Regulation of interleukin-1β by interferon-γ is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production
IFNgamma potentiates IL-1ß release from human cells, but transiently inhibits production of IL-1 ß from mouse cells—a finding that resolves previously conflicting reports concerning the pro- or anti-inflammatory responses to IFNgamma signalling in various models of disease.
Keywords: IFNγ, IL-17, IL-1β, inflammasome, SOCS1
Abstract
Reports describing the effect of interferon-γ (IFNγ) on interleukin-1β (IL-1β) production are conflicting. We resolve this controversy by showing that IFNγ potentiates IL-1β release from human cells, but transiently inhibits the production of IL-1β from mouse cells. Release from this inhibition is dependent on suppressor of cytokine signalling 1. IL-1β and Th17 cells are pathogenic in mouse models for autoimmune disease, which use Mycobacterium tuberculosis (MTB), in which IFNγ and IFNβ are anti-inflammatory. We observed that these cytokines suppress IL-1β production in response to MTB, resulting in a reduced number of IL-17-producing cells. In human cells, IFNγ increased IL-1β production, and this might explain why IFNγ is detrimental for multiple sclerosis. In mice, IFNγ decreased IL-1β and subsequently IL-17, indicating that the adaptive immune response can provide a systemic, but transient, signal to limit inflammation.
Introduction
The adaptive immune system can have a profound inhibitory effect on the innate immune system by regulating a multiprotein complex termed the inflammasome (Guarda et al, 2009). Conceptually, this might explain how a successful adaptive immune response limits potentially damaging inflammation due to ‘endogenous pyrogen' interleukin-1β (IL-1β). Such inhibition of the inflammasome was observed to require an interaction between the effector T cell and the antigen-presenting cell; however, T-cell-derived cytokines, such as interferon-γ (IFNγ), had no apparent effect. By contrast, several reports have demonstrated that IFNγ can inhibit IL-1β synthesis stimulated by lipopolysaccharide (LPS; Ghezzi & Dinarello, 1988; Ruschen et al, 1989; Schindler et al, 1989; de Boer et al, 2001) through a signal transducer and activator of transcription 1 (STAT1)-dependent mechanism (de Boer et al, 2001). Furthermore, other STAT1 activators, such as type I IFN, can also function in this capacity (Ghezzi & Dinarello, 1988). Despite the presence of potential STAT1-binding sites in the IL-1β promoter, STAT1 might not inhibit gene transcription directly. Instead, it might increase the function of a negative regulator, such as glycogen synthase kinase 3β, to inhibit cyclic AMP response element-binding protein and activator protein 1, both of which are key transcription factors at the IL-1β locus (Hu et al, 2006).
Opposing the reports that IFNγ can inhibit IL-1β synthesis, other investigators have reported increased IL-1β production after Toll-like receptor (TLR) stimulation (Boraschi et al, 1984; Arenzana-Seisdedos et al, 1985; Collart et al, 1986; Haq & Maca, 1986; Gerrard et al, 1987; Burchett et al, 1988; Schindler et al, 1990). Broadly, this would agree with effects seen on other genes that are induced by TLRs, which are all augmented by the addition of IFNγ. Mechanistically, this involves the priming of cells for TLR ligation by increasing the expression of TLRs themselves and of downstream signalling molecules, including nuclear factor-κB (NF-κB), a dominant transcription factor for the expression of TLR-induced genes, including IL1b. Together, these findings illustrate that control of IL-1β signalling by IFNγ is complex, tightly controlled and thus likely to be biologically relevant.
