Abstract
Background: Hidradenitis suppurativa (HS) is a chronic, relapsing inflammatory disease of skin, characterized by recurrent draining sinuses and abscesses, predominantly in skin folds carrying terminal hairs and apocrine glands.
Method: This study reviewed 54 sites in 27 patients with moderate to extensive chronic inflammatory skin lesions treated surgically in our hospital from 2004 through 2009, with a follow-up of at least 6 months.
Result: A total number of 54 operative procedures were performed during the study period with 42% (23 sites) involving the axilla, 20% (11 sites) involving the gluteal area, %24 (13 sites) involving the perineal area and 12% (7 sites) involving the inguinal region.
Conclusion: Conservative treatment methods have little or no effects especially on gluteal, perineal/perianal, axillary hidradenitis suppurativa. The morbidity associated with the established form of this disease is significant, and the only successful treatment is wide surgical excision.
Keywords: Hidradenitis Suppurativa, Surgery, Treatment, Gluteal, Axillary, Perianal
Introduction
Hidradenitis suppurativa (HS) is a chronic, relapsing inflammatory disease of skin, characterized by recurrent draining sinuses and abscesses, predominantly in skin folds carrying terminal hairs and apocrine glands.1 The incidence may be as high as one in 300.2
Hidradenitis suppurativa (from the Greek hidros, sweat and aden, glands), also known as acne inversa, was first described by Velpeau, a French physician in 1839, who reported a peculiar inflammation of the skin with the formation of superficial abscesses in the axillary, mammary and perianal areas.3 In 1854, this condition was termed 'hidrosade´nite phlegmoneuse' by Verneuil, a French surgeon who also suggested an association between HS and sweat glands, which had been described by Purkinje in 1833.1
Hidradenitis suppurative may affect any area of the body surface where apocrine glandular tissue is found, but most often it affects the skin of the axillae and inguinoperineal regions.2
Although the pathophysiology is understood poorly, it generally is believed that obstruction of the apocrine and/or follicular pores results in glandular dilatation and bacterial superinfection with subsequent gland rupture disseminating infection throughout the subcutaneous tissue plane.3 Consequently, hidradenitis is associated with chronic painful abscesses, multiple odiferous draining sinus tracts, and chronic fibrosis with range-limiting scar formation.4 Anogenital involvement most commonly affects the groins with extension to inguinal regions, mons pubis, inner thighs and sides of scrotum. The perineum, buttocks and perianal folds are often included. The sinuses can dissect deep into tissue, involving muscle, fascia and bowel forming a labyrinth of tracts in advanced cases.8
However, as the abscesses extend deeper into the subcutaneous tissue, intercommunicating sinus tracts develop, resulting in irregular hypertrophic scars.6 Rarely, the chronic inflammation results in malignant transformation to squamous cell carcinoma.10 In such a developed phase, antibiotics are usually ineffective alone and surgical treatment is required.4,9,14
The exact etiology of HS still remains unclear, genetic factors may play a role as a positive family history has been elicited in 26% of patients with HS. The role of endocrine factors in the etiology of HS has been controversial.1
There is no consensus about the relationship between HS and sex, race, and site of the lesions. Axillary location seems to be more frequent in women. The gluteal, inguinal, perineal, and perianal zones are more frequently involved in men. HS appears more commonly in young adults and is observed after puberty.3
In women, the condition frequently flares premenstrually and following pregnancy and it sometimes eases during pregnancy and after the menopause; these observations incriminate sex hormones. Children are never affected unless they have precocious puberty.8
Although exogenous factors such as the use of deodorants and shaving are thought to be causal, they have not been shown to be significantly responsible in a retrospective comparison of 40 patients with HS.13 Smoking is more common in patients with HS but the aetiological basis is unknown. From the exceedingly high rate of smokers among patients with this condition one may conclude that cigarette smoking is a major triggering factor of hidradenitis suppurativa.15 Wiltz et al. reported an association between smoking and perianal HS in 70% of patients.1 Obesity is an exacerbating factor, and weight loss can help control the disease severity.1,4
Early-stage treatment consists primarily of topical (clindamycin) or systemic antibiotics (tetracyclines, clindamycin, rifampicin), topical antiseptics and intralesional corticosteroids (triamcinolone acetonide). Systemic retinoids (isotretinoin, etretinate) antiandrogen therapy (cyproterone acetate, finasteride), immunotherapy (TNF alfa inhibitors), oral immunosuppressive agents (cyclosporin) have also shown a positive effect on disease progression.4,12 Radiotherapy and laser treatment applied cases available on literature.1 Currently available medical treatments are, however, insufficient and their efficacy is only transient. As a result, advanced-stage severe HS requires invasive surgical removal of all the involved tissue.1,5,8,11
In this report, we present our experience with moderate and extensive perineal, perianal, axillary and gluteal hidradenitis suppurativa cases, including our treatment methods and outcomes.
