Polo-like kinase 1 phosphorylation of G2 and S-phase-expressed 1 protein is essential for p53 inactivation during G2 checkpoint recovery
Contributing to our understanding of cell-cycle re-initiation after genomic stress, this study links Plk1 kinase activity and the p53 pathway through Plk1-mediated phosphorylation of GTSE1, a protein found to be essential for p53 inactivation during G2 checkpoint recovery.
Keywords: checkpoint recovery, p53, phosphorylation, Plk1, GTSE1
Abstract
In response to G2 DNA damage, the p53 pathway is activated to lead to cell-cycle arrest, but how p53 is eliminated during the subsequent recovery process is poorly understood. It has been established that Polo-like kinase 1 (Plk1) controls G2 DNA-damage recovery. However, whether Plk1 activity contributes to p53 inactivation during this process is unknown. In this study, we show that G2 and S-phase-expressed 1 (GTSE1) protein, a negative regulator of p53, is required for G2 checkpoint recovery and that Plk1 phosphorylation of GTSE1 at Ser 435 promotes its nuclear localization, and thus shuttles p53 out of the nucleus to lead to its degradation during the recovery.
Introduction
In response to DNA damage in G2 phase, activation of the DNA-damage cascade (ataxia telangiectasia mutated (ATM)/ataxia telangiectasia and Rad3-related (ATR)-Chk2/1-p53) leads to cell-cycle arrest (Smits & Medema, 2001; Taylor & Stark, 2001). During the subsequent recovery from the arrest, the DNA-damage cascade has to be silenced. As a crucial cell-cycle regulator, Polo-like kinase 1 (Plk1) has an essential role in G2 checkpoint recovery (van Vugt et al, 2004) through targeting various substrates: it silences the ATR–Chk1 pathway through phosphorylation-induced degradation of the adaptor protein Claspin; it also contributes directly to abolishing the inhibition of cyclin-dependent kinase 1 (Cdk1)/cyclin B through regulation of the phosphatase Cdc25B and the kinase Wee1 (van Vugt et al, 2004; Mamely et al, 2006). The tumour suppressor protein p53 was required to sustain G2 arrest after DNA damage (Bunz et al, 1998). However, how p53 is inactivated during G2 checkpoint recovery is unknown.
In this study, we identify G2 and S-phase-expressed 1 (GTSE1) protein, a negative regulator of p53, as a Plk1 target during the recovery process. The GTSE1 protein is expressed specifically during G2 and S phases of the cell cycle, and is localized mainly in the cytoplasm, apparently associated with microtubules (Utrera et al, 1998; Collavin et al, 2000). This protein is able to downregulate p53 by translocating into the nucleus, binding to and shuttling p53 out of the nucleus and inducing its degradation (Monte et al, 2003, 2004). In the later stage of DNA-damage-induced arrest, GTSE1 starts accumulating in the nucleus (Monte et al, 2004), suggesting that it might be involved in the checkpoint recovery process through downregulation of p53.
Results And Discussion
GTSE1 interacts with Plk1 in cells
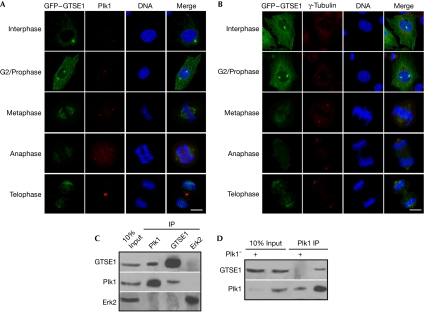
Proteomic studies suggest that GTSE1 is a potential Plk1 target (Beausoleil et al, 2004; Lowery et al, 2007). To test whether GTSE1 interacts with Plk1, we first generated a U2OS cell line stably expressing green fluorescent protein (GFP)–GTSE1. The expressed GFP–GTSE1 shows the characteristic microtubule-association pattern, indicating that it retains the property of endogenous GTSE1. Using immunofluorescence staining against Plk1 and γ-tubulin antibodies, we observed that GFP–GTSE1 co-localized with Plk1 in centrosomes from G2 phase to prophase, but not after metaphase (Fig 1A,B). Furthermore, the interaction between endogenous GTSE1 and Plk1 was detected in the co-immunoprecipitation experiment (Fig 1C). This interaction became undetectable on knockdown of Plk1 by RNA interference (RNAi; Fig 1D), suggesting that the interaction between GTSE1 and Plk1 is highly specific.