The biological effects of IFNγ vary markedly among models of T-cell-mediated autoimmune disease. IFNγ is pro-inflammatory in mouse models of diabetes, thyroiditis and myasthenia gravis, but anti-inflammatory in models such as experimental autoimmune encephalomyelitis, uveitis and collagen-induced arthritis (Kelchtermans et al, 2008). It is thought that the anti-inflammatory effect of IFNγ in these disease models is on Mac1+ myeloid cells stimulated by Mycobacterium tuberculosis (MTB), the active ingredient in complete Freund's adjuvant (CFA; Matthys et al, 1999). It has also been shown that IL-17 is pathogenic in these diseases and, interestingly, IL-1β has a central role in the induction of IL-17-producing CD4+ Th17 cells and γδ-T cells (Brereton et al, 2009). Thus, suppression of IL-1β might provide a mechanism whereby IFNγ can regulate autoimmune inflammation mediated by IL-17-producing cells.
As IL-1β is a pathogenic cytokine induced by MTB, but its regulation through STAT1 remains controversial, we have analysed carefully how myeloid-cell-derived IL-1β is regulated by IFNγ. We have discovered that in mice the biological effects of IFNγ on IL-1β production are regulated strictly by suppressor of cytokine signalling 1 (SOCS1), and that the inhibition of IL-1β synthesis by IFNγ reduces the production of inflammatory Th17 cells induced by MTB. By contrast, IFNγ promotes IL-1β production from human cells, suggesting why IFNγ therapy was not successful for multiple sclerosis (Panitch et al, 1987), whereas it does provide benefit in mouse models of the disease.
Results And Discussion
IFNγ inhibits the production of IL-1β from mouse cells
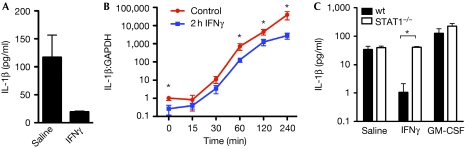
The discovery of danger-associated molecular patterns that can activate the inflammasome significantly advanced our understanding of IL-1β secretion. Before this, LPS alone was used to induce only small amounts of active IL-1β (Boraschi et al, 1984; Arenzana-Seisdedos et al, 1985; Collart et al, 1986; Haq & Maca, 1986; Gerrard et al, 1987; Burchett et al, 1988; Ghezzi & Dinarello, 1988; Ruschen et al, 1989; Schindler et al, 1989, 1990; de Boer et al, 2001). The most recent study that examined the influence of IFNγ on IL-1β production did activate the NLR family, pyrin domain containing 3 (Nlrp3) inflammasome with alum, but concluded that IFNγ had no effect (Guarda et al, 2009). We sought to verify this by investigating the effect of IFNγ priming on IL-1β secretion from mouse macrophages activated with muramyl dipeptide (MDP) conjugated to TiO2, which has been shown to activate the nucleotide-binding oligomerization domain containing 2 (Nod2)/Nlrp1 inflammasome (Hsu et al, 2008). We observed significant inhibition of IL-1β secretion when bone-marrow-derived macrophages were pretreated with IFNγ (Fig 1A). The suppression of IL-1β was confirmed at the messenger RNA (mRNA) level, at which pretreatment with IFNγ for 2 h significantly reduced endogenous and LPS-induced IL-1β mRNA by up to tenfold (Fig 1B). The inhibitory effect of IFNγ, where it has been reported, has been shown to depend on STAT1 (de Boer et al, 2001). Consistent with this, we observed that IFNγ-mediated inhibition of mature IL-1β induced through inflammasome activation was abrogated in macrophages from STAT1-deficient mice (Fig 1C). By contrast, granulocyte–macrophage colony-stimulating factor (GM-CSF), a cytokine that signals through STAT5 and not STAT1, increased the production of IL-1β by MDP–TiO2-treated macrophages from both wild-type and STAT1-deficient mice (Fig 1C).
Figure 1.
Interferon-γ inhibits interleukin-1β production by mouse macrophages. (A) Mouse macrophages were pretreated with IFNγ or saline for 2 h, then stimulated with LPS for 4 h and finally activated with MDP–TiO2 overnight to trigger IL-1β release through the inflammasome. (B) Macrophages were pretreated with IFNγ for 2 h followed by stimulation with LPS and then cells were lysed at various time points and analysed for IL-1β messenger RNA. (C) Wild-type (wt) or STAT1−/− macrophages were pretreated with IFNγ or GM-CSF for 2 h and then stimulated with LPS and MDP–TiO2 to trigger IL-1β release through the inflammasome. The inhibitory effect of IFNγ is not observed for cells deficient in STAT1. *P<0.05. GM-CSF, granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; STAT1, signal transducer and activator of transcription 1.