Patients and Methods
This study reviewed 54 sites in 27 patients with moderate to extensive chronic inflammatory skin lesions treated surgically in our hospital from 2004 through 2009, with a follow-up of at least 6 months. Nineteen (%70) patients were men and eight patients were women. The mean age at the time of presentation for operative management was 41.2 years (range, 24-58 y) and the average duration of symptomatic disease was 7.3 years (range, 0.9-30 y). None of these patients were detected to have any comorbid or associated conditions. According to answers about cleaning habits; personal hygiene was poor in most of the patients. 18 of the 21 (85%) male patients and 3 of the 8 (37%) female patients were smokers. 3 patients (2 female, 1 male) had insulin-dependent diabetes mellitus. (See Table 1) Most of the included patients had previously been prescribed a treatment by non surgical or inadequate surgical treatment modalities such as short term antibiotic treatments, local wound care and abscess drainage for long periods (up to 20 years). Seven patients previously were treated by limited local excisions and primary closure.
Table 1.
Patient characteristics.
| Patient No. | Sex | Age | Smoking,p/y | Site | Defect Size,cm | Duration of illness/y | Surgical Method | Follow-up,mo | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 50 | - | Gluteal | 10X15 | 5 | Skin grafting | 11 | - |
| 2 | F | 42 | - | Axilla | 8X12 | 0.9 | Parascapular fasciacutaneous flap | 33 | - |
| 3 | M | 39 | 0.5/20 | Inguinal | 3X10 | 3 | Local fasciacutaneous flap | 15 | + |
| 4 | M | 47 | 1/30 | Bilateral Axilla | 8X3,6X4 | 2 | Primary closure | 48 | - |
| 5 | F | 24 | - | Axilla | 3X5 | 1 | Primary closure | 10 | - |
| 6 | M | 38 | 1/12 | Gluteal | 9X13 | 1.5 | Skin grafting | 17 | - |
| 7 | M | 49 | 1.5/22 | Gluteal | 14X11 | 6 | Skin grafting | 16 | - |
| 8 | M | 56 | 1/30 | Gluteal | 14x12 | 20 | Skin grafting | 24 | - |
| Bilateral İnguinal | 3X15, 4X10 | Primary closure | - | ||||||
| Bilateral Perineal | 20X20,18X20 | Skin grafting | - | ||||||
| 9 | M | 47 | 1/23 | Gluteal,perianal | 30X20 | 3 | Skin grafting | 36 | - |
| Perineal | 5X15 | Primary closure | - | ||||||
| 10 | F | 57 | - | Gluteal,perianal | 42X40 | 30 | Skin grafting | 40 | - |
| Bilateral Perineal | 20X20,15X20 | Skin grafting | - | ||||||
| Bilateral axilla | 18X10,15X12 | Primary closure | |||||||
| 11 | F | 55 | - | Bilateral Perineal, | 15X10 | 2 | Primary closure | 36 | - |
| Bilateral İnguinal | 9X12 | Primary closure | - | ||||||
| Bilateral Axilla | 3X5 | Primary closure | |||||||
| 12 | M | 51 | 2/25 | Gluteal,perianal | 21X38 | 12 | Skin grafting | 21 | - |
