Abstract
Feedback inhibition of V(D)J recombination enforces antigen receptor allelic exclusion in mammalian lymphocytes. Yet, in-frame VβDJβ exons can assemble on both alleles in human and mouse αβ T lineage cells. To elucidate mechanisms that enforce TCRβ allelic exclusion in such cells, we analyzed Vβ expression and rearrangement in mice containing a functional Vβ14DJβ1.5Cβ1 gene (Vβ14NT) and/or Vβ8.2DJβ1.1Cβ1 transgene (Vβ8Tg). The majority of Vβ14NT and Vβ8Tg αβ T lineage cells expressed only Vβ14+ or Vβ8+ TCRβ chains, respectively, and lacked Vβ rearrangements on wild-type TCRβ loci. Yet, endogenous Vβ rearrangements and αβ T lineage cells expressing endogenous Vβs from wild-type alleles alone or with the pre-rearranged Vβ in cell surface TCRβ chains were observed in Vβ14NT and Vβ8Tg mice. Although nearly all Vβ8Tg:Vβ14NT thymocytes and splenic αβ T cells expressed Vβ8+ TCRβ chains, only half of these lymphocytes expressed Vβ14+ TCRβ chains even though similar steady state levels of Vβ14NT mRNA were expressed in both Vβ8+Vβ14+ and Vβ8+Vβ14+ populations. Our data demonstrate that post-transcriptional silencing of functionally assembled endogenous VβDJβCβ genes can enforce TCRβ allelic exclusion and reveal another mechanism that contributes to the development of lymphocytes with mono-specific antigen receptors.
Introduction
The adaptive immune systems of jawed vertebrates consist of T and B lymphocytes that express cell surface T cell antigen receptor (TCR) or B cell antigen receptor complexes. TCR and immunoglobulin (Ig) variable region exons are assembled in developing lymphocytes through the recombination of germline variable (V), diversity (D), and joining (J) gene segments (1). In mammals, the combination of possible rearrangement events within single genetic loci encoding each TCR and Ig chain contributes to diversification of antigen receptor binding specificities. However, in cartilaginous fish, each individual type of Ig chain is encoded by fully pre-assembled, partially pre-assembled, and un-assembled germline gene segments located within hundreds of independent genetic loci (2). Most lymphocytes in jawed vertebrates express cell surface antigen receptor chains from a single allele or locus, a phenomenon that is referred to as antigen receptor allelic or haplotypic exclusion. For example, approximately 99% of mouse and human αβ T cells express cell surface TCRβ chains from a single allele (3–5). The majority of lymphocytes in mice and humans assemble a single in-frame exon within TCRβ, IgH, and IgL loci due to feedback inhibition of V(D)J recombination, which is signaled by the expression of functional TCR or Ig chains and enforces antigen receptor allelic exclusion (6). In contrast, restricted expression of functional Ig genes from a single genetic locus appears to be the major mechanism that mediates haplotype exclusion in lymphocytes of cartilaginous fish (7).
In humans and mice, αβ T lymphocytes develop through a differentiation program that involves the assembly, expression, and selection of a functional VβDJβCβ gene from one allele (8). TCRβ genes are assembled through DJβ intermediates in CD4−CD8− (double-negative, DN) thymocytes(9). Transcription through a functional VβDJβ rearrangement generates TCRβ chains that can pair with pTα molecules to form pre-TCRs (8). These receptors signal feedback inhibition of further Vβ rearrangement to enforce TCRβ allelic exclusion and select DN cells for differentiation into CD4+CD8+ (double-positive, DP) thymocytes (8). DN cells that assemble an out-of-frame VβDJβ rearrangement on the first allele can attempt Vβ rearrangement on the second allele (9). In DP cells, TCRα genes are assembled on both alleles from Vα and Jα segments (10). In-frame VαJα rearrangements generate TCRα chains that can associate with TCRβ molecules to form αβ TCRs (8). Positive selection of αβ TCRs promotes further differentiation of DP cells into CD4+ or CD8+ (single positive, SP) thymocytes (8). These cells exit the thymus and migrate to the spleen and other peripheral locations as naive mature αβ T cells. However, DP thymocytes expressing auto-reactive αβ TCRs are frequently eliminated by apoptosis (8). TCRβ allelic exclusion has been hypothesized to prevent auto-immunity by facilitating the development and selection of cells with αβ TCRs of a single specificity (11).
Generation and analysis of mice containing different pre-assembled VβDJβCβ transgenes demonstrated that expression of a functional TCRβ chain can inhibit rearrangement and expression of endogenous Vβ segments (12). Enforcement of allelic exclusion by such feedback inhibition predicts that ~60% of αβ T cells contain DJβ intermediates and ~40% contain out-of-frame VβDJβ rearrangements on their non-selected alleles (13). Yet, sequence analyses of TCRβ joins or mRNA have revealed the presence of two in-frame VβDJβ rearrangements in 5–10% of mouse and human αβ T cells that exhibit allelic exclusion (14, 15). In addition, in-frame endogenous Vβ14Jβ rearrangements were found but not expressed in approximately 10% of αβ T cell hybridomas generated from mice with a modified TCRβ locus that permits direct Vβ14-to-Jβ rearrangement (16). Moreover, VβDJβCβ genes that were assembled in-frame within a transgenic TCRβ mini-locus were not expressed on αβ T cells of mice containing a pre-assembled TCRβ transgene (17). Furthermore, although TCRβ-mediated feedback inhibition is blocked in pTα−/− thymocytes, TCRβ allelic exclusion is maintained in the αβ T lineage cells of pTα−/− mice (18, 19). Collectively, these data indicate that additional mechanisms must restrict the cell surface expression of functionally assembled TCRβ genes; however the absence of an allotypic Cβ marker in either humans or mice has prevented definitive conclusions. Thus, to elucidate mechanisms that enforce allelic exclusion in cells with two functional VβDJβCβ genes, we analyzed Vβ expression and rearrangement in αβ T lineage cells of mice containing one allelic copy of a pre-assembled functional endogenous TCRβ gene and/or classical TCRβ transgene.
Materials and Methods
Mice
Generation and characterization of Vβ8Tg mice and LN2 embryonic stem cells containing the pre-assembled Vβ14Dβ1Jβ1.5Cβ gene were previously described (20, 21). Vβ8Tg mice were bred onto a 129SvEv (Taconic) background. LN2 cells were used to generate mice with the Vβ14NT allele transmitted through the germline. These mice were mated with 129SvEv mice to isolate the Vβ14NT allele from the other rearranged TCRβ and TCRα alleles. These Vβ14NT/+ mice were maintained on a 129SvEv background and mated with one another to generate the Vβ14NT/+, Vβ14NT/NT, and wild-type mice used in experiments. All experiments were performed on 4–6 week old mice in accordance relevant institutional and national guidelines and regulations and approved by the Children's Hospital of Philadelphia IACUC committee. None of the individual or compound mutant mice appeared or exhibited phenotypes by which they could be distinguished from wild-type littermate or age-matched controls.
Flow cytometry
Single cell suspensions of lymphocytes from thymuses and spleens were incubated with red blood cell lysis buffer (0.7 M NaCl and 17 mM Tris HCl). Cells were washed with FACS staining buffer (PBS containing 0.5% BSA) and stained with the following BD Pharmigen antibodies: APC-anti-Cβ (553174), APC-cy7-anti-B220 (552094), FITC-anti-Vβ14 (553258), PE-anti-Vβ8 (553862), PE-anti-Vβ10 (553285), biotin-anti-Vβ6 (553192), biotin-anti-Vβ5 (553188), and PE-Cyc7-SA (557598). Cells were stained in FACS staining buffer. Live cells were gated on the basis of forward or side scatter and DAPI exclusion (D1306; Invitrogen). Data were collected on an LSR II and were analyzed using FlowJo. 500,000 events were collected for each sample file. All displayed events were gated on single DAPI−B220−TCRβ+ cells.
