CHK2-independent induction of telomere dysfunction checkpoints in stem and progenitor cells
This study provides evidence that CHK2-independent checkpoints limit the function of stem and progenitor cells in response to telomere dysfunction.
Keywords: telomeres, senescence, stem cells, CHK2, DNA damage
Abstract
Telomere shortening limits the proliferation of primary human fibroblasts by the induction of senescence, which is mediated by ataxia telangiectasia mutated-dependent activation of p53. Here, we show that CHK2 deletion impairs the induction of senescence in mouse and human fibroblasts. By contrast, CHK2 deletion did not improve the stem-cell function, organ maintenance and lifespan of telomere dysfunctional mice and did not prevent the induction of p53/p21, apoptosis and cell-cycle arrest in telomere dysfunctional progenitor cells. Together, these results indicate that CHK2 mediates the induction of senescence in fibroblasts, but is dispensable for the induction of telomere dysfunction checkpoints at the stem and progenitor cell level in vivo.
Introduction
Telomere shortening limits the proliferative capacity of human cells by the induction of senescence or crisis (Wright & Shay, 1992). Studies on telomerase knockout mice (Terc−/−) have shown that telomere dysfunction enhances tissue atrophy and thus impairs organ maintenance, resulting in a shortened lifespan of the mice (Rudolph et al, 1999). Telomere shortening and an increase in the expression level of markers of telomere dysfunction are associated with shortened lifespan and the evolution of age-associated diseases in humans (Cawthon et al, 2003; Lansdorp, 2009), indicating that telomere shortening can contribute to ageing and disease in humans.
Tissue atrophy in telomere dysfunctional mice correlates with activation of the p53 pathway (Chin et al, 1999; Wong et al, 2003; Choudhury et al, 2007; Schaetzlein et al, 2007). It has been shown that deletion of p21 elongates the lifespan of primary human fibroblast cultures (Brown et al, 1997) and improves stem-cell function and organ maintenance in telomere dysfunctional mice (Choudhury et al, 2007), indicating that checkpoint responses to telomere dysfunction are conserved in cell culture and in vivo models.
CHK2 is the downstream target of the ataxia telangiectasia mutated (ATM) kinase, which is the primary mediator of checkpoint induction in response to DNA double-strand breaks. Short hairpin RNA (shRNA)-mediated knockdown of CHK2 was sufficient to abrogate the induction of p53 and senescence in cultured human fibroblasts (Gire et al, 2004). To decipher the in vivo role of CHK2 in telomere-driven ageing, we crossed CHK2 knockout mice (Hirao et al, 2002) with Terc−/− (Rudolph et al, 1999). This study shows that CHK2 deletion cannot rescue the progenitor cell function, organ maintenance and lifespan of telomere dysfunctional mice, indicating that CHK2-independent checkpoints limit the function of stem and progenitor cells in response to telomere dysfunction.
Results And Discussion
Stem-cell function, organ maintenance and lifespan
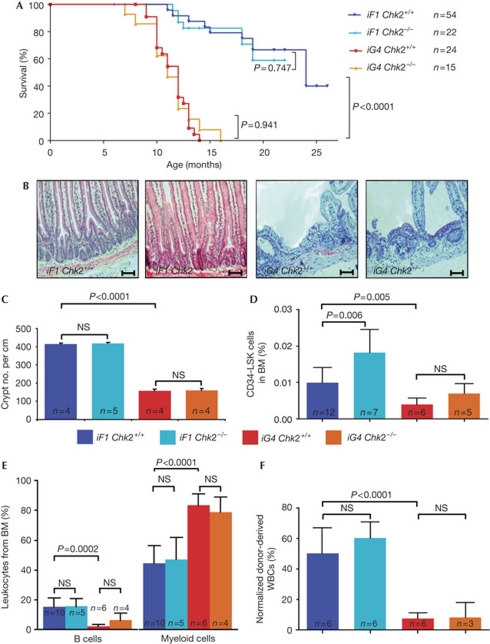
Terc+/−Chk2+/− double-heterozygous mice were crossed with third-generation Terc−/−Chk2+/− mice to generate the experimental cohorts (supplementary Fig S1A online). On using this mating scheme, intercross G4 (iG4) Terc−/− mice show telomere dysfunction, impaired organ maintenance and a shortened lifespan, whereas intercross Terc+/− (iF1 Terc+/−) littermates show a rescue in telomere function, organ maintenance and lifespan (Hemann et al, 2001; Choudhury et al, 2007). In agreement with previous studies, iG4 Terc−/− mice from our cohorts showed a shortened lifespan compared with iF1 Terc+/− mice (Fig 1A). Of note, CHK2 deletion did not affect the lifespan of iF1 Terc+/− or iG4 Terc−/− mice (Fig 1A) and CHK2 deletion did not lead to an increase in cancer formation (data not shown).