| 13 | M | 24 | 1/5 | Axilla | 3X10 | 3 | Primary closure | 6 | - |
| 14 | M | 24 | - | Bilateral Axilla | 20X15,15X15 | 6 | Parascapular fasciacutaneous | 12 | - |
| 15 | M | 32 | 2/10 | Axilla | 10X10, | 4 | Parascapular fasciacutaneous | 20 | - |
| İnguinal | 2X10 | Primary closure | - | ||||||
| Perineal | 10X18 | Gracilis musculocutaneous flap | - | ||||||
| 16 | M | 42 | 1/20 | Axilla | 12X12 | 16 | Parascapular fasciacutaneous flap | 18 | - |
| 17 | M | 39 | 1/15 | Axilla | 5x8 | 4 | Primary closure | 10 | - |
| Gluteal | 16X20 | Skin grafting | - | ||||||
| Perineal | 2X8 | Primary closure | - | ||||||
| 18 | F | 45 | - | Axilla | 10X5 | 5 | Parascapular fasciacutaneous flap | 20 | - |
| 19 | M | 58 | 1/30 | Gluteal,perianal | 15X20 | 20 | Fasciacutaneous V-Y advancement | 10 | - |
| 20 | M | 40 | 2/20 | Gluteal,perianal | 20X42 | 7 | Skin grafting | 48 | - |
| Perineal | 15x12 | Fasciacutaneous flap | - | ||||||
| 21 | M | 45 | 3/22 | Bilateral axilla | 15X8,15x10 | 12 | Parascapular fasciacutaneous flap | 20 | - |
| 22 | M | 55 | 1/30 | Gluteal | 10X12 | 7 | Local transposition flap | 8 | - |
| 23 | F | 30 | 1/12 | Axilla | 3X9 | 15 | Primary closure | 16 | - |
| İnguinal | 4X10 | Primary closure | - | ||||||
| 24 | F | 32 | 0.5/5 | Bilateral axilla | 4X8,3X8 | 8 | Primary closure | 9 | - |
| 25 | M | 45 | 1/22 | Bilateral axilla | 3X7,12X6 | 5 | Parascapular fasciacutaneous flap | 7 | - |
| Bilateral perineal | 20X10,8X15 | Fasciacutaneous flap | + | ||||||
| 26 | M | 24 | - | Perineal | 4x5 | 2 | Primary closure | 12 | - |
| 27 | F | 25 | - | Axilla | 3X7 | 3 | Primary closure | 10 | - |
Total surgical excisions under general anaesthesia were performed on all patients. All patients were operated on in the lithotomy, jackknife, supine or prone positions. Rectal tubes were used for prevention of the surgical field from contamination with stool in the patients with perianal or gluteal lesions, colostomy was not used in any patients for this purpose. The operative technique was based on the complete excision of the entire diseased skin and subcutaneous fatty tissue and down to the muscular fascia on aggressive cases. Patients with limited disease involving the axilla or inguinal region were selected for excision and primary closure if the skin and soft tissue could be mobilized adequately. The size of the defects created after excision of the lesions ranged from 15 cm2 up to 1680 (42X40) cm2. Preoperative and postoperative antibiotherapy is administered for all patients according to wound tissue culture tests results. Culture results were Proteus Mirabilis, Staphylococcus Aureus and E. Coli predominantly, and were sensitive to Ciprofloxacin and Seftazidim antibiotic protocol mostly.
