Abstract
Mutations in the zebrafish gene moonshine, encoding the ortholog of tif1γ, cause profound anemia and embryonic lethality. In a recent issue of Cell, Bai et al. (2010) provide evidence that these defects arise from inefficient transcription elongation, implicating elongation as an important point of regulation during cell differentiation and development.
Development depends critically upon the establishment and maintenance of tissue-specific gene expression programs. Although much is known about the signaling pathways and transcription factors that influence cell fate decisions, the mechanisms underlying the precise temporal and spatial regulation of transcription during development remain unclear. One paradigm for understanding the differentiation of multipotent, self-renewing stem cells into lineage-committed cells is hematopoiesis. This process is conserved throughout vertebrate evolution, and the events that program hematopoietic stem cells to undergo lineage-restricted differentiation have been the subject of extensive research. Recently, work by Bai et al. (2010), published in Cell, sheds new light on the changes in gene expression that occur during erythropoiesis by revealing a decisive role for regulated transcription elongation.
This exciting finding comes on the heels of work indicating that transcription elongation is highly regulated during development (Nechaev and Adelman, 2008). In particular, promoter-proximal pausing of RNA polymerase II (Pol II) was recently shown to be widespread across metazoan genomes (Core et al., 2008; Nechaev and Adelman, 2008). Pausing occurs shortly after transcription initiation, when Pol II comes under the influence of negative transcription factors such as DSIF (DRB Sensitivity Inducing Factor). DSIF, comprised of Spt4 and Spt5 proteins, plays dual roles in transcription elongation: DSIF inhibits early elongation by inducing pausing but stimulates productive transcript synthesis further downstream (Price, 2008). This switch in DSIF activity and the release of paused Pol II into productive elongation is triggered by the recruitment of the P-TEFb (Positive Transcription Elongation Factor b) kinase. P-TEFb rapidly stimulates elongation by phosphorylating the C-terminal domains of both Spt5 and the largest subunit of Pol II (Price, 2008). Paused Pol II is prevalent at developmentally-regulated promoters, suggesting that having a polymerase “waiting” near the promoter might facilitate precise, synchronous expression of developmental genes (Boettiger and Levine, 2009; Nechaev and Adelman, 2008).
This idea is consistent with earlier studies of the murine β-globin gene cluster, which demonstrated that the locus control region (LCR) regulates gene activation primarily at the level of transcription elongation (Sawado et al., 2003) (Figure 1A). This pioneering work established that although deletion of the LCR resulted in a >90% reduction in β-globin transcript levels, it had only a modest affect on the recruitment of Pol II to the β-globin promoter. Importantly, presence of the LCR substantially increased phosphorylation of promoter-proximal Pol II and polymerase density within the β-globin gene, suggesting that gene expression is controlled through the release of paused polymerase.
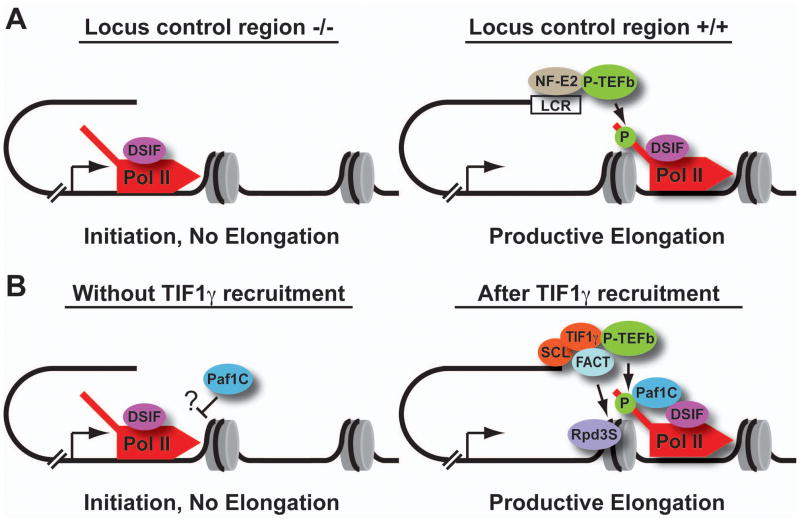
Figure 1. The elongation phase of transcription is highly regulated during hematopoiesis.
(A) Studies of the murine β-globin gene cluster have demonstrated that the critical locus control region (LCR) is not essential for recruitment of Pol II (red) to the β-globin promoter, but that productive elongation and phosphorylation of Pol II (green P) require an intact LCR. This finding implicates the transcription factor NF-E2 (brown) which binds the LCR, in delivery of P-TEFb (green) to the β-globin promoter, and release of Pol II from pausing mediated by DSIF (purple). (B) A similar model is proposed for erythroid gene transcription, wherein recruited Pol II would not efficiently transcribe blood genes in the absence of TIF1γ due to inhibition of transcription by DSIF and/or Paf1C (blue). Binding of TIF1γ and the SCL complex (orange) would stimulate release from pausing through recruitment of P-TEFb. Notably, FACT, Paf1C, and Rpd3S would work together both to permit transcription elongation through chromatin and to re-assemble nucleosomes over the transcribed gene, perhaps limiting subsequent rounds of transcription elongation.