Figure 1.
CHK2 deletion does not rescue progeroid phenotypes of telomere dysfunctional mice. (A) Survival curves for iF1 Terc+/−Chk2+/+ mice (n=54), iF1 Terc+/−Chk2−/− mice (n=22), iG4 Terc−/−Chk2+/+ mice (n=24) and iG4 Terc−/−Chk2−/− mice (n=15). (B) Representative photographs of haematoxylin and eosin-stained longitudinal sections of the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bars, 200 μm. (C) Histogram of the number of basal crypts per vision field (original magnification × 100) in the small intestine of 9–12-month-old mice of the indicated genotypes (n=4–5 mice per group). (D) Histogram of the percentage of haematopoietic stem cells (that is, CD34−/loLSK cells) in total BM cells of 9–12-month-old mice of the indicated genotypes (n=4–6 mice per group; data are shown as mean; error bars represent s.d.). (E) Histogram showing percentage of B cells and myeloid cells in WBCs of the BM of 9–12-month-old mice of the indicated genotypes (n=4–6 mice per group; data are shown as mean; error bars represent s.d.). (F) Histogram showing percentage of donor-derived WBCs, 3 months after competitive transplantation for the indicated genotypes (n=3–6 mice per group; data are shown as mean; error bars represent s.d.). BM, bone marrow; iF1, intercross; iG4, intercross G4; NS, non-significant; WBC, white blood cell.
The reduction in the lifespan of iG4 Terc−/− mice was associated with impaired maintenance of intestinal epithelium and reduction in the body weight of 9–12-month-old iG4 compared with age-matched iF1 Terc+/− mice, but CHK2 deletion did not rescue these phenotypes (Fig 1B,C; supplementary Fig S1B–D online). CHK2 deletion also did not rescue telomere shortening in iG4 Terc−/− mice (supplementary Fig S2A online). Similarly, the quantification of anaphase bridges—a tissue marker of telomere dysfunction (Kirk et al, 1997; Rudolph et al, 2001)—showed no rescue by CHK2 deletion (supplementary Fig S2B,C online). Chromosome fluorescence in situ hybridization on basal intestinal crypts showed that CHK2 deletion did not provoke an increase in chromosomal aberration (ploidy change) in iG4 Terc−/− mice (supplementary Fig S2D online), as it was detected in response to intestinal deletion of p53 in previous studies (Begus-Nahrmann et al, 2009).
Impairments in haematopoietic stem-cell (HSC) function and lymphopoiesis are further age-related phenotypes that are induced by telomere dysfunction in both mice and humans ( Ju et al, 2007; Rossi et al, 2007). Previous studies have shown a reduced number of HSCs (c-Kit-positive, Sca1-positive, Lineage-negative, CD34 low/negative=CD34lo/−LSK cells) in the bone marrow of aged telomere dysfunctional mice compared with age-matched wild-type mice (Choudhury et al, 2007; Ju et al, 2007; Schaetzlein et al, 2007). The deletion of CHK2 did not rescue the maintenance of HSCs or skewing of haemato-lymphopoiesis (that is, a decrease in B-lymphopoiesis and increase in myelopoiesis) in the bone marrow of iG4 Terc−/− mice (Fig 1D,E). HSCs from 9–12-month-old iG4 Terc−/− mice had a significantly reduced repopulation capacity compared with those from iF1 Terc+/− mice, which was not rescued by CHK2 deletion (Fig 1F). Together, these data show that CHK2-independent mechanisms impaired the function of intestinal and haematopoietic stem and progenitor cells in response to telomere dysfunction.