The inhibition of IL-1β by IFNγ is limited by SOCS1
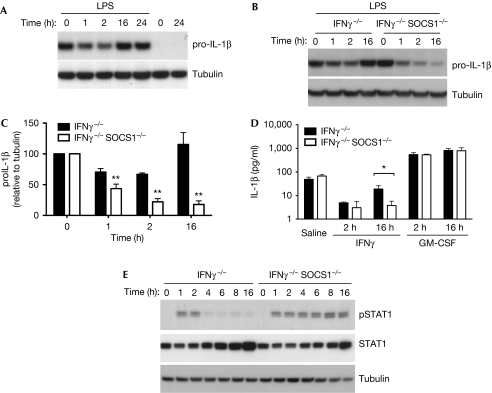
A closer examination of the duration of IFNγ priming showed that the decrease in the level of IL-1β, as measured at the protein level, was only evident transiently, and that these levels returned to baseline when priming was extended to 16 h (Fig 2A). Accordingly, researchers who observed no effect of IFNγ pretreatment on IL-1β mRNA and protein expression in mouse cells added IFNγ for periods between 16 h and 96 h before stimulation with LPS (Burchett et al, 1988; Guarda et al, 2009). A potent negative regulator of the IFNγ signal that could account for a rebound in the IL-1β message is SOCS1, which is induced strongly and promptly by IFNγ to inhibit signalling in a STAT1-dependent manner (Alexander et al, 1999). The SOCS1 protein is absolutely required to prevent lethal IFNγ-mediated hepatitis in neonates; however, its physiological role in regulating the inflammasome in vivo has not yet been examined. Owing to the neonatal lethality of SOCS1−/− mice, we stimulated doubly deficient SOCS1−/−/IFNγ−/− mouse macrophages with exogenous IFNγ and observed IL-1β protein levels by immunoblotting. In this case, IFNγ−/− macrophages were used as controls, and behaved similarly to wild type, with a rebound in IL-1β levels at 16 h. However, in macrophages that also lack SOCS1, the signal from IFNγ to downregulate IL-1β is not only maintained beyond 16 h but is also more significantly decreased over this timeframe (Fig 2B–D). Furthermore, this decrease results in a less mature IL-1β that can be generated after the inflammasome is activated with MDP–TiO2 (Fig 2D), and corresponds to prolonged IFNγ-induced pSTAT1 levels in macrophages lacking SOCS1 (Fig 2E). These data indicate that SOCS1 can have a pro-inflammatory role by interfering with IFNγ-mediated inhibition of IL-1β production. Our results suggest that a careful appraisal of SOCS1 deletion specifically from myeloid cells could reveal its function during IL-1β-dependent innate and adaptive immune responses in mice.
Figure 2.
Suppressor of cytokine signalling 1 can increase interleukin-1β production by regulating the inhibitory effects of interferon-γ. (A) Mouse macrophages were pretreated with IFNγ for 1–24 h before stimulation with LPS for 4 h. Cells were then lysed and pro-IL-1β analysed by immunoblot. Pretreatment of macrophages with IFNγ alone did not induce IL-1β production. Tubulin is shown as a loading control. (B) Western blotting was performed as in (A) using IFNγ−/− (control) and IFNγ−/−SOCS1−/− macrophages. The IL-1β level returns to normal after 16 h IFNγ pretreatment in control mice, but is decreased greatly at this time point in SOCS1-deficient cells. (C) The expression levels of IL-1β relative to tubulin from three independent experiments. **P<0.01. (D) IFNγ−/− and IFNγ−/−SOCS1−/− macrophages were primed with IFNγ or GM-CSF for different times, followed by stimulation with LPS for 4 h. IL-1β release after inflammasome activation using MDP–TiO2 was decreased in SOCS1-deficient cells at the 16 h time point when quantified by ELISA. *P<0.05. (E) IFNγ−/− and IFNγ−/−SOCS1−/− macrophages were stimulated with IFNγ for 1–16 h and western blot performed for pSTAT1. STAT1 and tubulin are shown as loading controls. ELISA, enzyme-linked immunosorbent assay; GM-CSF; granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; SOCS1, suppressor of cytokine signalling 1; STAT1, signal transducer and activator of transcription 1.