Expanding on this idea, the results in Bai et al. implicate regulated elongation in hematopoiesis from zebrafish to humans. The researchers investigated moonshine, a mutant zebrafish with defects in erythroid maturation due to deficiency of TIF1γ, a key regulator of hematopoietic gene expression (Ransom et al., 2004). Through elegant suppressor screens in zebrafish, they identified the sunrise mutant, which carries a mutation in the cdc73 gene. cdc73 is an integral component of the Paf1 complex (Paf1C), which functions in transcription elongation and chromatin modification. Furthermore, Bai et al. show that loss of any subunit of Paf1C suppresses the moonshine phenotype, conclusively demonstrating a functional antagonism between Paf1C and TIF1γ in erythroid gene transcription.
Dissecting the opposing contributions of TIF1γ and Paf1C to hematopoiesis, however, is a tricky business. Controversy exists over the function of TIF1γ, which has been suggested to either promote or inhibit TGF-β signaling during blood development. Arguing that TIF1γ plays a stimulatory role, Bai et al. identified two positive transcription elongation factors as binding partners of TIF1γ: P-TEFb and FACT (Facilitates Access to Chromatin Templates). Intriguingly, their data suggest that TIF1γ could release paused Pol II by actively recruiting P-TEFb. Moreover, FACT is known to facilitate elongation through chromatin, which would further enhance elongation efficiency at TIF1γ target genes.
Thus, TIF1γ appears to promote transcription elongation. But how might the absence of Paf1C suppress mutations in TIF1γ To address these questions, Bai et al. considered the myriad roles of Paf1C, including stimulation of elongation, histone modifications, and recruitment of 3’-RNA processing factors. Using additional genetic assays, Bai et al. ruled out a need for several Paf1C-dependent histone modifications and RNA processing factors in suppression of blood defects, narrowing the possibilities down to Paf1C’s role in transcription elongation, or in recruitment of the Rpd3S histone de-acetylation complex. Notably, all previous evidence indicates that the direct effects of Paf1C on transcription elongation are positive in nature and occur well downstream from the promoter (Kim et al., 2010), which is seemingly at odds with its ability to antagonize TIF1γ function.
In contrast, the indirect effect of Paf1C on recruitment of Rpd3S is generally thought to be inhibitory, since Rpd3S removes stimulatory acetyl groups from histones and helps re-assemble nucleosomes in the wake of elongating polymerase. Interestingly, in S. cerevisiae, mutations in a Paf1 subunit or Rpd3S suppress growth defects caused by deficiencies in the yeast homologs of P-TEFb (Bur1) or FACT (Buratowski, 2009). This raises striking parallels with the results in Bai et al., where loss of Paf1 suppresses deficiencies in TIF1γ, a factor that recruits P-TEFb and FACT. Thus, the negative role of Paf1C on erythroid gene transcription in sunrise mutants may occur through manipulation of promoter-proximal chromatin structure.
Bai et al. also provide support for Pol II pausing at erythroid genes. Blood defects in moonshine were rescued by mutations in foggy, the zebrafish Spt5 gene. Foggy was first isolated in genetic screens for factors that affected neuronal development (Guo et al., 2000), and the mutant identified caused early lethality through deficits in blood circulation and neuronal function. In vitro transcription assays revealed that the foggy mutant was unable to elicit Pol II pausing but was fully functional in its stimulatory activities, thereby suggesting that pausing was critical for early zebrafish development (Guo et al., 2000). Accordingly, the finding that this foggy mutant suppresses defects in moonshine argues that TIF1γ does indeed impact paused Pol II.
Thus, Bai et al. put forth the appealing model that defects in the moonshine mutants are manifested at the level of pausing (Figure 1B). This hypothesis makes several important predictions that await testing. For instance, if TIF1γ is required to overcome pausing at blood genes, then paused Pol II should be observed at these promoters in TIF1γ-deficient cells. Furthermore, if the loss of DSIF or Paf1C relieves the need for TIF1γ to release paused Pol II, then erythroid genes should experience aberrant transcription in these mutants, prior to receiving appropriate developmental cues. Finally, the intriguing negative role of Paf1C remains to be elucidated. Previous work has failed to detect Paf1C associated with paused Pol II (Kim et al., 2010), suggesting that Paf1C might affect early elongation indirectly. Regardless of the exact mechanisms, Bai et al. reveal a sophisticated interplay between multiple transcription factors, and expose transcription elongation as a critical checkpoint during cell differentiation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai X, Kim J, Yang Z, Jurynec MJ, Akie TE, Lee J, LeBlanc J, Sessa A, Jiang H, DiBlase A, Zhou, et al. Cell. doi: 10.1016/j.cell.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger AN, Levine M. Science. 2009;325:471–473. doi: 10.1126/science.1173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S. Mol Cell. 2009;36:541–546. doi: 10.1016/j.molcel.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Yamaguchi Y, Schilbach S, Wada T, Lee J, Goddard A, French D, Handa H, Rosenthal A. Nature. 2000;408:366–369. doi: 10.1038/35042590. [DOI] [PubMed] [Google Scholar]
- Kim J, Guermah M, Roeder RG. Cell. 2010;140:491–503. doi: 10.1016/j.cell.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechaev S, Adelman K. Cell cycle. 2008;7:1539–1544. doi: 10.4161/cc.7.11.6006. [DOI] [PubMed] [Google Scholar]
- Price DH. Mol Cell. 2008;30:7–10. doi: 10.1016/j.molcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ransom DG, Bahary N, Niss K, Traver D, Burns C, Trede NS, Paffett-Lugassy N, Saganic WJ, Lim CA, Hersey C, et al. PLoS biology. 2004;2:E237. doi: 10.1371/journal.pbio.0020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawado T, Halow J, Bender MA, Groudine M. Genes & development. 2003;17:1009–1018. doi: 10.1101/gad.1072303. [DOI] [PMC free article] [PubMed] [Google Scholar]