Figure 1.
G2 and S-phase-expressed 1 protein interacts with Polo-like kinase 1. (A,B) U2OS cells were transfected with GFP–GTSE1 and transfection-positive cells were selected by G418 (400 μg/ml) for 2 weeks. Cells in different stages of the cell cycle were subjected to immunofluorescence staining with antibodies against (A) Plk1 or (B) γ-tubulin. DNA was stained with DAPI. Scale bars, 10 μm. (C) U2OS cells were treated with nocodazole for 14 h and collected for anti-Plk1, anti-GTSE1 or anti-Erk2 immunoprecipitation (IP), followed by western blot analysis. Erk2 IP was used as a non-specific binding control. (D) U2OS cells were depleted of Plk1 with double-stranded RNA, treated with nocodazole for 14 h and collected for anti-Plk1 IP, followed by western blot analysis. DAPI, 4',6-diamidino-2-phenylindole; Erk2, extracellular signal-related kinase 2; GFP, green fluorescent protein; GTSE1, G2 and S-phase-expressed 1 protein; Plk1, Polo-like kinase 1.
Plk1 phosphorylates GTSE1 at Ser 435
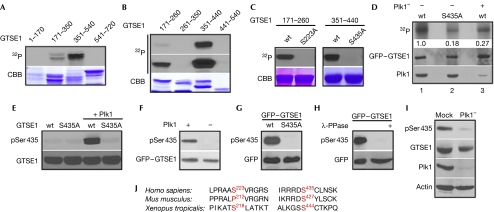
Next we asked whether GTSE1 is a substrate of Plk1. In the in vitro kinase assay, both GTSE1-amino acids (aa) 171–350 and GTSE1-aa 351–540 were phosphorylated by Plk1, but the latter acted as a much more robust substrate for Plk1 (Fig 2A). The phosphorylation region was narrowed down further as GTSE1-aa 171–260 and GTSE1-aa 351–440 (Fig 2B). Subsequently, Ser 233 and Ser 435 were identified by site-directed mutagenesis as two Plk1 phosphorylation sites in vitro (Fig 2C). However, we focused on Ser 435, as Ser 233 is not conserved between human and mouse sequences (Fig 2J). To test whether Ser 435 of GTSE1 is phosphorylated by Plk1 in cells, we first performed metabolic labelling experiments in 293T cells transfected with GFP–GTSE1. As shown in Fig 2D, introduction of the S435A mutation reduced the in vivo phosphorylation signal of GTSE1 fivefold, indicating that Ser 435 is phosphorylated in cells. The knockdown of Plk1 also decreased the phosphorylation signal, indicating that this phosphorylation is Plk1-dependent. Next, a polyclonal antibody directed against a peptide encompassing phospho-Ser 435 (pSer 435) was generated. On incubation with Plk1, only wild-type GTSE1 (GTSE1-wt), but not GTSE1-S435A, was recognized by the pSer 435 antibody, suggesting that Ser 435 of GTSE1 is phosphorylated directly by Plk1 in vitro (Fig 2E,F). Moreover, GFP–GTSE1-wt, but not GFP–GTSE1-S435A, expressed in 293T cells was recognized specifically by the pSer 435 antibody (Fig 2G), confirming that Ser 435 is phosphorylated in cells. The pSer 435 epitope was lost on phosphatase treatment of lysates from cells expressing GFP–GTSE1, verifying the specificity of this antibody for phosphorylated GTSE1 (Fig 2H). Most importantly, anti-pSer 435 identified the phosphorylated GTSE1 band in the cell lysates from U2OS cells treated with nocodazole, but not from Plk1-depleted cells (Fig 2I), suggesting that endogenous GTSE1 is phosphorylated at Ser 435 in a Plk1-dependent manner.
Figure 2.