IFNγ inhibits IL-1β production by MTB in mouse cells
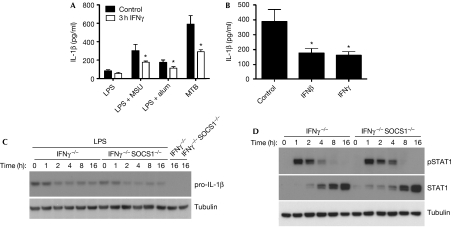
In addition to the MDP–TiO2 conjugate that might activate a Nod2/Nlrp1 inflammasome complex, our data predict that, in mouse cells, IFNγ should transiently inhibit IL-1β production resulting from activation of the Nlrp3 inflammasome by stimuli, such as alum and crystalline uric acid (monosodium urate; MSU). We tested the effect of MSU and alum on IFNγ-primed mouse dendritic cells (DCs) stimulated for 3 h in 10 ng/ml LPS and observed significantly less induction of secreted IL-1β levels compared with controls (Fig 3A). No such inhibition was observed for other pro-inflammatory cytokines driven by LPS, such as IL-6 (supplementary Fig S1 online). Other TLR ligands can increase pro-IL-1β levels sufficiently to allow the secretion of mature IL-1β after Nlrp3 activation, and complex mixtures of TLR ligands, as presented by whole heat-inactivated bacteria, such as MTB, can cause IL-1β release in this way (Koo et al, 2008). We observed that the effect of IFNγ priming on DCs activated with MTB was even more significant than for cells treated with LPS and MSU or alum (Fig 3A). This is particularly relevant because most mouse models for autoimmune disease in which IFNγ has been observed to have an anti-inflammatory role rely on CFA, with MTB as the active ingredient to promote disease (Matthys et al, 1999). Furthermore, in instances in which the role of IL-1β has been investigated in these models, it has been observed to be pathogenic.
Figure 3.
Interferon-β inhibits interleukin-1β production in response to Mycobacterium, independent of suppressor of cytokine signalling 1. (A) Mouse DCs were pretreated with IFNγ for 3 h and stimulated with MTB overnight, LPS for 3 h, followed by MSU crystals or alum overnight. Supernatants were then analysed by ELISA for IL-1β secretion. *P<0.05. (B) Mouse DCs were pretreated with IFNγ or 100 U/ml IFNβ for 3 h, stimulated with MTB overnight and IL-1β was measured by ELISA. *P<0.05. (C) IFNγ−/− and IFNγ−/−SOCS1−/− macrophages were pretreated with IFNβ for 1–16 h, followed by LPS stimulation for 3 h. Western blot analysis was performed for pro-IL-1β showing a decrease due to IFNβ, but did not return to levels seen without IFNβ. (D) Macrophages were treated with IFNβ for the indicated times and then analysed by immunoblot using antibodies specific for pSTAT1, STAT1 and tubulin. Unlike pretreatment with IFNγ, pSTAT1 was not prolonged in SOCS1-deficient cells. DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; MSU, monosodium urate; MTB, Mycobacterium tuberculosis; SOCS1, suppressor of cytokine signalling 1; STAT1, signal transducer and activator of transcription 1.