PCR Analysis of Vβ Rearrangements
Total thymocytes or splenocytes were lysed in rapid lysis buffer (0.1 M Tris pH 8.5, 0.2% SDS, 0.005 M EDTA, 0.2 M NaCl, and 250µg/µl Proteinase K). Genomic DNA was isolated by isopropanol precipitation. PCR conditions for a final volume of 25µL were 10 X PCR Buffer (Qiagen), 0.2 mM dNTPs (ABI), 0.2 mM each primer, 5 units of Hot Star Taq polymerase (Qiagen), and 500 ng DNA. PCR cycles were: 94°C for 3 minutes; 40 cycles of 94°C for 45 seconds, 60°C for 1:30 minutes, 72°C for 2:30 minutes; and 72°C for 10 minutes. The Vβ specific primers and the 3'Jβ1.2 primer (P2) were previously described (22). The 3'Jβ2.2 primer is 5'-CTCCAACCCTGACTCAGATCCCCACC-3'. The Cβ2 primers are 5'-CAAACAAAAGGCTACCCTCGTG-3' and 5'- GCAGACAGAACCCCCTGATGATAG −3'.
Generation and Analysis of Hybridomas
The generation and analysis of TCRβ gene rearrangements in Vβ14NT/+ and Vβ14NT/NT αβ T cell hybridomas were conducted as previously described (16, 23, 24).
Analysis of Vβ14DJβ1.5Cβ1 mRNA Expression
The sort-purification of Vβ14+Vβ8+ and Vβ14+Vβ8− thymocytes from Vβ8Tg:Vβ14NT/+ mice was conducted on a FACS Aria with staining and gating strategy identical to that described above for flow cytometry. RNA was isolated using Trizol and poly-A cDNA was generated using the NEB Protoscript II cDNA synthesis kit. Expression levels of Vβ14DJβ1.5Cβ1 and GAPDH mRNAs were determined by qPCR on an ABI 7500 Fast Real-Time PCR machine using the following primer pairs: Vβ14F 5'-AGGCCACAATGCTATGTATTGGT-3' and Vβ14R 5'-TGAGGTTGG AAGCGACTTGA-3' primers or GAPDHF 5'-CTTCACCACCATGGAGAAGGC-3' and GAPDHR 5'-GGCATGGACTGTGGTCATGAG-3'.
Results
Expression of Endogenous Vβ Segments in αβ T Lineage Cells of Mice Containing a Pre-Assembled Functional VβDJβCβ Gene
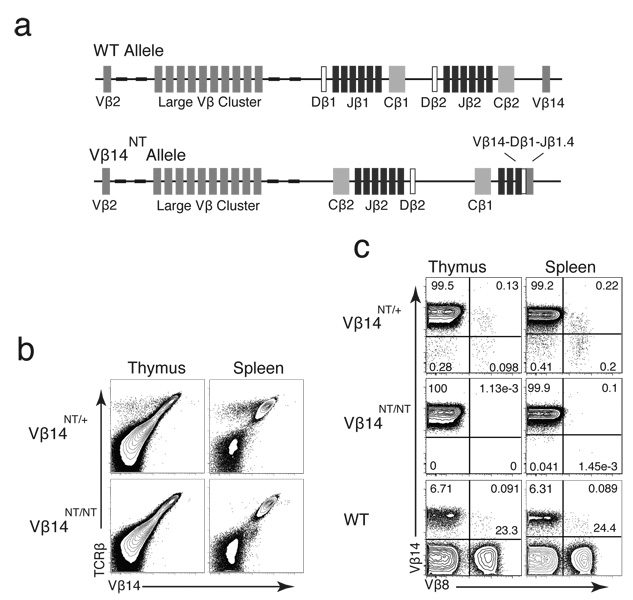
Most investigations of TCRβ allelic exclusion have been conducted through analyses of mice expressing pre-assembled functional VβDJβCβ transgenes. Physiologic relevance of such studies has been questioned due to the varying extents at which transgenes enforce allelic exclusion, the high copy number of transgenes often required for allelic exclusion, and other potential transgenic artifacts (19, 25, 26). Thus, we sought to study TCRβ allelic exclusion in mice containing a single allelic copy of a pre-assembled functional endogenous VβDJβCβ gene. Chimeric mice containing pre-assembled in-frame endogenous TCR genes have been generated through the transfer of αβ T cell nuclei into embryonic stem cells (20). We used stem cells reconstituted with the nucleus of a Vβ14+ αβ T cell to establish mice containing a pre-assembled functional endogenous Vβ14DJβ1.5Cβ1 gene (Vβ14NT) within their germline . These mice were bred with wild-type (WT) mice to separate the Vβ14NT allele from the other pre-rearranged TCR alleles and their offspring were inter-crossed to establish mice containing the Vβ14NT gene on one (Vβ14NT/+) or two (Vβ14NT/NT) alleles (Figure 1a). FACS analysis of Vβ14NT/+ and Vβ14NT/NT thymocytes and splenocytes with anti-Vβ14 and anti-Cβ antibodies revealed that most Vβ14NT/+ cells and all Vβ14NT/NT cells expressed Vβ14 within surface TCRβ chains (Figure 1b). Notably, cell populations lacking Vβ14 within surface TCRβ chains (Vβ14−Cβ+) were detectable in Vβ14NT/+, but not Vβ14NT/NT, mice (Figure 1b). These data indicate that expression of the pre-assembled functional Vβ14DJβ1.5Cβ1 gene within cell surface TCRβ chains can be silenced in Vβ14NT/+ mice.
Figure 1. Mice with a Pre-assembled Functional Vβ14DJβ1.4Cβ1 Gene Develop αβ T Cells Expressing Vβ14 and Vβ8 Segments.
(a) Schematic representations of the genomic organization of the wild-type and Vβ14NT TCRβ loci. The relative locations of germline Vβ, Dβ, Jβ, and Cβ gene segments and the pre-assembled Vβ14DJβ1.4 rearrangement are indicated. (b) FACS analysis of Vβ14 expression in Vβ14NT/+ mice. This analysis was conducted on mice of each genotype five independent times. Shown are representative plots of anti-Cβ and anti-Vβ14 stains conducted on thymocytes and splenocytes isolated from Vβ14NT/+ and Vβ14NT/NT mice. (c) FACS analysis of Vβ8 expression in Vβ14NT/+ mice. This analysis was conducted on mice of each genotype three independent times. Shown are representative plots of anti-Vβ14 and anti-Vβ8 stains conducted on thymocytes and splenocytes isolated from Vβ14NT/+, Vβ14NT/NT, and wild-type (WT) mice. The percentages of cells within each quadrant are indicated.
In mice, Vβ8 is the most highly represented Vβ within cell surface αβ TCR since three individual Vβ segments (Vβ8.1, Vβ8.2, and Vβ8.3) exist (27). The presence of Vβ8+Vβ3− and Vβ8+Vβ3+ splenic T cells has been observed in mice containing a Vβ3+ TCRβ transgene that prevents the expression of other endogenous Vβ segments (5). Thus, in an initial attempt to characterize the Vβ14−Cβ+ αβ T cell populations in Vβ14NT/+ mice, we conducted FACS analysis of Vβ14NT/+ and Vβ14NT/NT thymocytes and splenocytes with combinations of anti-Vβ8, anti-Vβ14, and anti-Cβ antibodies. In Vβ14NT/+ mice, we detected populations expressing only Vβ14, only Vβ8, or both Vβ14 and Vβ8 within surface TCRβ chains (Figure 1c). The frequencies of Vβ8+ αβ T lineage cells were significantly lower in Vβ14NT/+ mice as compared to wild-type mice (107 fold lower in thymocytes and 57 fold lower in splenocytes) (Figure 2b,c). Yet, we found only Vβ14+Vβ8−Cβ+ populations in Vβ14NT/NT mice (Figure 1c), indicating that the Vβ14−Cβ+ populations in Vβ14NT/+ mice represent bona fide αβ T cells rather than staining artifacts. These data demonstrate that Vβ8+ TCRβ chains from the wild-type allele can be expressed on the surface of Vβ14NT/+ αβ T lineage cells with or without Vβ14+ chains from the Vβ14NT allele, the former which results in TCRβ allelic inclusion.