Polo-like kinase 1 phosphorylates G2 and S-phase-expressed 1 protein at Ser 435. (A) Plk1 was incubated with four purified GST–GTSE1 regions (amino acids (aa) 1–170, 171–350, 351–540 and 541–720) in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS–PAGE, stained with Coomassie brilliant blue (CBB), and detected by autoradiography. (B) Plk1 was incubated with the indicated GST–GTSE1 regions as in (A). A longer exposure of 32P signal is shown in the middle panel. (C) Plk1 was incubated with the indicated forms of GST–GTSE1 fragments (aa 171–260 or 351–440). (D) 293T cells were depleted of Plk1 with dsRNA (lane 3), then transfected with GFP–GTSE1-wt (lanes 1 and 3) or S435A (lane 2), treated with nocodazole, and metabolically labelled with [32P]orthophosphate. Phosphoproteins were immunoprecipitated with GFP antibodies, resolved by SDS–PAGE and detected by autoradiography. Numbers indicate the relative intensity of 32P-labelled GTSE1. (E) Plk1 was incubated with GST–GTSE1-aa 351–440 (wt or S435A) in the presence of unlabelled ATP, followed by an anti-pSer 435 western blot. (F) 293T cells were transfected with GFP–GTSE1 in the presence of Plk1 inhibitor (BI 2536) and collected for anti-GFP immunoprecipitation (IP). Then the IP products were incubated with purified Plk1 in the presence of unlabelled ATP, followed by anti-pSer 435 western blot analysis. (G) 293T cells were transfected with GFP–GTSE1 constructs (wt or S435A), treated with nocodazole and immunoblotted. (H) Lysates from mitotic 293T cells transfected with GFP–GTSE1 were treated with λ-phosphatase and immunoblotted. (I) U2OS cells were depleted of Plk1 with dsRNA, treated with nocodazole and immunoblotted. (J) Alignment of GTSE1 protein sequences containing Ser 233 and Ser 435 in different species. dsRNA, double-stranded RNA; GFP, green fluorescent protein; GST, glutathione-S-transferase; GTSE1, G2 and S-phase-expressed 1 protein; Plk1, Polo-like kinase 1; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis; wt, wild type.
GTSE1 is essential for G2 checkpoint recovery
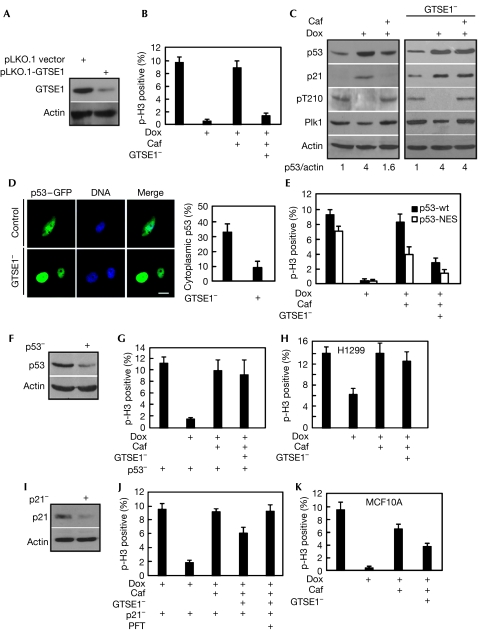
Nuclear accumulation of GTSE1 occurs in the later phase after DNA damage (Monte et al, 2004), suggesting that GTSE1 might be involved in the checkpoint recovery process to downregulate the protein levels of p53. Thus, we examined whether GTSE1 is required for G2 checkpoint recovery. Endogenous GTSE1 can be knocked down efficiently by RNAi (Fig 3A), and GTSE1 depletion did not affect normal mitotic progression (supplementary Fig S1A online). As shown in Fig 3B, DNA damage blocked the cells at G2 and treatment with caffeine, an inhibitor of ATM/ATR to override the DNA-damage checkpoint, drove the cells into mitosis. By contrast, GTSE1-depleted cells lost the ability to re-enter mitosis after caffeine treatment, suggesting that GTSE1 is required for G2 checkpoint recovery. To understand the molecular mechanism of the recovery defects in GTSE1-depleted cells, we analysed the oscillation of the level of p53 protein and its transcriptional target p21 under the same conditions. Both the levels of p53 and p21 proteins were elevated markedly on doxorubicin treatment, and reduced during caffeine-induced recovery in control U2OS cells (Fig 3C, left panel). By contrast, the level of p53 was not reduced significantly and the level of p21 remained easily detectable in GTSE1-depleted cells after caffeine treatment (Fig 3C, right panel). As a consequence of highly active p53, the elevated level of p21 contributed to Cdk1/cyclin B inhibition, thus preventing these cells from entering mitosis.
Figure 3.