Patient reports
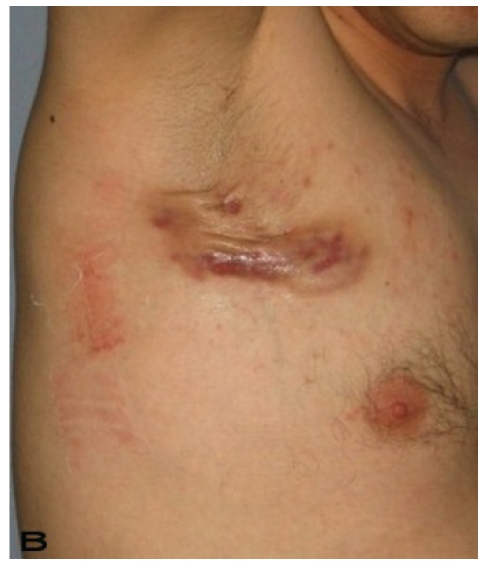
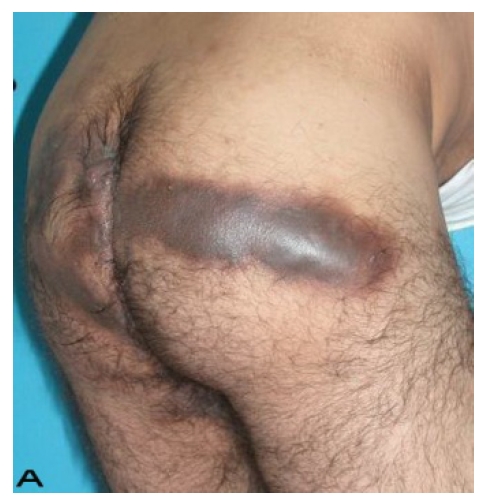
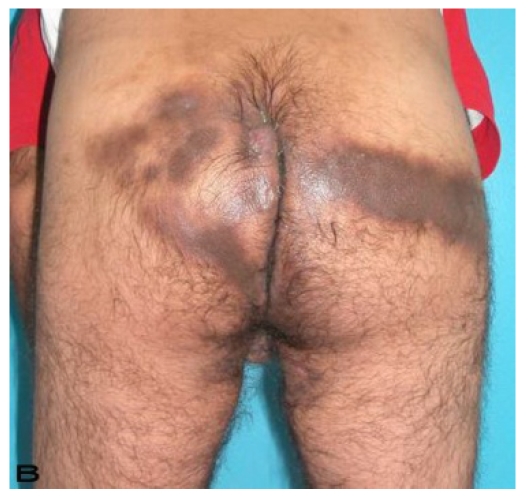
Patient 8
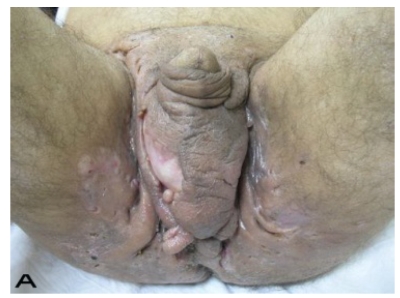
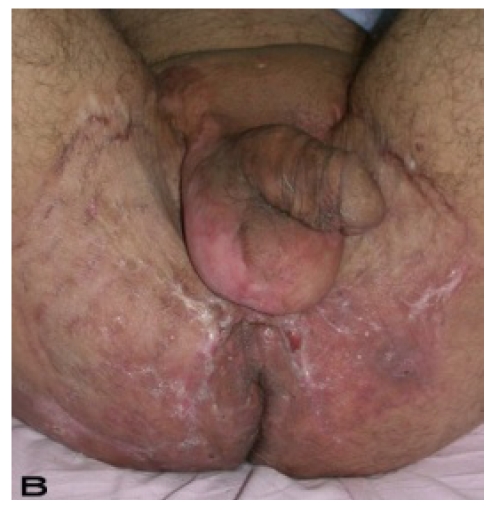
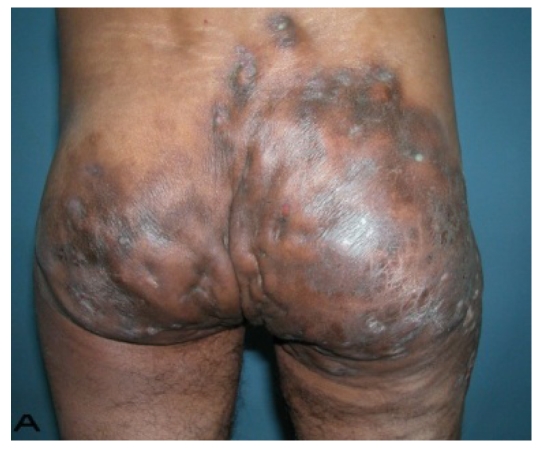
A 56-year old male patient had recurrent and progressive lesions on his perineal, inguinal, perianal and gluteal regions for 20 years. Drainage, local wound care and antibiotherapy was used previously every time the lesions were exacerbated. Perineal lesions also affected the scrotal skin. (See Figure 1) The lesions were excised widely and the scrotal skin down to the dartos fascia anal sphincter was preserved. The Inguinal defect was closed primarily and the perineal lesions were reconstructed by split thickness skin grafts. Gluteal lesions were excised and skin grafted 8 months later at a second stage.
Figure 1.