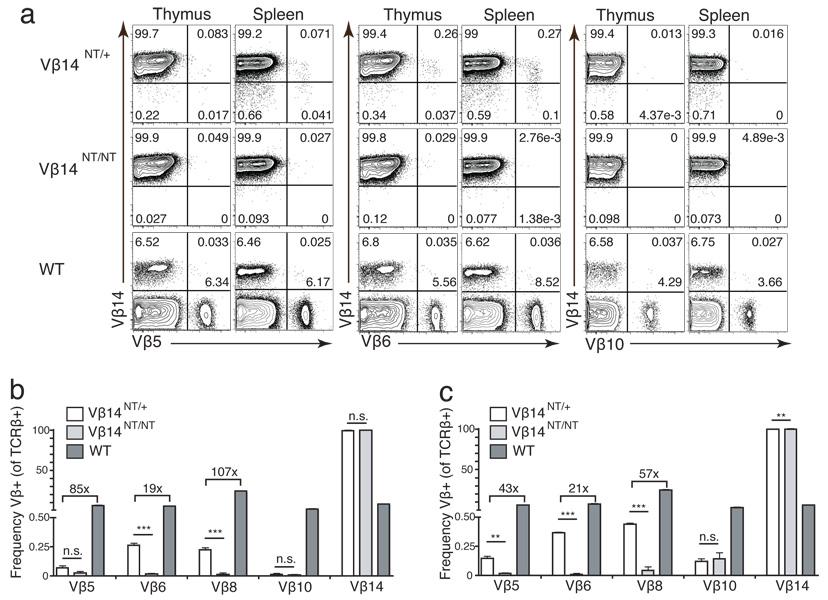
Figure 2. Vβ14NT/+ Mice Develop αβ T Cells Expressing a Limited Repertoire of Endogenous Vβ Segments.
(a) FACS analysis of Vβ expression in Vβ14NT/+ mice. Shown are representative plots of anti-Vβ14 and anti-Vβ5, anti-Vβ6, or anti-Vβ10 stains conducted on thymocytes and splenocytes isolated from Vβ14NT/+, Vβ14NT/NT, and WT mice. The percentages of cells within each quadrant are indicated. (b-c) Bar graphs depicting Vβ usage in (b) thymocytes or (c) splenocytes isolated from Vβ14NT/+, VB14NT/NT, and WT mice. These analyses were conducted on mice of each genotype three independent times. The numbers above the bars represent fold differences in expression of the particular Vβ segment between the indicated genotypes. Error bars are SEM and two-tailed Student’s t tests were performed. n.s., not significant; **, P=0.001–0.01; ***, P<0.001.
The murine TCRβ locus contains 20 functional Vβ segments that can be expressed as part of cell surface αβ TCRs (28). Thus, to evaluate whether endogenous Vβ segments other than Vβ8 are expressed within TCRβ chains on αβ T cells of Vβ14NT/+ mice, we conducted FACS analysis of Vβ14NT/+ and Vβ14NT/NT thymocytes and splenocytes with combinations of anti-Vβ5, anti-Vβ6, anti-Vβ10, anti-Vβ12, anti-Vβ14, and anti-Cβ antibodies. We found cell populations expressing Vβ5 or Vβ6 segments within surface TCRβ chains on both Vβ14+ and Vβ14− cells in Vβ14NT/+ mice (Figure 2a). Yet, we observed only Vβ14+Cβ+ populations in Vβ14NT/NT mice (Figure 2a), indicating that the Vβ5+Cβ+ and Vβ6+Cβ+ cells in Vβ14NT/+ mice also represent bona fide αβ T cells rather than staining artifacts. We were unable to detect bona fide αβ T lineage cells expressing Vβ10 or Vβ12 within TCRβ chains on Vβ14NT/+ or Vβ14NT/NT mice (Figure 2c, data not shown). The frequency of αβ T lineage cells expressing Vβ5 and Vβ6 were significantly lower in Vβ14NT/+ mice as compared to wild-type mice (Vβ5 was 85 fold lower in thymocytes and 43 fold lower in splenocytes, Vβ6 was 19 fold lower in thymocytes and 21 fold lower in splenocytes) (Figure 2c). These data demonstrate that a limited repertoire of endogenous Vβ segments are expressed within TCRβ chains on Vβ14NT/+ αβ T lymphocytes, which either results in TCRβ allelic inclusion or occurs in association with silenced cell surface expression of the pre-assembled functional Vβ14DJβ1.5Cβ1 gene.
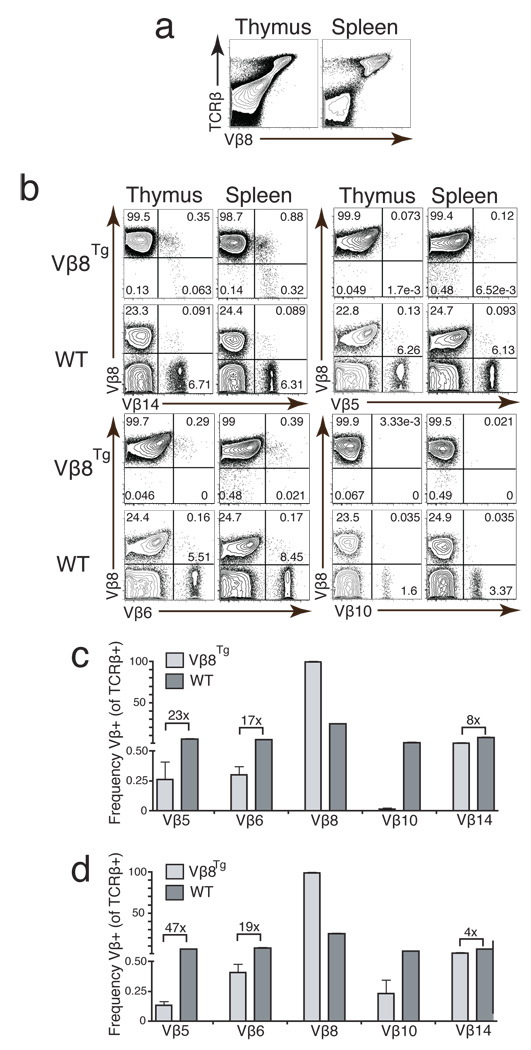
For purpose of comparison between Vβ14NT and TCRβ transgenic mice, we conducted the same FACS analyses on thymocytes and splenocytes of mice expressing a pre-assembled functional Vβ8.2DJβ1.1Cβ1 transgene from one allele (Vβ8Tg)(21). Vβ8Tg mice have been shown to exhibit feedback inhibition of Vβ rearrangement and TCRβ allelic exclusion (25, 29). Consistent with these published findings, we observed that almost all Vβ8Tg αβ T cells expressed Vβ8 within cell surface TCRβ chains (Figure 3a). We failed to detect significant populations of αβ T cells expressing Vβ10 in Vβ8Tg mice (Figure 3b). Although we observed cell populations expressing Vβ5, Vβ6, and Vβ14 segments within surface TCRβ chains of Vβ8Tg αβ T lineage cells (Figure 3b), the frequencies of cells expressing these Vβ segments in Vβ8Tg mice were significantly lower than those in wild-type mice (Vβ5 was 23 fold lower in thymocytes and 47 fold lower in splenocytes, Vβ6 was 17 fold lower in thymocytes and 19 fold lower in splenocytes, Vβ14 was 8 fold lower in thymocytes and 4 fold lower in splenocytes) (Figure 3c,d). TCRβ chains with these Vβ segments are observed predominantly on cells that also express surface Vβ8+ chains resulting in TCRβ allelic inclusion (Figure 3b). Since similar Vβ5+Cβ+ and Vβ6+Cβ+ populations were observed in Vβ14NT/+ mice, these findings demonstrate that a limited repertoire of endogenous Vβ segments also is expressed within surface TCRβ chains on Vβ8Tg αβ T lymphocytes. Collectively, our data and published observations (5) indicate that the incomplete down-regulation of endogenous Vβ expression and silenced cell surface expression of functional TCRβ chains is not unique to TCRβ trangenic mice, but rather a general phenomenon of mice containing pre-assembled functional VβDJβCβ genes.