G2 and S-phase-expressed 1 protein is essential for G2 checkpoint recovery. (A) U2OS cells were transfected with pLKO.1-GTSE1 or pLKO.1 vector for 24 h and immunoblotted. (B) U2OS cells were synchronized with the double thymidine block (16 h treatment with thymidine, 8 h of release and a second 16 h block with thymidine) at the G1/S boundary and GTSE1 was depleted by transfection with pLKO.1-GTSE1 during the 8 h interval and second 16 h blocking period. After release from the double thymidine block for 6 h, the cells were treated with 1.0 μM doxorubicin (Dox) for 1 h, incubated in fresh medium with or without caffeine (Caf) for an additional 6 h. Phospho-histone H3 positivity was determined. (C) Cells as described in (B) were immunoblotted with various antibodies indicated on the left. pT210 is an antibody that recognizes the activated form of Plk1. Numbers at the bottom indicate the relative intensity of p53 compared with actin. (D) On transfection with p53–GFP construct, U2OS cells were subject to synchronization and doxorubicin/caffeine treatment as described in (B). DNA was stained with DAPI. Cells displaying cytoplamsic localization of p53–GFP were quantified in the right panel. Scale bar, 10 μm. (E) U2OS cells were transfected with p53–GFP-wt or p53–GFP-NES (NES-deficient mutant), processed as in (B) and stained with phospho-histone H3. (F) U2OS cells were infected with lentivirus targeting p53, selected by puromycin (10 μg/ml) for 2 weeks, and immunoblotted. (G) The p53-depleted U2OS cells were processed as in (B). (H) H1299 cells were processed as in (B). (I) U2OS cells were depleted of p21 with dsRNA for 24 h, treated with doxorubicin for 1 h and immunoblotted. (J) U2OS cells were depleted of p21 with dsRNA and then processed as in (B). (K) MCF10A cells were processed as in (B). DAPI, 4′,6-diamidino-2-phenylindole; dsRNA, double-stranded RNA; GFP, green fluorescent protein; GTSE1, G2 and S-phase-expressed 1 protein; NES, nuclear export signal; PFT, pifithrin 2.
Considering that GTSE1 downregulates p53 through shuttling it to the cytoplasm, we analysed the subcellular localization of GFP-tagged p53 during recovery. The depletion of GTSE1 markedly reduced the cytoplasmic localization of GFP–p53 (Fig 3D), suggesting that shuttling of p53 to the cytoplasm is an important aspect for GTSE1 to promote checkpoint recovery. To examine the impact of cytoplasmic localization of p53 on checkpoint recovery, we compared the recovery abilities of U2OS cells expressing p53–GFP-wt or p53–GFP-NES (nuclear export signal-deficient mutant). Blocking the shuttling of p53 to the cytoplasm partly suppressed the ability to recover (Fig 3E; supplementary Fig S2A online), indicating that the cytoplasmic localization of p53 contributes to the checkpoint recovery.
To further evaluate the contribution of GTSE1 in checkpoint recovery, we analysed the impact of GTSE1 on checkpoint recovery in p53-depleted U2OS cells and H1299 cells (p53−/−). Under the same conditions as in Fig 3B, GTSE1 depletion did not affect the recovery process in these cells, suggesting that the essential role of GTSE1 during G2 checkpoint recovery depends on p53 (Fig 3F–H, supplementary Fig S2B online). Moreover, we analysed the impact of GTSE1 on checkpoint recovery in p21-depleted U2OS cells. p21 can be knocked down efficiently on the activation of p53 by the DNA-damage signal (Fig 3I). We observed that knock down of p21 partly rescued the GTSE1 depletion-induced checkpoint defect and that inhibition of p53 transcriptional activation by Pifithrin-α fully rescued the recovery defect (Fig 3J), suggesting that p21 is the main effector of active p53, but not the only one contributing to G2 arrest. Finally, we evaluated the impact of GTSE1 during G2 checkpoint recovery in a normal non-transformed human cell line. Partial depletion of GTSE1 by RNAi in MCF10A cells led to moderate recovery deficiency (Fig 3K; supplementary Fig S1B online).