Telomere dysfunction induced checkpoints
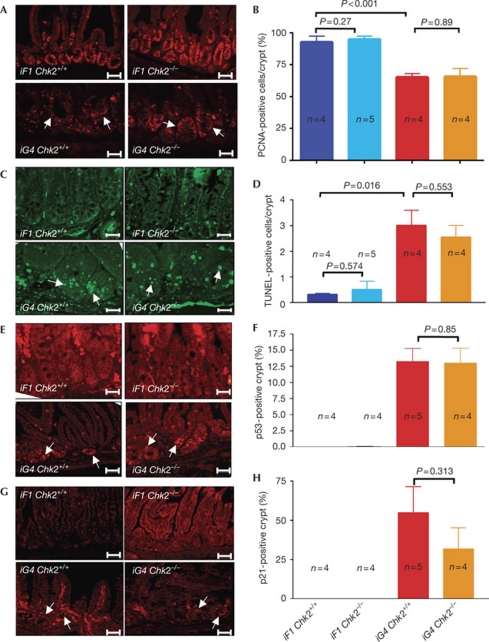
To elucidate the failure of CHK2 deletion to rescue organ maintenance in ageing telomere dysfunctional mice, we focused on the intestinal epithelium, which shows high rates of cell turnover during a lifetime (Barker et al, 2007). Intestinal atrophy in ageing iG4 mice has been associated with impaired cell proliferation in progenitor cells located in the basal crypts of the intestinal epithelium (Choudhury et al, 2007; Schaetzlein et al, 2007). An analysis of cell proliferation markers—proliferating cell nuclear antigen (PCNA) and Ki67—in intestinal basal crypts revealed that CHK2 deletion did not rescue the induction of cell-cycle arrest in telomere dysfunctional mice (Fig 2A,B; supplementary Fig S3 online). CHK2 deletion also did not rescue the elevated rates of apoptosis in 9–12-month-old iG4 Terc−/− mice (Fig 2C,D), or the activation of p53 and p21 in the telomere dysfunctional intestine (Fig 2E–H). Together, these results indicate that CHK2-independent mechanisms mediate the activation of p53 and p21 as well as the induction of cell-cycle arrest and apoptosis in response to telomere dysfunction in intestinal progenitor cells. Owing to the absence of transplantation assays for the assessment of intestinal stem-cell function, it was not possible to prove formally the CHK2-independent induction of checkpoints in response to telomere dysfunction at the intestinal stem-cell level.
Figure 2.
CHK2 deletion does not ameliorate checkpoint induction in response to telomere dysfunction. (A) Representative photographs of PCNA-stained crypts in the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bars, 200 μm. (B) Histogram showing the percentage of PCNA-positive cells per crypt in the small intestine of 9–12-month-old mice of the indicated genotypes. (C) Representative photographs of apoptotic cells (green nuclei) in basal crypts of the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bars, 200 μm. (D) Histogram showing the number of TUNEL-positive nuclei per basal crypt in the small intestine of 9–12-month-old mice of the indicated genotypes. (E) Representative photographs of p53 staining in crypts in the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bars, 200 μm. (F) Histogram showing the percentage of p53-positive crypts in the small intestine of 9–12-month-old mice of the indicated genotypes. (G) Representative photographs of p21-stained crypts in the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bars, 200 μm. (H) Histogram showing the percentage of p21-positive crypts in the small intestine of 9–12-month-old mice of the indicated genotypes (n=4–5 mice per group; data are shown as mean; error bars represent s.d.). PCNA, proliferating cell nuclear antigen; TUNEL, TdT-mediated dUTP nick end-labelling.
CHK2-dependent senescence in fibroblasts
The results on CHK2-independent induction of checkpoints in progenitor cells of mice with dysfunctional telomeres stood in contrast to the significant role of CHK2 in the induction of senescence in primary human fibroblasts (Takai et al, 2002; Gire et al, 2004). Infection of late-passage, primary human BJ fibroblasts with lentiviral vectors expressing a CHK2-directed shRNA confirmed that CHK2 knockdown abrogated the induction of senescence, resulting in positive selection of CHK2-knockdown cells and improved proliferation rates of human fibroblast cultures at late passage (supplementary Fig S4A–C online). Similar results were obtained for mouse ear fibroblasts. Genetic deletion of CHK2 rescued proliferation of iG4 Terc−/− fibroblasts (supplementary Fig S4D online). These experiments on mouse fibroblasts were carried out under low-stress conditions (3% oxygen), suggesting that CHK2 deletion can rescue telomere-dependent senescence in cell culture. However, the improvement in cell proliferation of Terc+/+Chk2−/− fibroblasts compared with Terc+/+Chk2+/+ fibroblasts indicates that stress-induced culture conditions (independent of oxygen) can limit fibroblast proliferation in a CHK2-dependent manner. The relative contribution of CHK2 to the induction of telomere-dependent and telomere-independent senescence in fibroblast senescence remains to be analysed. A challenge for these experiments is that oxygen-independent stress factors that contribute to induction of telomere-independent senescence in fibroblasts are not well defined.