Figure 3. Vβ8Tg Mice Develop αβ T Cells Expressing a Limited Repertoire of Endogenous Vβ Segments.
(a) FACS analysis of Vβ8 expression in Vβ8Tg mice. Shown are representative plots of anti-Cβ and anti-Vβ8 stains conducted on thymocytes and splenocytes isolated from Vβ8Tg mice. Shown are representative plots of anti-Vβ8 and anti-Vβ14, anti-Vβ5, anti-Vβ6, or anti-Vβ10 stains conducted on thymocytes and splenocytes isolated from Vβ8Tg and WT mice. The percentages of cells within each quadrant are indicated. (c-d) Bar graphs depicting Vβ usage in (c) thymocytes or (d) splenocytes isolated from Vβ8Tg and WT mice. These analyses were conducted on mice of each genotype three independent times. The numbers above the bars represent fold differences in expression of the particular Vβ segment between the two genotypes. Error bars are SEM.
A Larger Repertoire of Endogenous Vβ Segments Rearranges in Thymocytes Containing Pre-Assembled Functional VβDJβCβ Genes
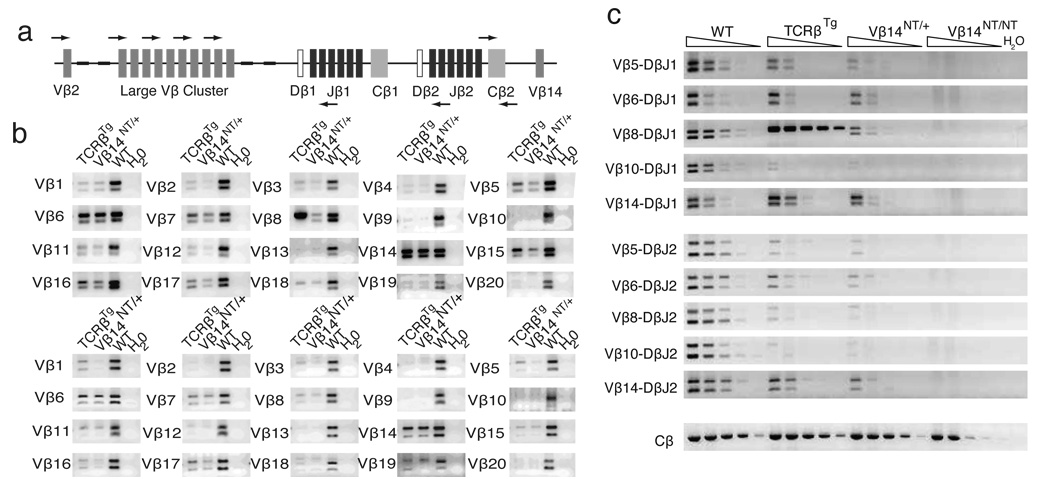
Our observations that endogenous Vβ segments are expressed within TCRβ chains on the surface of Vβ14NT/+ and Vβ8Tg αβ T cells indicate that Vβ rearrangements must have occurred on wild-type alleles in developing Vβ14NT/+ and Vβ8Tg thymocytes. Since surface expression of pre-assembled functional VβDJβCβ chains can be silenced, FACS analysis with anti-Vβ specific antibodies cannot be used as an accurate readout of Vβ-to-DJβ rearrangements. Thus, to ascertain the repertoire of Vβ rearrangements in αβ T lineage cells of Vβ14NT/+ and Vβ8Tg mice, we conducted PCR-based analysis of VβDJβ joins in wild-type, Vβ8Tg, Vβ14NT/+, and Vβ14NT/NT thymocytes. The murine TCRβ locus contains 20 functional Vβ segments and two Dβ-Jβ clusters (Dβ1-Jβ1 and Dβ2-Jβ2), each with one Dβ segment and six functional Jβ segments (Figure 4a). We used combinations of Vβ and Jβ specific primers to amplify potential rearrangements of each functional endogenous Vβ segment to DJβ complexes involving Jβ1.1/Jβ1.2 or Jβ2.1/Jβ2.2 segments (Figure 4a). We found that the levels of rearrangements involving many Vβ segments to DJβ1.1/DJβ1.2 and DJβ2.1/DJβ2.2 complexes were either undetectable or substantially reduced in Vβ8Tg and Vβ14NT/+ cells, as compared to in wild-type cells (Figure 4b). In contrast, we found that the levels of rearrangements involving Vβ5, Vβ6, Vβ7, Vβ8, Vβ14, Vβ15, Vβ16, and Vβ17 segments to DJβ1.1/DJβ1.2 complexes were unchanged or slightly reduced in Vβ8Tg and Vβ14NT/+ cells, as compared to in wild-type cells (Figure 4b). These data demonstrate that a limited repertoire of endogenous Vβ segments can rearrange at appreciable levels in Vβ14NT/+ and Vβ8Tg thymocytes despite the presence of a pre-assembled functional VβDJβCβ gene/transgene.
Figure 4. Endogenous Vβ Segments Rearrange in Vβ14 NT/+ and Vβ8Tg Thymocytes.
(a) PCR strategy for amplification of Vβ rearrangements to DJβ1.1/DJβ1.2 and DJβ2.1/DJβ2.2 complexes. Shown is a schematic representation of the wild-type TCRβ locus depicting the relative locations of representative Vβ, Dβ, Jβ, and Cβ gene segments, as well as the primers located just downstream of Jβ1.2 or Jβ2.2 and the Cβ2 primers. (b) PCR analysis of potential Vβ-to-DJβ1 and Vβ-to-DJβ2 rearrangements. Shown are representative PCR amplifications of Vβ rearrangements to (top panel) DJβ1.1/DJβ1.2 and (bottom panel) DJβ2.1/DJβ2.2 complexes for the indicated Vβ segments performed on DNA isolated from Vβ8Tg, Vβ14NT/+, or WT thymocytes. The amounts of DNA and numbers of PCR cycles used were previously demonstrated to amplify rearrangements within the linear range for the wild-type sample. (c) Quantitative PCR analysis of Vβ-to-DJβ1 and Vβ-to-DJβ2 rearrangements. Shown are representative PCR amplifications of Vβ rearrangements to DJβ1.1/DJβ1.2 and DJβ2.1/DJβ2.2 complexes for the indicated Vβ segments using serial 1:5 dilutions of DNA isolated from WT, Vβ8Tg, Vβ14NT/+, or Vβ14NT/NT thymocytes. Also shown is a representative PCR amplification of Cβ2 as a control for DNA content.