Plk1 phosphorylation of GTSE1 is required for recovery
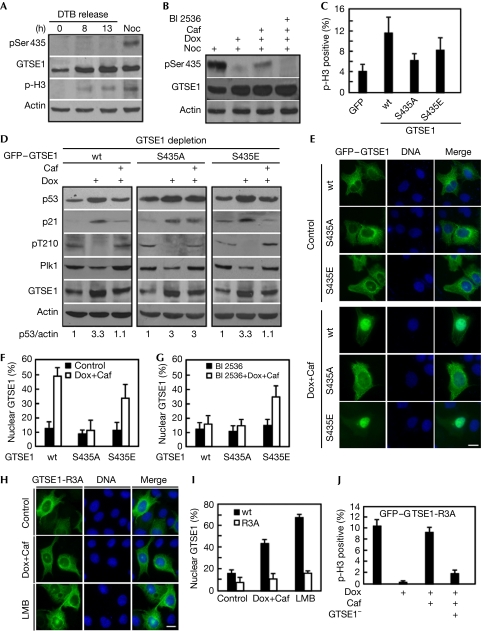
Next we examined whether the essential role of GTSE1 during G2 checkpoint recovery is regulated by Plk1 phosphorylation. We first examined GTSE1-Ser 435 phosphorylation during normal cell-cycle progression. Although we were not able to detect the pSer 435 epitope in U2OS cells released for 0 h (G1/S), 8 h (G2) and 13 h (mitosis) after the double thymidine block, we did detect it in nocodazole-treated cells (Fig 4A). Failure to detect the pSer 435 epitope after 13 h release from the double thymidine block is probably due to the low synchronization efficiency of U2OS cells by this protocol, and we did detect the pSer 435 epitope in HeLa cells by the same protocol (supplementary Fig S3C online). Nevertheless, we were also able to detect the pSer 435 epitope in U2OS cells with immunofluorescence, and it was detected only during late G2 and mitosis (supplementary Fig S3A,B online). Furthermore, we observed that phosphorylation of Ser 435 decreased markedly in response to DNA damage, consistent with the fact that Plk1 is a DNA-damage target (Smits et al, 2000). Significantly, GTSE1 was rephosphorylated at Ser 435 during the caffeine-induced checkpoint recovery, but remained unphosphorylated in the presence of BI 2536, a Plk1 inhibitor (Fig 4B; supplementary Fig S3E online), suggesting that the phosphorylation of Ser 435 during the G2 checkpoint recovery occurs in a Plk1-dependent manner. Under a physiological recovery process (without caffeine treatment), we also observed the phosphorylation of Ser 435 in 48 h time points after doxorubicin treatment (supplementary Fig S3D online). To evaluate the significance of Ser 435 phosphorylation, endogenous GTSE1 was replaced with RNAi-resistant GFP–GTSE1 constructs (wt, S435A or S435E). Under the same conditions as in Fig 3B, cells expressing GFP–GTSE1-wt and GFP–GTSE1-S435E were able to recover from G2 arrest. By contrast, cells expressing GFP–GTSE1-S435A showed the recovery defect (Fig 4C), whereas overexpression of GFP–GTSE1-wt, GFP–GTSE1-S435A or GFP–GTSE1-S435E showed a similar mitotic progression pattern in the absence of DNA damage (supplementary Fig S4A,B online). In agreement with the abilities of cells expressing GTSE1-wt and GTSE1-S435E to enter mitosis, both the p53 and p21 protein levels were lower than those of cells expressing GTSE1-S435A after caffeine treatment (Fig 4D). In summary, these data clearly demonstrate that phosphorylation of GTSE1 by Plk1 regulates its essential function during G2 checkpoint recovery.
Figure 4.