IFNβ inhibits IL-1β production from mouse cells
In mouse models of autoimmune disease, which rely on MTB, other molecules that activate STAT1, such as type I IFN, also have an anti-inflammatory role (Brod & Burns, 1994). Therefore, we tested whether IFNβ could suppress the induction of IL-1β in the same manner as IFNγ by using DCs activated with MTB, with or without priming from IFNβ (Fig 3B). These data reveal that IFNβ decreased IL-1β expression to the same extent as IFNγ in mouse cells, whereas IL-6 expression was unaffected (supplementary Fig S1B online). In separate experiments, macrophages were treated with IFNβ before stimulation with LPS, followed by western blotting to measure pro-IL-1β, pSTAT1 and STAT1 levels (Fig 3C,D). These data demonstrate that IFNβ induces Stat1 phosphorylation and inhibits IL-1β expression, but that neither of these is regulated by SOCS1.
IFNγ impairs IL-17 production via effects on IL-1β
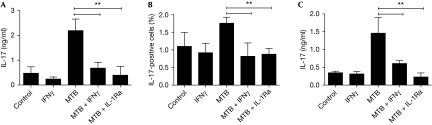
Th17 cells are now recognized as pathogenic effector cells induced by IL-1β in mouse models of autoimmune disease mediated by MTB. As we find that IFNγ can decrease the level of MTB-induced IL-1β, we next investigated the manner in which this influenced the production of IL-17. We obtained supernatants from mouse DCs primed with IFNγ and then treated with MTB. To exclude differences from other cytokines that could influence IL-17 production, we measured IL-6, IL-12p70 and IL-23 levels, which were unchanged or undetectable, whereas TGF-β was enhanced greatly by IFNγ, which might be expected to increase IL-17 production (supplementary Fig S2 online). Similarly to IL-1β, IL-1α levels were also decreased, probably because of paracrine effects. These supernatants were then added to spleen cells, activated with anti-CD3 and anti-CD28, and cultured with IL-6, IL-23 and anti-IFNγ to aid in the differentiation of Th17 cells. We observed that addition of supernatants from MTB-stimulated DCs substantially augmented IL-17 production, and blocking this signal using IL-1Ra reduced the expression of IL-17 to baseline, indicating that IL-1 is crucial for this effect (Fig 4A). Supernatants from DCs that had been pretreated with IFNγ before the addition of MTB, which we have already shown to reduce IL-1β production, were significantly less effective at induction of IL-17 production from activated spleen cells (Fig 4A).
Figure 4.
Mycobacterium-induced interleukin-1β increases interleukin-17 production, which is inhibited by interferon-γ in mouse cells. (A) Supernatants from mouse DCs pretreated with or without IFNγ for 2 h, washed and then stimulated with MTB overnight were transferred to spleen cells activated with anti-CD3 and anti-CD28 in the presence of IL-6, IL-23 and anti-IFNγ. In some cultures, the IL-1 receptor antagonist (IL-1Ra) was used to block IL-1 signalling. After 3 days, IL-17 levels were measured by ELISA. (B) Cells were cultured as in (A), then intracellular staining and FACS analysis of IL-17-producing cells were performed. (C) DCs were pretreated with IFNγ for 2 h, washed, then activated with MTB in the presence of CD4+ T cells specific for the MHC class II-restricted OVA323–339 peptide and ovalbumin peptide for 3 days. IL-17 levels in the supernatant were measured by ELISA. **P<0.01. DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IFNγ, interferon-γ; IL-1β; interleukin-1β; MTB, Mycobacterium tuberculosis.
We also performed intracellular staining that revealed an increase in the percentage of IL-17-producing cells because of cytokines released from mouse DCs after MTB treatment (Fig 4B). Consistently, both blocking this signal by using IL-1Ra and pretreatment of the MTB-stimulated DCs with IFN-γ reduced the number of cells expressing IL-17. Similar results were observed when TGF-β was also added to spleen cells with IL-6, IL-23 and anti-IFNγ (supplementary Fig S3 online). Finally, we performed an in vitro co-culture experiment to assess the ability of IFNγ-primed DCs to directly activate CD4+ T cells producing IL-17. For this experiment, mouse DCs were primed with IFNγ, washed, then stimulated with MTB and provided with ovalbumin peptide to directly activate purified CD4+ T cells specific for the MHC class II-restricted OVA323–339 peptide (OTII). At day 3, the amount of IL-17 produced in these DC/T-cell co-cultures was measured, and a significant decrease in IL-17 production was observed due to IFNγ pretreatment, similarly to the effect of adding IL-1Ra (Fig 4C).