Our PCR data appear in conflict with previous studies concluding that the levels of rearrangements involving all endogenous Vβ segments are substantially reduced in αβ T lineage cells of Vβ8Tg mice (25, 29). These previous experiments quantified Vβ rearrangements only by PCR amplification of VβDJβ2 joins since, theoretically, VβDJβ1 joins can form on extra-chromosomal excision circles that might not be subject to feedback inhibition. To our knowledge, the direct quantification of chromosomal VβDJβ rearrangements in αβ T cells of mice containing pre-assembled TCRβ transgenes/genes has not been reported. Therefore, we generated panels of Vβ14NT/+ and Vβ8Tg αβ T cell hybridomas and quantified chromosomal TCRβ rearrangements by Southern blot analysis on EcoRI-digested genomic DNA using a series of TCRβ locus probes. Of the 82 Vβ14NT/+ hybridomas analyzed, 66 (81%) contained DJβ rearrangements and 10 (12%) contained VβDJβ rearrangements on the wild-type TCRβ allele. Similarly, of the 129 Vβ8Tg hybridomas analyzed, 102 (79%) contained DJβ rearrangements on one or both wild-type alleles and 12 (9.3%) contained VβDJβ rearrangements on one or both TCRβ alleles (Table I). The remaining 6 (7%) Vβ14NT/+ and 15 (11%) Vβ8Tg αβ T cell hybridomas contained germline TCRβ loci, Vβ-to-Dβ rearrangement, or rearranged loci with Southern patterns suggesting aberrant Dβ-to-Jβ rearrangements that deleted Jβ coding sequences (Table I). In addition to VβDJβ joins on selected alleles, ~60% of normal αβ T cells contain DJβ joins and ~40% contain VβDJβ joins on non-selected alleles. Accordingly, the overall level of chromosomal VβDJβ rearrangements is reduced only approximately four fold in Vβ14NT/+ and Vβ8Tg αβ T lineage cells, as compared to wild-type cells.
Table I. Analysis of TCRβ Rearrangements in Vβ14NT/+ and Vβ8Tg αβ T Cell Hybridomas.
Southern blot analysis using a series of TCRβ locus probes was used to characterize and quantify TCRβ rearrangements on wild-type alleles in panels of Vβ14NT/+ and Vβ8Tg αβ T cell hybridomas.
| Total | Dβ-to-Jβ | Vβ-to-DJβ | Other | |
|---|---|---|---|---|
| Vβ14NT/+ | 82 | 66 | 10 | 6 |
| 80.5% | 12.2% | 6.1% | ||
| TCRβTg | 129 | 102 | 12 | 15 |
| 79% | 9.3% | 11.6% | ||
This modest reduction in the overall level of chromosomal Vβ rearrangements, as compared to the substantial decrease in the numbers of cells expressing endogenous Vβ segments, indicates that not all VβDJβCβ genes assembled in-frame on wild-type alleles are expressed within TCRβ chains on Vβ14NT/+ and Vβ8Tg αβ T lineage cells. To further demonstrate this point, we conducted PCR analysis on serially diluted thymocyte DNA to quantify the levels of endogenous Vβ5, Vβ6, Vβ8, Vβ10, and Vβ14 rearrangements to DJβ1.1/DJβ1.2 and DJβ2.1/DJβ2.2 complexes in Vβ8Tg and Vβ14NT/+ cells, as compared to wild-type cells. We found that the levels of Vβ6 and Vβ14 rearrangements to DJβ1.1/DJβ1.2 complexes were comparable among Vβ8Tg, Vβ14NT/+, and wild-type cells, while Vβ6 and Vβ14 rearrangements to DJβ2.1/DJβ2.2 complexes were reduced ~5 fold in Vβ8Tg cells and ~25 fold in Vβ14NT/+ cells (Figure 4c). The levels of Vβ5 rearrangements to DJβ1.1/DJβ1.2 complexes were reduced ~5 fold in Vβ8Tg cells and ~25 fold in Vβ14NT/+ cells, and the levels of Vβ5 rearrangements to DJβ2.1/DJβ2.2 complexes were reduced greater than 25 fold in Vβ8Tg and Vβ14NT/+ cells (Figure 4c). Vβ8 rearrangements to DJβ1.1/DJβ1.2 and DJβ2.1/DJβ2.2 complexes were reduced ~25 fold and ~100 fold, respectively, in Vβ14NT/+ cells (Figure 4c). Due to the genomic organization of Vβ8Tg, we were unable to amplify endogenous Vβ8 rearrangements to DJβ1.1/DJβ1.2 complexes in Vβ8Tg cells; Vβ8 rearrangements to DJβ2.1/DJβ2.2 complexes were reduced greater than 25 fold (Figure 4c). Consistent with our ability to detect only Vβ14+Vβ8−Cβ+ populations in Vβ14NT/NT mice, we observed no PCR amplicons of Vβ-to-DJβ rearrangements in Vβ14NT/NT cells (Figure 4c). Our data indicate that the levels of chromosomal Vβ6 and Vβ14 rearrangements in Vβ14NT/+ and Vβ8Tg αβ T lineage cells are reduced to a lesser extent than are the numbers of cells expressing Vβ6 and Vβ14 within surface TCRβ chains.
Post-Transcriptional Silencing of Functionally Assembled Endogenous VβDJβCβ Genes Contributes to TCRβ Allelic Exclusion
Since αβ T lineage cells expressing two functional TCRβ genes are not selected against (30), our observations are consistent with the notion that not all VβDJβCβ genes assembled in-frame on wild-type alleles are expressed on Vβ14NT/+ and Vβ8Tg αβ T cells. The inability of VHDJHCH chains to form functional pre-BCR and promote differentiation can result in their lack of expression on the surface of B cells, ensuring IgH allelic exclusion (31). Yet, the silencing of Vβ14 and Vβ8 expression on Vβ14NT/+ and Vβ8Tg cells, respectively, cannot be due to defects in pairing with pTα because the Vβ14DJβ1.5Cβ1 and Vβ8.2DJβ1.1Cβ1 genes were isolated from selected TCRβ alleles. Mature αβ T lineage cells frequently express intracellular TCRα chains from both alleles, but exhibit TCRα allelic exclusion through post-translational mechanisms that appear to include competition between TCRα chains for a single TCRβ chain or inability of one TCRα chain to pair with the expressed TCRβ chain (13, 32). Thus, we next conducted intracellular FACS analysis of Vβ14NT/+, Vβ8Tg, and Vβ14NT/NT αβ T lineage cells with anti-Vβ14 and anti-Vβ8 antibodies to evaluate whether analogous mechanisms may restrict cell surface expression of TCRβ chains. We found Vβ8+ TCRβ chains inside of Vβ14NT/+, but not Vβ14NT/NT, thymocytes, and Vβ14+ TCRβ chains inside of Vβ8Tg thymocytes (Figure 5a). The percentages of Vβ14NT/+ and Vβ8Tg thymocytes with intracellular and extracellular Vβ8+ and Vβ14+ TCRβ chains, respectively, were equivalent (Compare Figures 1c and 5a). These data suggest that not all Vβ8DJβCβ and Vβ14DJβCβ genes assembled in-frame on wild-type alleles are expressed as TCRβ chains within or on the surface of Vβ14NT/+ and Vβ8Tg cells, respectively.