Phosphorylation of G2 and S-phase-expressed 1 protein by Polo-like kinase 1 is required for G2 checkpoint recovery. (A) U2OS cells were synchronized by the double thymidine block (DTB) protocol, released for 0, 8 and 13 h (left three lanes) or treated with nocodazole (Noc) for 24 h and immunoblotted. (B) U2OS cells were subjected to synchronization/DNA damage as in Fig 3B, released into nocodazole-containing medium with or without caffeine (Caf) and BI 2536 for 6 h and immunoblotted. (C) U2OS cells stably expressing RNAi-resistant GFP–GTSE1 constructs (wt, S435A or S435E) were processed as in Fig 3B with endogenous GTSE1 depleted. Phospho-histone H3 positivity was determined. (D) Samples as in (C) were immunoblotted with antibodies as indicated on the left. (E–G) Plk1 phosphorylation of GTSE1 promotes its nuclear accumulation during G2 checkpoint recovery. (E) U2OS cells transfected with GFP–GTSE1 constructs (wt, S435A or S435E) were subjected to synchronization, DNA damage and caffeine treatment as in Fig 3B and stained with DAPI to determine the subcellular localization of GFP–GTSE1. Scale bar, 10 μm. (F) Quantification of the results in (E). (G) U2OS cells were processed as in (E), but released into medium containing both caffeine and BI 2536. (H–I) Plk1 targets the nuclear localization signal of GTSE1. (H) U2OS cells were transfected with GFP–GTSE1 constructs (wt or R3A) and processed as in (E). Alternatively, GTSE1-expressing cells were incubated with leptomycin B (LMB) for 12 h. Scale bar, 10 μm. (I) Quantification of the results in (H). (J) U2OS cells expressing GFP–GTSE1-R3A were depleted of endogenous GTSE1 and processed as in (C). DAPI, 4',6-diamidino-2-phenylindole; GFP, green fluorescent protein; GTSE1, G2 and S-phase-expressed 1 protein; Plk1, Polo-like kinase 1; R3A, R431A/R432A/R433A; RNAi, RNA interference; wt, wild type.
To understand how Plk1-mediated phosphorylation of GTSE1 downregulates p53 during G2 checkpoint recovery, we examined the subcellular localization of GFP–GTSE1 (wt, S435A or S435E) during the recovery process. The GFP–GTSE1 fusion protein (wt, S435A and S435E) localized mainly in the cytoplasm in the absence of DNA damage (Fig 4E, upper panel). Notably, during the caffeine-induced recovery, GFP–GTSE1 (wt and S434E) accumulated in the nucleus, but GFP–GTSE1-S435A remained in the cytoplasm (Fig 4E, bottom panel; Fig 4F), suggesting that Plk1 phosphorylation of GTSE1 is essential for its nuclear accumulation during the recovery. The inactivation of Plk1 by BI 2536 treatment abolished GTSE1-wt accumulation in the cytoplasm, confirming that Plk1 activity is crucial for this process (Fig 4G). Doxorubicin/caffeine treatment also has a marked effect on the nuclear accumulation of GFP–GTSE1-S435E, suggesting that an additional Plk1-independent mechanism also contributes to the nuclear accumulation of GTSE1 during the recovery (Fig 4G). Therefore, Plk1 phosphorylation of GTSE1 is a crucial step to promote its nuclear accumulation during checkpoint recovery. However, other Plk1-indepentdent mechanisms also contribute to nuclear accumulation of GTSE1 in the process.
Considering that one typical nuclear localization signal (NLS) sequence R431RR433 exists upstream of the phosphorylation site Ser 435, we speculated that Plk1-mediated phosphorylation might lead to exposure of this NLS to the cellular transport machinery, thus promoting its nuclear localization. To test this hypothesis, we generated an NLS-deficient mutant (GFP–GTSE1-R3A) by mutating the three arginines to alanines (supplementary Fig S5A online). As indicated in Fig 4H,I, the R3A mutation completely abolished the response of GTSE1 to leptomycin B, a nuclear export inhibitor, suggesting that R431RR433 is a bona fide NLS of GTSE1. In addition, the R3A mutant failed to accumulate in the nucleus during caffeine-induced checkpoint recovery, suggesting that the NLS sequence is essential for the function of GTSE1 during checkpoint recovery. Consistent with a nuclear accumulation defect, neither the protein level of p53 nor p21 was downregulated in U2OS cells expressing GFP–GTSE1-R3A during the recovery (supplementary Fig S5B online), whereas the phosphorylation of GFP–GTSE1-R3A at Ser 435 was not affected (supplementary Fig S5C online). As expected, the GFP–GTSE1-R3A mutant failed to rescue the GTSE1 depletion-induced defect during G2 checkpoint recovery (Fig 4J).