IFNγ potentiates IL-1β release from human cells
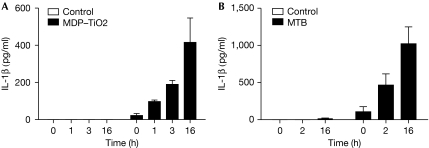
To this point, our analyses concentrated on mouse cells for which the Th17 differentiation protocols are well established. Genetically modified mice are commonly used to model human disease. However, to confirm the effect of IFNγ on IL-1β production in humans, we investigated the response of the human monocytic cell line Thp-1, and primary human monocyte-derived DCs. Thp-1 cells were primed with IFNγ and stimulated with MDP–TiO2 (Fig 5A), whereas human DCs were primed with IFNγ and stimulated with MTB (Fig 5B). In contrast to mouse cells, IFNγ increased IL-1β production in a time-dependent manner in human cells. These data agree with previously published studies on IFNγ, inducing IL-1β, which were predominantly conducted using human cells (Arenzana-Seisdedos et al, 1985; Haq & Maca, 1986; Gerrard et al, 1987; Burchett et al, 1988; Schindler et al, 1990). Recently, it has been shown that IFNβ decreases IL-1β levels and IL-17 production in human cells (Ramgolam et al, 2009); hence the increase in IL-1β might be specific for IFNγ. This is also a potential explanation for the observation that, although IFNγ is anti-inflammatory in mouse models for autoimmune disease, it exacerbates disease in patients with multiple sclerosis (Panitch et al, 1987).
Figure 5.
Interferon-γ potentiates interleukin-1β secretion from human cells. (A) Thp-1 cells were pretreated with 100 ng/ml IFNγ for different times, then stimulated with 10 ng/ml LPS for 3 h and activated with MDP–TiO2 overnight, after which the level of IL-1β was measured by ELISA. (B) Primary human dendritic cells were pretreated with IFNγ for different times, followed by stimulation with 1 μg/ml MTB and IL-1β was measured by ELISA. ELISA, enzyme-linked immunosorbent assay; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; MTB, Mycobacterium tuberculosis.
Concluding remarks
IFN-γ is considered to be an important pro-inflammatory cytokine, mediating protective immunity to infection and cancer. Recent evidence has suggested that it might also have anti-inflammatory properties, especially, though not exclusively, in mouse models for autoimmune disease. One mechanism by which IFNγ could provide an anti-inflammatory signal is through the inhibition of IL-1β, which is not only a major downstream mediator of inflammation but also an upstream activator of IL-17-producing cells that are now considered to be central factors in autoimmune inflammation. However, many reports about the regulation of IL-1β production by IFNγ have been conflicting. Our results provide new insight into the suppression of IL-1β by IFNγ and the consequences of this for production of the inflammatory cytokine IL-17. We reveal that, in mice, IFNγ can constrain IL-17 production by inhibiting IL-1β generated after activation of the inflammasome by MTB. As IFNγ could provide a systemic signal to limit IL-1β, the temporal control of this effect to maintain an adequate innate immune response requires careful regulation. We observe that this regulation is carried out by SOCS1, which rapidly returns IL-1β levels to normal by limiting the IFNγ signal. This is in contrast to human cells, in which IFNγ promotes IL-1β secretion, and is in agreement with the results from clinical trials of IFNγ for multiple sclerosis in which it is deleterious. Our findings resolve a long-standing controversy over the role of IFNγ in IL-1β production, and further explain how STAT1 activators can provide anti-inflammatory signals in mouse models of auto-inflammatory and autoimmune disease.