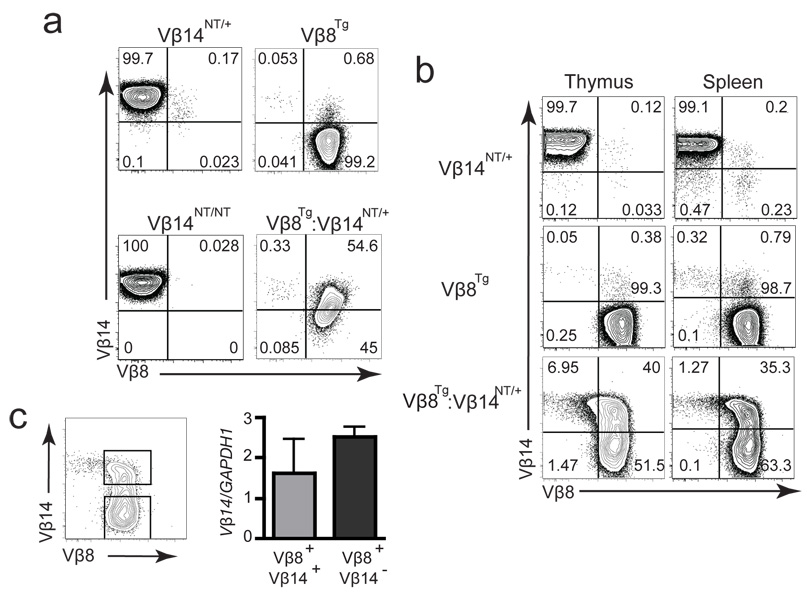
Figure 5. Silencing of Functional VβDJβCβ Genes in αβ T Lineage Cells.
(a) Intracellular FACS analysis of Vβ14 and Vβ8 expression. This analysis was conducted on mice of each genotype three independent times. Shown are representative plots of anti-Vβ14 and anti-Vβ8 intracellular stains conducted on thymocytes isolated from Vβ14NT/+, Vβ8Tg, Vβ14NT/NT, and Vβ8Tg:Vβ14NT/+ mice. The percentages of cells within each quadrant are indicated. (b) Extracellular FACS analysis of Vβ14 and Vβ8 expression. This analysis was conducted on mice of each genotype three independent times. Shown are representative plots of anti-Vβ14 and anti-Vβ8 stains conducted on thymocytes and splenocytes isolated from Vβ14NT/+, Vβ8Tg, and Vβ8Tg:Vβ14NT/+ mice. The percentages of cells within each quadrant are indicated. (c) qPCR analysis of Vβ14NT mRNA expression in Vβ8Tg:Vβ14NT/+ αβ T cells. The gating strategy for sorting of Vβ14+Vβ8+ and Vβ14+Vβ8− αβ T cells is shown on the left. A bar graph depicting the Vβ14NT mRNA levels relative to GAPDH mRNA levels in Vβ14+Vβ8+ and Vβ14+Vβ8− αβ T cells sort-purified from Vβ8Tg:Vβ14NT/+ mice is shown on the right. This experimental analysis was conducted three independent times. Error bars are SEM.
To demonstrate that the expression of functionally assembled endogenous VβDJβCβ genes within TCRβ chains can be silenced, we bred together Vβ14NT/+ and Vβ8Tg mice to generate Vβ8Tg:Vβ14NT/+ mice. Extracellular FACS analysis of Vβ8Tg:Vβ14NT/+ thymocytes and splenocytes with anti-Vβ14, anti-Vβ8, and anti-Cβ antibodies revealed substantial populations of Vβ8+Vβ14+Cβ+ and Vβ8+Vβ14−Cβ+ cells and a minor population of Vβ8−Vβ14+Cβ+ cells (Figure 5b). Nearly all Vβ8Tg:Vβ14NT/+ cells expressed Vβ8, but only half expressed Vβ14, within cell surface TCRβ chains. Intracellular FACS analysis of Vβ8Tg:Vβ14NT/+ thymocytes and splenocytes with anti-Vβ14, anti-Vβ8, and anti-Cβ antibodies showed populations of Vβ8+Vβ14+Cβ+, Vβ8+Vβ14−Cβ+, and Vβ8−Vβ14+Cβ+ cells (Figure 5a), which were present at similar numbers as those observed with extracellular FACS analyses (compare Figures 5a and b). Notably, almost all Vβ8Tg:Vβ14NT/+ cells expressed Vβ8, but only half expressed Vβ14, as part of intracellular TCRβ chains. These data indicate that the expression of functionally assembled endogenous VβDJβCβ genes within TCRβ chains can be silenced in mouse lymphocytes.
The silenced expression of functionally assembled VβDJβCβ genes within TCRβ chains could occur at the level of mRNA or protein expression. To determine the level at which the pre-assembled functional endogenous Vβ14DJβ1.5Cβ1 gene is silenced, we used qPCR to quantify the steady-state levels of mature Vβ14+ mRNA in sort-purified Vβ8+Vβ14+ and Vβ8+Vβ14− splenic αβ T cells of Vβ8Tg:Vβ14NT/+ mice. We found the steady state levels of Vβ14+ transcripts were comparable between each population of cells (Figure 5c). These data demonstrate that expression of the Vβ14NT gene can be silenced at the level of protein. Thus, we conclude that post-transcriptional silencing of functionally assembled endogenous VβDJβCβ genes can contribute to the enforcement of TCRβ allelic exclusion in mammalian lymphocytes.
Discussion
Here, we have investigated VβDJβCβ gene/transgene expression and the rearrangement and expression of endogenous Vβ segments in αβ T lineage cells of mice containing the Vβ14NT gene and/or Vβ8Tg transgene. We found that most Vβ14NT/+ αβ T lineage cells isolated from 4–6 week old mice express only Vβ14 within cell surface TCRβ chains. These data provide direct evidence that expression of a functionally assembled VβDJβCβ gene from one allele can enforce TCRβ allelic exclusion, as would be expected from prior analyses of TCRβ trangenic mice. Yet, despite TCRβ feedback inhibition of Vβ rearrangements, a limited repertoire of endogenous Vβ segments is expressed within TCRβ chains on Vβ14NT/+ αβ T lymphocytes. Expression of these Vβ segments can result in TCRβ allelic inclusion or correlate with silenced expression of the functional Vβ14NT gene within cell surface TCRβ chains. We obtained analogous data through the analysis of Vβ8Tg mice. Similar observations have been published for mice containing a Vβ3+ TCRβ transgene (5). Thus, our current study indicates that incomplete down-regulation of endogenous Vβ expression and silenced cell surface expression of pre-assembled TCRβ chains is a general phenomenon in αβ T lineage cells containing functional VβDJβCβ genes/transgenes. However, as discussed below, the expression of endogenous Vβ segments within such mice might be attributable to a common non-physiologic aspect of V(D)J recombination in DN thymocytes with pre-assembled functional TCRβ genes/transgenes.
Consistent with the incomplete down-regulation of endogenous Vβ expression, we found chromosomal VβDJβ1 joins on wild-type TCRβ alleles in ~10% of Vβ14NT/+ and Vβ8Tg αβ T lineage cells. Since ~40% of normal αβ T cells contain VβDJβ joins on non-selected TCRβ alleles, our data reveals that the overall level of endogenous Vβ rearrangements may be reduced only four-fold lower in Vβ14NT/+ and Vβ8Tg αβ T lineage cells as compared to wild-type cells. A precise quantification cannot be made since VβDJβ2 joins in wild-type cells could occur arise through primary or secondary Vβ rearrangements. This modest reduction seems at odds with published studies demonstrating that TCRβ transgenes such as Vβ8Tg inhibit endogenous Vβ rearrangements to an apparently greater extent (25, 29). However, analyses of Vβ rearrangements in TCRβ transgenic mice predominantly have been conducted by PCR amplification of VβDJβ2 joins since, theoretically, VβDJβ1 joins can assemble on extra-chromosomal circles that might not be subject to feedback inhibition. Considering the results of our analysis of TCRβ rearrangements in αβ T cell hybridomas generated from mice containing a pre-assembled TCRβ gene or transgene, reappraisals of conclusions gained from some previous studies of TCRβ mediated feedback inhibition may be warranted. Still, the frequency of Vβ rearrangements on wild-type alleles in Vβ14NT/+ and Vβ8Tg cells is higher than we expected considering the accepted model of TCRβ mediated feedback inhibition. DNA cleavage during V(D)J recombination activates ATM-dependent responses that may regulate lymphocyte differentiation and antigen receptor gene rearrangements (33, 34). We have recently found that Atm−/− αβ T lineage cells exhibit a higher frequency of TCRβ allelic inclusion than wild-type cells (N. S. and C.H.B., unpublished observations). In this context, perhaps the ability of TCRβ genes/transgenes to bypass the necessity of assembling VβDJβCβ genes through DNA cleavage prevents the activation of ATM-dependent signals that inhibit endogenous Vβ rearrangements.