In contrast to the cell culture data, the deletion of CHK2 did not rescue organ maintenance in ageing telomere dysfunctional mice (Figs 1,2). These findings demonstrate that CHK2 is dispensable for the induction of DNA-damage checkpoints in stem and progenitor cell compartments in response to telomere dysfunction in vivo.
Localization of CHK2 in response to telomere dysfunction
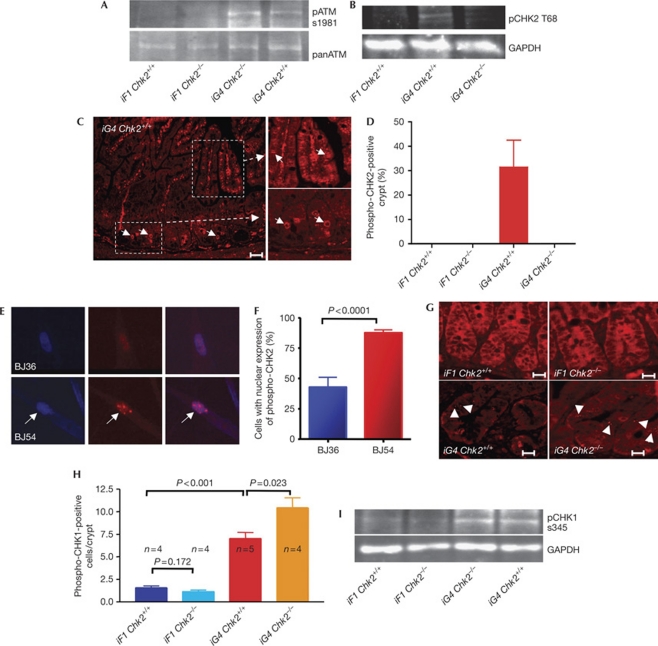
Previous studies have shown that ATM is activated in response to telomere dysfunction in fibroblasts (d'Adda di Fagagna et al, 2003). In agreement with these data, phosphorylation of ATM was seen in western blot analysis and immunofluorescence staining of the intestinal epithelium of iG4 and iG4 Chk2−/− mice (Fig 3A; supplementary Fig S5A,B online). In line with ATM activation, western blot and immunofluorescence staining showed an upregulation of phospho-CHK2 (T68) in intestinal progenitor cells of 9–12-month-old iG4 Terc−/− mice compared with iF1 Terc+/− mice (Fig 3B–D; supplementary Fig S5C,D online). Interestingly, the expression of activated CHK2 in ageing telomere dysfunctional intestine was restricted to the cytoplasm, indicating that activated CHK2 did not translocate to the nucleus (Fig 3C; supplementary Fig S5D online). In contrast, intra-nuclear expression of phospho-CHK2 was readily detectable in terminally differentiated epithelial cells of the intestinal villi of iG4 mice (Fig 3C), as well as in senescent human fibroblasts (Fig 3E,F).
Figure 3.
Localization of activated CHK2 and CHK1 in the intestine of telomere dysfuctional mice. (A) Western blot showing the expression of phospho-ATM s1981 in intestinal biopsies of 9–12-month-old mice of the indicated genotypes. The lower panel shows panATM expression for the loading control. (B) Western blot showing activation of phospho-CHK2 T68 in the small intestinal lysates of 9–12-month-old mice of the indicated genotypes. The lower panel shows GAPDH expression for the loading control. (C) Representative photographs showing cytoplasmic localization of phosphorylated CHK2 in the intestinal crypts and nuclear localization of phosphorylated CHK2 in villi of the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bar, 400 μm. (D) Histogram showing the percentage of phospho-CHK2-positive cells per crypt in the small intestine of 9–12-month-old mice of the indicated genotypes. (E) Representative photographs of phosphorylated CHK2 staining in human BJ fibroblasts at the indicated population doubling. (F) Histogram showing the percentage of phospho-CHK2-positive fibroblasts at the indicated population doublings. (More than 100 cells were counted.) (G) Representative photographs of phospho-CHK1-stained crypts in the small intestine of 9–12-month-old mice of the indicated genotypes. Scale bars, 400 μm. (H) Histogram showing the percentage of phospho-CHK1-positive cells per crypt in the small intestine of 9–12-month-old mice of the indicated genotypes (n=4–5 mice per group; data are shown as mean; error bars represent s.d.). (I) Western blot on small-intestinal protein lysates from 9–12-month-old mice of the indicated genotypes. The lower panel shows GAPDH expression for the loading control. ATM, ataxia telangiectasia mutated; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; iF1, intercross; iG4, intercross G4.