Methods
Mice. C57BL/6, IFNγ−/−, IFNγ−/−SOCS1−/−, Balb/c and Balb/c OTII mice were purchased from Harlan or obtained from the Walter and Eliza Hall Institute. All experiments were carried out in accordance with local animal ethics guidelines.
DC and macrophage culture. Mouse bone-marrow-derived DCs (BMDCs) were prepared by culturing bone marrow cells in a medium containing 20 ng/ml GM-CSF for 10 days. Macrophages were prepared by culturing mouse bone marrow cells in medium with 20% L929-conditioned media for 5 days. Human DCs were prepared by fractionating monocytes with anti-CD14-labelled magnetic beads (Miltenyi Biotec) from buffy coats of healthy donors. The monocytes were cultured with GM-CSF (50 ng/ml) and IL-4 (40 ng/ml; Immunotools) for 7 days.
Cell stimulation and immunoblotting. Thp-1 cells (ATCC), macrophages and DCs were pretreated with human or mouse IFNγ (100 ng/ml), GM-CSF (10 ng/ml) or IFNβ (1–100 ng/ml) for 1–24 h. Cells were then stimulated with 1 μg/ml heat-inactivated MTB overnight or with LPS (1–10 ng/ml) for 2–4 h and an inflammasome activator (20 μg/ml MSU crystals, 10 μg/ml alum or 10 μg/ml MDP conjugated to 20 μg/ml TiO2) overnight. Cell lysates were prepared in radioimmunoprecipitation assay lysis buffer supplemented with Complete Protease Inhibitor Cocktail (Roche) and were analysed using antibodies specific for IL-1β (R&D Systems), pSTAT1 (Cell Signaling Technology), STAT1 (Transduction Laboratories) and tubulin (Sigma).
RNA analysis. The expression of IL-1β mRNA was analysed using commercially available primer/probe sets (Applied Biosystems) and Applied Biosystems 7500 real-time PCR system.
Enzyme-linked immunosorbent assay. The concentrations of IL-1α, IL-1β, IL-6, IL-12p70, IL-17, IL-23 and TGF-β in supernatants were quantified by enzyme-linked immunosorbent assay (R&D Systems, BD Biosciences and eBioscience).
T cells and co-culture. The CD4+ T cells were purified using a CD4+ T-cell isolation kit as per the manufacturer's instructions (Miltenyi Biotec). The spleen cells or purified CD4+ T cells were cultured with plate-bound anti-CD3 and anti-CD28 (1 μg/ml) for 3 days with BMDC supernatants and anti-IFNγ with or without the addition of IL-1β (10 ng/ml), IL-1Ra (10 μg/ml), IL-6 (10 ng/ml), IL-23 (10 ng/ml) or TGF-β (5 ng/ml) from Peprotech. For DC/T-cell co-culture experiments, Balb/c BMDCs were stimulated with or without IFNγ for 2 h. Cells were placed in fresh medium and stimulated with MTB (1 μg/ml). Ovalbumin peptide and purified CD4+ OTII T cells were added to BMDCs and cultured together for 3 days. Intracellular staining for IL-17 and IFNγ was performed after phorbol myristate acetate and ionomycin (Sigma) stimulation for 4 h in the presence of brefeldin A (Sigma).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This study was supported by a National Health and Medical Research Council Program Grant (461219); National Health and Medical Research Council Independent Research Institutes Support Scheme Grant (361646); Victorian State Government Operational Infrastructure Support Grant and National Institutes of Health Grant CA022556; National Health and Medical Research Council Overseas Biomedical Fellowship (SLM, 516783); National Health and Medical Research Council Career Development Award (to B.A.C., 575531); National Health and Medical Research Council Peter Doherty Fellowship (to A.L.C.); National Health and Medical Research Council Practitioner Fellowship and Reid Charitable Trusts (to I.P.W.); National Health and Medical Research Council Practitioner Fellowship (to A.W.R., 637309) and a Victorian Cancer Agency Fellowship (to A.W.R.). The part of the study conducted at Trinity College, Dublin, was supported by Science Foundation Ireland.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alexander WS et al. (1999) SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98: 597–608 [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F, Virelizier JL, Fiers W (1985) Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol 134: 2444–2448 [PubMed] [Google Scholar]