We have also discovered that TCRβ allelic exclusion in mouse lymphocytes can be enforced through silencing the expression of functionally assembled VβDJβCβ genes within cell surface TCRβ chains. In Vβ14NT/+ and Vβ8Tg αβ T lineage cells, TCRβ allelic exclusion of some endogenous Vβs mainly occurs through silencing of assembled VβDJβCβ genes involving these Vβ segments. Differential regulation of Vβ expression by inhibition of rearrangement versus silencing in Vβ14NT/+ and Vβ8Tg αβ T lineage cells reinforces previous conclusions that germline transcription and recombinational accessibility of each Vβ segment is regulated individually (35, 36) and distinct cis acting elements control Vβ rearrangement and TCRβ allelic exclusion (37). The endogenous Vβ segments that are regulated by feedback inhibition versus silencing in Vβ14NT/+ and Vβ8Tg cells are interspersed throughout the TCRβ locus and reside both proximal and distal from Dβ-Jβ segments. Our comparison of Vβ promoter, coding, and recombination signal sequences failed to reveal any similarities within or differences between these two groups of Vβs that could provide insight into the mechanistic basis for their distinct regulation. Yet, we did observe a low density of transposons and repetitive genomic sequences directly upstream or downstream of the Vβs that rearranged in Vβ14NT/+ and Vβ8Tg cells. Since these types of DNA elements promote epigenetic changes that inhibit site-specific genomic recombination events in Schizosaccharomyces pombe and Tetrahymena thermophila (38, 39), and possibly DH-to-JH rearrangements in developing B cells (40), there is reason to speculate that Vβ-to-DJβ rearrangements may be down regulated by similar mechanisms. Considering that VHDJHCH transgenes can enforce IgH allelic exclusion without preventing the rearrangement of proximal VH segments (41, 42), post-recombination silencing of antigen receptor genes may contribute to enforce allelic exclusion of more than TCRβ genes.
Post-transcriptional silencing of the Vβ14NT gene contributes to TCRβ allelic exclusion in approximately half of Vβ14NT:Vβ8Tg αβ T lineage cells. We demonstrated that this silencing occurs at the protein level, indicating that either the Vβ14NT mRNA is not translated or the Vβ14NT chain is rapidly degraded in ~50% of αβ T cells expressing the Vβ8.2DJβ1.1Cβ1 transgene. In Vβ14+Vβ8+ cells, the Vβ8Tg and Vβ14NT proteins must each form stable αβ TCR complexes with the TCRα chains expressed in Vβ14+Vβ8+ cells. Perhaps intrinsic properties or high expression of the Vβ8Tg protein outcompetes the Vβ14NT protein for association with TCRα chains in Vβ14−Vβ8+ cells, leading to the rapid degradation of free Vβ14NT chains. Transcripts of in-frame VβDJβCβ genes from both alleles have been isolated from wild-type αβ T cells that exhibit TCRβ allelic exclusion (15), however experiments to determine potential expression of both genes within intracellular TCRβ were never reported. Consequently, future experiments are needed to evaluate whether the regulation of TCRβ allelic exclusion at the protein level is a general mechanism that extends to other endogenous VβDJβCβ genes and occurs in cells lacking pre-assembled TCRβ transgenes. Although Vβ14NT can be silenced at the protein level in Vβ14NT:Vβ8Tg cells, our data cannot exclude contributions of other mechanisms, such as transcriptional or translational silencing, to enforce TCRβ allelic exclusion in αβ T lineage cells that have assembled in-frame VβDJβCβ genes on both alleles. Consistent with this notion, TCRβ allelic exclusion of a Vβ13 gene segment inserted upstream of the endogenous Dβ1 segment can be mediated through transcriptional down-regulation post-recombination (37). Thus, our findings should alert the field that, to reach unequivocal conclusions, future studies of TCRβ allelic exclusion and feedback inhibition might need to include assays that quantify chromosomal Vβ-to-DJβ rearrangements, VβDJβCβ mRNA, and intracellular TCRβ protein.
Acknowledgments
This work was supported by the Department of Pathology and Laboratory Medicine and the Center for Childhood Cancer Research of the Children's Hospital of Philadelphia (C.H.B.), and the Abramson Family Cancer Research Institute (C.H.B.). B.L.B. is supported by Training Grant TG GM-07229 of the University of Pennsylvania. C.H.B. was a Pew Scholar in the Biomedical Sciences and is a Leukemia and Lymphoma Society Scholar.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109 Suppl:S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 2.Eason DD, Litman GW. Haplotype exclusion: the unique case presented by multiple immunoglobulin gene loci in cartilaginous fish. Semin Immunol. 2002;14:145–152. doi: 10.1016/s1044-5323(02)00038-6. discussion 220. [DOI] [PubMed] [Google Scholar]
- 3.Davodeau F, Peyrat MA, Romagne F, Necker A, Hallet MM, Vie H, Bonneville M. Dual T cell receptor beta chain expression on human T lymphocytes. J Exp Med. 1995;181:1391–1398. doi: 10.1084/jem.181.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padovan E, Giachino C, Cella M, Valitutti S, Acuto O, Lanzavecchia A. Normal T lymphocytes can express two different T cell receptor beta chains: implications for the mechanism of allelic exclusion. J Exp Med. 1995;181:1587–1591. doi: 10.1084/jem.181.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balomenos D, Balderas RS, Mulvany KP, Kaye J, Kono DH, Theofilopoulos AN. Incomplete T cell receptor V beta allelic exclusion and dual V beta-expressing cells. J Immunol. 1995;155:3308–3312. [PubMed] [Google Scholar]
- 6.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Eason DD, Litman RT, Luer CA, Kerr W, Litman GW. Expression of individual immunoglobulin genes occurs in an unusual system consisting of multiple independent loci. Eur J Immunol. 2004;34:2551–2558. doi: 10.1002/eji.200425224. [DOI] [PubMed] [Google Scholar]
- 8.von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84:201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 9.Jackson AM, Krangel MS. Turning T-cell receptor beta recombination on and off: more questions than answers. Immunol Rev. 2006;209:129–141. doi: 10.1111/j.0105-2896.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 10.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 11.Khor B, Sleckman BP. Allelic exclusion at the TCRbeta locus. Curr Opin Immunol. 2002;14:230–234. doi: 10.1016/s0952-7915(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 12.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 13.Malissen M, Trucy J, Jouvin-Marche E, Cazenave PA, Scollay R, Malissen B. Regulation of TCR alpha and beta gene allelic exclusion during T-cell development. Immunol Today. 1992;13:315–322. doi: 10.1016/0167-5699(92)90044-8. [DOI] [PubMed] [Google Scholar]