Together, these data indicate that CHK2 is phosphorylated in response to telomere dysfunction. In contrast to senescent fibroblasts, phosphorylated CHK2 did not translocate to the nucleus in telomere dysfunctional progenitor cells of the intestinal basal crypts. The cytoplasmic localization could be one of the reasons for the failure of activated CHK2 to contribute to activation of p53-dependent checkpoint responses in intestinal basal crypt cells. This interpretation is in line with previous findings showing that the failure of embryonic stem cells to induce G1 cell-cycle arrest in response to γ-irradiation is associated with an impaired nuclear localization of activated CHK2 in response to DNA damage (Hong & Stambrook, 2004). The current data suggest that this observation could also hold true for intestinal progenitor cells in vivo.
The formation of DNA-damage foci represents the initial response to telomere dysfunction leading to activation of ATM and ataxia telangiectasia and rad3-related (ATR) kinases (Nalapareddy et al, 2008). ATM and ATR have both been implicated in the induction of replicative senescence in fibroblast cultures (Herbig et al, 2004) and it has been shown that these two pathways are partly redundant in telomere maintenance (Bi et al, 2005). The deletion of ATM accelerated the ageing of telomere dysfunctional mice (Wong et al, 2003). However, this accelerated ageing possibly involved the role of ATM in the metabolism of reactive oxygen species and telomere maintenance (Ito et al, 2004; Silva et al, 2004) and thus did not allow us to draw a direct conclusion on the role of ATM/CHK2 signalling in checkpoint induction in response to telomere dysfunction. In contrast to ATM, CHK2 has no direct role in anti-oxidant defence or telomere capping but can rescue cell-cycle arrest induced by oxidative DNA damage (Liu et al, 2009). The current study indicates that CHK2 is dispensable for the induction of DNA damage checkpoints in response to telomere dysfunction at the stem and progenitor cell level and that CHK2 deletion cannot rescue the organ maintenance and lifespan of telomere dysfunctional mice.
It is known that ATR-dependent activation of CHK1 is an alternative route of p53 activation in response to various kinds of DNA damage (d'Adda di Fagagna et al, 2003; Zou & Elledge, 2003; Jazayeri et al, 2006). Studies on exonuclease 1 showed that exonuclease 1 deletion prevented the induction of ATR and p53 in telomere dysfunctional mice (Schaetzlein et al, 2007). To analyse the possible involvement of CHK1 activation in telomere dysfunction-induced checkpoints in progenitor cells, the expression of phospho-CHK1 was determined in intestinal basal crypts. Immunofluorescence staining of phosphorylated CHK1 showed nuclear staining of phospho-CHK1 in intestinal basal crypts and progenitor cells of iG4 mice (Fig 3G,H). Immunofluorescence on CHK1-shRNA-treated cells confirmed the staining specificity (supplementary Fig S6A online). In addition, western blot analysis of intestinal epithelium reconfirmed the activation of CHK1 in iG4 and iG4 Chk2−/− mice (Fig 3I). These data are in agreement with our previous observation on increased expression of phospho-ATR in the intestinal basal crypt cells of telomere dysfunctional mice (Schaetzlein et al, 2007). However, the functional importance of CHK1 for the induction of DNA-damage checkpoints in response to telomere dysfunction remains to be demonstrated in vivo.
Together, these data show that CHK2 is dispensable for the induction of DNA damage checkpoints in telomere dysfunctional intestinal progenitor cells in vivo. Moreover, CHK2 (Fig 1F) deletion could not rescue the repopulation capacity of telomere dysfunctional HSCs as it was reported for p21 deletion (Choudhury et al, 2007). The study supports the concept that CHK2-independent pathways can contribute to the induction of telomere dysfunction-induced checkpoints in stem and progenitor cells. Together, these findings have an implication for regenerative therapies aimed at improving the function of endogenous stem and progenitor cells in the context of telomere dysfunction and ageing.