In conclusion, our results demonstrate that Plk1 phosphorylates GTSE1 at Ser 435 both during normal cell-cycle progression and G2 checkpoint recovery. As a negative regulator of p53, GTSE1 is essential for eliminating active p53 from the nucleus in the later stage of the recovery process. Thus, the sustenance of G2 arrest by p53 is released, thereby allowing increased Cdk1/cyclin B activity to promote mitotic entry. The function of Plk1-mediated phosphorylation of GTSE1 is to activate the nuclear import signal of GTSE1 and promote its nuclear accumulation. Furthermore, the phosphorylation-deficient mutant GTSE1-S435A-expressing cells failed to enter mitosis after caffeine treatment, suggesting that phosphorylation of GTSE1 is required for G2 checkpoint recovery. Our finding provides the first evidence that Plk1 facilitates p53 elimination during the recovery process. Recently, Medema and co-workers showed that wild-type p53-induced phosphatase (Wip1) is responsible for retaining checkpoint recovery competence by counteracting p53 function (Lindqvist et al, 2009). Our future study will aim to determine whether GTSE1 contributes to recovery competence through balancing the protein levels of p53.
Methods
Vector construction, RNAi, cell culture and transfection. Detailed information on the construction of various expression or RNAi vectors, cell culture and DNA transfection are described in the supplementary information online.
Antibodies. The phospho-specific antibody against Ser 435 of GTSE1 was generated by Proteintech. We also purchased the following antibodies: Plk1 (sc-17783, Santa Cruz Biotechnology), γ-tubulin (T-6557, Sigma), β-actin (A-5441, Sigma), p21 (sc-397, Santa Cruz Biotechnology), phospho-T210 of Plk1 (558400, BD), phospho-histone H3 (06-570, Millipore).
Protein purification. Various glutathione-S-transferase-tagged human GTSE1 constructs were subcloned into pGEX-KG, expressed in Escherichia coli and purified. Point mutations were generated by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene).
Kinase assays. Purified recombinant GTSE1 was incubated with purified Plk1 in kinase reaction buffer (50 mM Tris (pH 7.5), 10 mM MgCl2, 2 mM EGTA, 0.5 mM Na3VO4, 100 mM para-nitrophenylphosphate, 25 mM dithiothreitol, 125 μM ATP) supplemented with 10 μCi of [γ-32P] ATP at 30°C for 30 min. After the reaction mixtures were resolved by sodium dodecyl sulphate polyacrylamide gel electrophoresis, the gels were stained with Coomassie brilliant blue, dried and subjected to autoradiography.
Immunoprecipitation, immunoblotting and immunofluorescence staining. For detailed information, see the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank R. Erikson for his constant support. We also thank Y. Zhang at the University of North Carolina at Chapel Hill for providing p53–GFP constructs. X.L. is a recipient of the Howard Temin Award from the National Cancer Institute (K01 CA114401).
Footnotes
The authors declare that they have no conflict of interest.
References
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP (2004) Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA 101: 12130–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501 [DOI] [PubMed] [Google Scholar]
- Collavin L, Monte M, Verardo R, Pfleger C, Schneider C (2000) Cell-cycle regulation of the p53-inducible gene B99. FEBS Lett 481: 57–62 [DOI] [PubMed] [Google Scholar]
- Lindqvist A, de Bruijn M, Macurek L, Bras A, Mensinga A, Bruinsma W, Voets O, Kranenburg O, Medema RH (2009) Wip1 confers G2 checkpoint recovery competence by counteracting p53-dependent transcriptional repression. EMBO J 28: 3196–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB (2007) Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J 26: 2262–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, Perrakis A, Medema RH, Freire R (2006) Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol 16: 1950–1955 [DOI] [PubMed] [Google Scholar]
- Monte M, Benetti R, Buscemi G, Sandy P, Del Sal G, Schneider C (2003) The cell cycle-regulated protein human GTSE1 controls DNA damage-induced apoptosis by affecting p53 function. J Biol Chem 278: 30356–30364 [DOI] [PubMed] [Google Scholar]
- Monte M, Benetti R, Collavin L, Marchionni L, Del Sal G, Schneider C (2004) hGTSE1 expression stimulates cytoplasmic localization of p53. J Biol Chem 279: 11744–11752 [DOI] [PubMed] [Google Scholar]
- Smits VA, Medema RH (2001) Checking out the G2/M transition. Biochim Biophys Acta 1519: 1–12 [DOI] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH (2000) Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol 2: 672–676 [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815 [DOI] [PubMed] [Google Scholar]
- Utrera R, Collavin L, Lazarevic D, Delia D, Schneider C (1998) A novel p53-inducible gene coding for a microtubule-localized protein with G2-phase-specific expression. EMBO J 17: 5015–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt MA, Bras A, Medema RH (2004) Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell 15: 799–811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.