- 14.Aifantis I, Buer J, von Boehmer H, Azogui O. Essential role of the pre-T cell receptor in allelic exclusion of the T cell receptor beta locus [published erratum appears in Immunity 1997 Dec;7(6):following 895] Immunity. 1997;7:601–607. doi: 10.1016/s1074-7613(00)80381-7. [DOI] [PubMed] [Google Scholar]
- 15.Smith CA, Graham CM, Thomas DB. Productive re-arrangement at both alleles of the T-cell receptor beta-chain locus in CD4 T-cell clones specific for influenza haemagglutinin. Immunology. 1994;81:502–506. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Ranganath S, Gleason M, Woodman BB, Borjeson TM, Alt FW, Bassing CH. Restriction of endogenous TCRbeta rearrangements to Vbeta14 through selective recombination signal sequence modifications. Proc Natl Acad Sci U S A. 2007;104:4002–4007. doi: 10.1073/pnas.0700081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Meerwijk JP, Iglesias A, Hansen-Hagge T, Bluethmann H, Steinmetz M. Allelic exclusion of a T cell receptor-beta minilocus. J Immunol. 1991;147:3224–3228. [PubMed] [Google Scholar]
- 18.Xu Y, Davidson L, Alt FW, Baltimore D. Function of the pre-T-cell receptor alpha chain in T-cell development and allelic exclusion at the T-cell receptor beta locus. Proc Natl Acad Sci U S A. 1996;93:2169–2173. doi: 10.1073/pnas.93.5.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krotkova A, von Boehmer H, Fehling HJ. Allelic exclusion in pTalpha-deficient mice: no evidence for cell surface expression of two T cell receptor (TCR)-beta chains, but less efficient inhibition of endogeneous Vbeta--> (D)Jbeta rearrangements in the presence of a functional TCR-beta transgene. J Exp Med. 1997;186:767–775. doi: 10.1084/jem.186.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- 21.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 22.Bassing CH, Alt FW, Hughes MM, D'Auteuil M, Wehrly TD, Woodman BB, Gartner F, White JM, Davidson L, Sleckman BP. Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature. 2000;405:583–586. doi: 10.1038/35014635. [DOI] [PubMed] [Google Scholar]
- 23.Ranganath S, Carpenter AC, Gleason M, Shaw AC, Bassing CH, Alt FW. Productive coupling of accessible Vbeta14 segments and DJbeta complexes determines the frequency of Vbeta14 rearrangement. J Immunol. 2008 doi: 10.4049/jimmunol.180.4.2339. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C, Bassing CH, Jung D, Woodman BB, Foy D, Alt FW. Dramatically increased rearrangement and peripheral representation of Vbeta14 driven by the 3'Dbeta1 recombination signal sequence. Immunity. 2003;18:75–85. doi: 10.1016/s1074-7613(02)00515-0. [DOI] [PubMed] [Google Scholar]
- 25.Gartner F, Alt FW, Monroe R, Chu M, Sleckman BP, Davidson L, Swat W. Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity. 1999;10:537–546. doi: 10.1016/s1074-7613(00)80053-9. [DOI] [PubMed] [Google Scholar]
- 26.Storb U. Transgenic mice with immunoglobulin genes. Annu Rev Immunol. 1987;5:151–174. doi: 10.1146/annurev.iy.05.040187.001055. [DOI] [PubMed] [Google Scholar]
- 27.Wilson A, Marechal C, MacDonald HR. Biased V beta usage in immature thymocytes is independent of DJ beta proximity and pT alpha pairing. J Immunol. 2001;166:51–57. doi: 10.4049/jimmunol.166.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Glusman G, Rowen L, Lee I, Boysen C, Roach JC, Smit AF, Wang K, Koop BF, Hood L. Comparative genomics of the human and mouse T cell receptor loci. Immunity. 2001;15:337–349. doi: 10.1016/s1074-7613(01)00200-x. [DOI] [PubMed] [Google Scholar]
- 29.Agata Y, Tamaki N, Sakamoto S, Ikawa T, Masuda K, Kawamoto H, Murre C. Regulation of T cell receptor beta gene rearrangements and allelic exclusion by the helix-loop-helix protein, E47. Immunity. 2007;27:871–884. doi: 10.1016/j.immuni.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 30.van Meerwijk JP, Romagnoli P, Iglesias A, Bluethmann H, Steinmetz M. Allelic exclusion at DNA rearrangement level is required to prevent coexpression of two distinct T cell receptor beta genes. J Exp Med. 1991;174:815–819. doi: 10.1084/jem.174.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ten Boekel E, Melchers F, Rolink AG. Precursor B cells showing H chain allelic inclusion display allelic exclusion at the level of pre-B cell receptor surface expression. Immunity. 1998;8:199–207. doi: 10.1016/s1074-7613(00)80472-0. [DOI] [PubMed] [Google Scholar]
- 32.Alam SM, Gascoigne NR. Posttranslational regulation of TCR Valpha allelic exclusion during T cell differentiation. J Immunol. 1998;160:3883–3890. [PubMed] [Google Scholar]
- 33.Bredemeyer AL, Helmink BA, Innes CL, Calderon B, McGinnis LM, Mahowald GK, Gapud EJ, Walker LM, Collins JB, Weaver BK, Mandik-Nayak L, Schreiber RD, Allen PM, May MJ, Paules RS, Bassing CH, Sleckman BP. DNA double-strand breaks activate a multi-functional genetic program in developing lymphocytes. Nature. 2008;456:819–823. doi: 10.1038/nature07392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hewitt SL, Yin B, Ji Y, Chaumeil J, Marszalek K, Tenthorey J, Salvagiotto G, Steinel N, Ramsey LB, Ghysdael J, Farrar MA, Sleckman BP, Schatz DG, Busslinger M, Bassing CH, Skok JA. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nat Immunol. 2009;10:655–664. doi: 10.1038/ni.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathi R, Jackson A, Krangel MS. A change in the structure of Vbeta chromatin associated with TCR beta allelic exclusion. J Immunol. 2002;168:2316–2324. doi: 10.4049/jimmunol.168.5.2316. [DOI] [PubMed] [Google Scholar]
- 36.Chen F, Rowen L, Hood L, Rothenberg EV. Differential transcriptional regulation of individual TCR V beta segments before gene rearrangement. J Immunol. 2001;166:1771–1780. doi: 10.4049/jimmunol.166.3.1771. [DOI] [PubMed] [Google Scholar]
- 37.Sieh P, Chen J. Distinct control of the frequency and allelic exclusion of the V beta gene rearrangement at the TCR beta locus. J Immunol. 2001;167:2121–2129. doi: 10.4049/jimmunol.167.4.2121. [DOI] [PubMed] [Google Scholar]
- 38.Yao MC, Chao JL. RNA-guided DNA deletion in Tetrahymena: an RNAi-based mechanism for programmed genome rearrangements. Annu Rev Genet. 2005;39:537–559. doi: 10.1146/annurev.genet.39.073003.095906. [DOI] [PubMed] [Google Scholar]
- 39.Klar AJ. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu Rev Genet. 2007;41:213–236. doi: 10.1146/annurev.genet.39.073103.094316. [DOI] [PubMed] [Google Scholar]
- 40.Chakraborty T, Chowdhury D, Keyes A, Jani A, Subrahmanyam R, Ivanova I, Sen R. Repeat organization and epigenetic regulation of the DH-Cmu domain of the immunoglobulin heavy-chain gene locus. Mol Cell. 2007;27:842–850. doi: 10.1016/j.molcel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Costa TE, Suh H, Nussenzweig MC. Chromosomal position of rearranging gene segments influences allelic exclusion in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:2205–2208. doi: 10.1073/pnas.89.6.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roldan E, Fuxa M, Chong W, Martinez D, Novatchkova M, Busslinger M, Skok JA. Locus 'decontraction' and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]