Methods
Mouse crosses and survival. The Chk2 knockout mice were provided by T.W. Mak. For PCR genotyping, in the wild-type mice a 600 bp fragment was amplified and in Chk2 knockout mice, exons 8–11 were deleted. The PCR primers flanking exons 8–11 resulted in a product of 900 bp (supplementary Fig S6B online). Chk2+/− mice were crossed with Terc+/− mice to generate double-heterozygous mice, which in turn were intercrossed through successive generations to produce G3 Terc−/−Chk2+/− mice. Intercrosses between Terc+/−Chk2+/− and G3 Terc−/−Chk2+/− mice generated the following experimental cohorts: Terc+/−Chk2+/+(iF1 Chk2+/+), Terc+/−Chk2−/− (iF1 Chk1−/−), Terc−/−Chk2+/+(iG4 Chk2+/+) and Terc−/−Chk2−/− (iG4 Chk2−/−). Mice were bred on a C57BL/6J background.
Stainings for PCNA, Ki67, p53, p21CIP, phospho-CHK1 and phospho-CHK2. Immunofluorescence was performed on 3 μm-thick paraffin sections of the small intestine. Sections were deparaffinized, rehydrated and permeabilized in 1 mM sodium citrate buffer. Primary antibodies were used either overnight at 4°C or for 2 h at room temperature (22–25°C): PCNA (Calbiochem, 1:150 dilution), Ki67 (Monosan, 1:800 dilution), p53 (Vector Labs, 1:500), p21CIP (Santa Cruz, 1:50), phospho CHK1 (Cell Signaling, 1:100) and phospho-CHK2 T68 (Cell Signaling, Abcam, 1:100). The following secondary antibodies were used: p21CIP and PCNA: anti-mouse-Cy3 (Zymed, 1:200); ki67 and p53: anti-rabbit-Cy3 ( Jackson Laboratories, 1:500); phospho-CHK1 and phospho-CHK2: anti-rabbit-Cy3 or FITC (Jackson Laboratories, 1:200) for 1–2 h at room temperature (22–25°C).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
K.L.R. is supported by the funding from the German Research Foundation (RU745/10-1, RU745/7-1, RU7415/13-1), the Deutsche Krebshilfe e.V. (project programme grant on tumor stem cells) and the European Union (GENINCA and TELOMARKER). Z.J. is supported by the funding from the National Science and Technology Major Projects (2009ZX09501-026) and by the Partner Group programme of the Max Planck Society.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Begus-Nahrmann Y et al. (2009) p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet 41: 1138–1143 [DOI] [PubMed] [Google Scholar]
- Bi X, Srikanta D, Fanti L, Pimpinelli S, Badugu R, Kellum R, Rong YS (2005) Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc Natl Acad Sci USA 102: 15167–15172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Wei W, Sedivy JM (1997) Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277: 831–834 [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361: 393–395 [DOI] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA (1999) p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97: 527–538 [DOI] [PubMed] [Google Scholar]
- Choudhury AR et al. (2007) Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet 39: 99–105 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V (2004) DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J 23: 2554–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann MT, Strong MA, Hao LY, Greider CW (2001) The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107: 67–77 [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol Cell 14: 501–513 [DOI] [PubMed] [Google Scholar]
- Hirao A et al. (2002) Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol 22: 6521–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Stambrook PJ (2004) Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci USA 101: 14443–14448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K et al. (2004) Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431: 997–1002 [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP (2006) ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8: 37–45 [DOI] [PubMed] [Google Scholar]
- Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL (2007) Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med 13: 742–747 [DOI] [PubMed] [Google Scholar]
- Kirk KE, Harmon BP, Reichardt IK, Sedat JW, Blackburn EH (1997) Block in anaphase chromosome separation caused by a telomerase template mutation. Science 275: 1478–1481 [DOI] [PubMed] [Google Scholar]
- Lansdorp PM (2009) Telomeres and disease. EMBO J 28: 2532–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J et al. (2009) Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K, Jiang H, Gutierrez LM, Rudolph KL (2008) Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging—what markers can we use? Exp Gerontol 43: 998–1004 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729 [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA (1999) Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96: 701–712 [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Millard M, Bosenberg MW, DePinho RA (2001) Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28: 155–159 [DOI] [PubMed] [Google Scholar]
- Schaetzlein S et al. (2007) Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell 130: 863–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Tiong S, Pedersen M, Homola E, Royou A, Fasulo B, Siriaco G, Campbell SD (2004) ATM is required for telomere maintenance and chromosome stability during Drosophila development. Curr Biol 14: 1341–1347 [DOI] [PubMed] [Google Scholar]
- Takai H et al. (2002) Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J 21: 5195–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA (2003) Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421: 643–648 [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW (1992) The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol 27: 383–389 [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300: 1542–1548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.