- Boraschi D, Censini S, Tagliabue A (1984) Interferon-gamma reduces macrophage-suppressive activity by inhibiting prostaglandin E2 release and inducing interleukin 1 production. J Immunol 133: 764–768 [PubMed] [Google Scholar]
- Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH (2009) Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J Immunol 183: 1715–1723 [DOI] [PubMed] [Google Scholar]
- Brod SA, Burns DK (1994) Suppression of relapsing experimental autoimmune encephalomyelitis in the SJL/J mouse by oral administration of type I interferons. Neurology 44: 1144–1148 [DOI] [PubMed] [Google Scholar]
- Burchett SK, Weaver WM, Westall JA, Larsen A, Kronheim S, Wilson CB (1988) Regulation of tumor necrosis factor/cachectin and IL-1 secretion in human mononuclear phagocytes. J Immunol 140: 3473–3481 [PubMed] [Google Scholar]
- Collart MA, Belin D, Vassalli JD, de Kossodo S, Vassalli P (1986) Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med 164: 2113–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer ML, Hu J, Kalvakolanu DV, Hasday JD, Cross AS (2001) IFN-γ inhibits lipopolysaccharide-induced interleukin-1β in primary murine macrophages via a Stat1-dependent pathway. J Interferon Cytokine Res 21: 485–494 [DOI] [PubMed] [Google Scholar]
- Gerrard TL, Siegel JP, Dyer DR, Zoon KC (1987) Differential effects of interferon-alpha and interferon-gamma on interleukin 1 secretion by monocytes. J Immunol 138: 2535–2540 [PubMed] [Google Scholar]
- Ghezzi P, Dinarello CA (1988) IL-1 induces IL-1. III. Specific inhibition of IL-1 production by IFN-gamma. J Immunol 140: 4238–4244 [PubMed] [Google Scholar]
- Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J (2009) T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature 460: 269–273 [DOI] [PubMed] [Google Scholar]
- Haq AU, Maca RD (1986) Role of IFN-gamma and alpha in IL 1 synthesis and secretion of in vitro differentiated human macrophages: a comparative study. Immunobiology 171: 451–460 [DOI] [PubMed] [Google Scholar]
- Hsu LC et al. (2008) A NOD2–NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA 105: 7803–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB (2006) IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24: 563–574 [DOI] [PubMed] [Google Scholar]
- Kelchtermans H, Billiau A, Matthys P (2008) How interferon-gamma keeps autoimmune diseases in check. Trends Immunol 29: 479–486 [DOI] [PubMed] [Google Scholar]
- Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ (2008) ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol 10: 1866–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Schols D, de Wolf-Peeters C, Billiau A (1999) Enhanced autoimmune arthritis in IFN-γ receptor-deficient mice is conditioned by mycobacteria in Freund's adjuvant and by increased expansion of Mac-1+ myeloid cells. J Immunol 163: 3503–3510 [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP (1987) Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology 37: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S (2009) IFN-β inhibits human Th17 cell differentiation. J Immunol 183: 5418–5427 [DOI] [PubMed] [Google Scholar]
- Ruschen S, Lemm G, Warnatz H (1989) Spontaneous and LPS-stimulated production of intracellular IL-1β by synovial macrophages in rheumatoid arthritis is inhibited by IFN-γ. Clin Exp Immunol 76: 246–251 [PMC free article] [PubMed] [Google Scholar]
- Schindler R, Ghezzi P, Dinarello CA (1989) Interferons as inhibitors of interleukin 1 induced interleukin 1 synthesis. Lymphokine Res 8: 275–280 [PubMed] [Google Scholar]
- Schindler R, Ghezzi P, Dinarello CA (1990) IL-1 induces IL-1. IV. IFN-γ suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol 144: 2216–2222 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.