 and p73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol 3: 199–207 [DOI] [PubMed] [Google Scholar]
- Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH (2000) Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 26: 633–646 [DOI] [PubMed] [Google Scholar]
- Zukerberg LR et al. (2004) Loss of Cables, a cyclin-dependent kinase regulatory protein, is associated with the development of endometrial hyperplasia and endometrial cancer. Cancer Res 64: 202–208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.