Patient 8 (56 years). (A) Preoperative view: Preineal area with putrid productive infection. (B) Postoperative result after 1 year, no scar contracture, no recurrence.
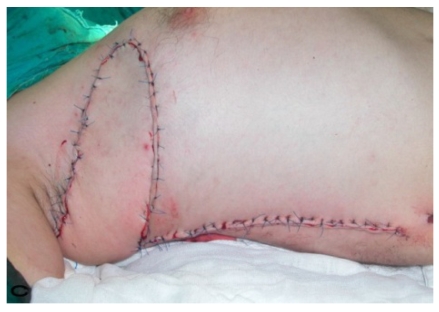
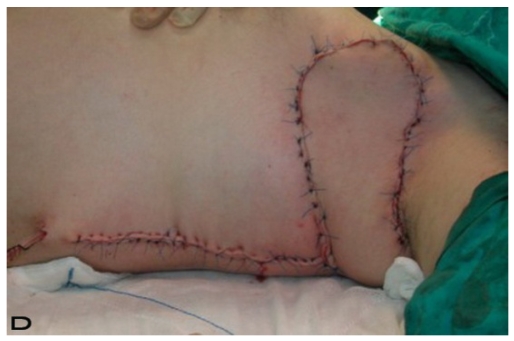
Patient 10
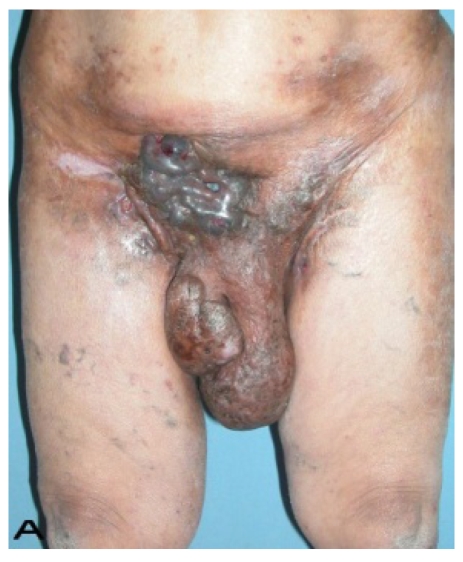
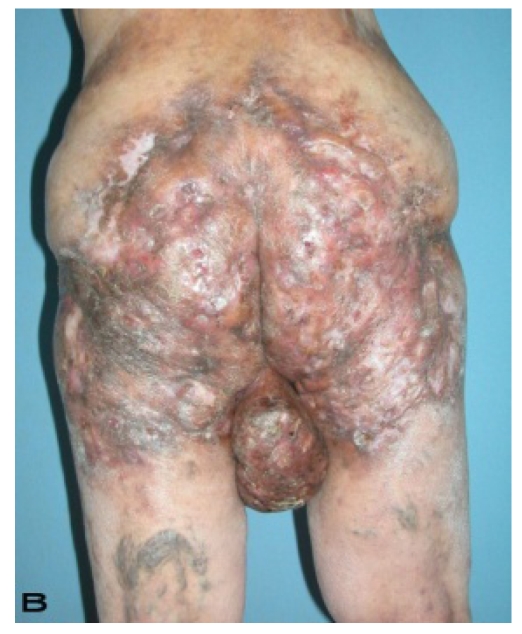
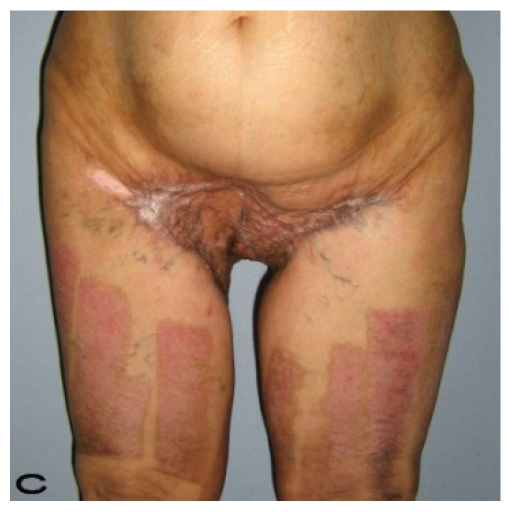
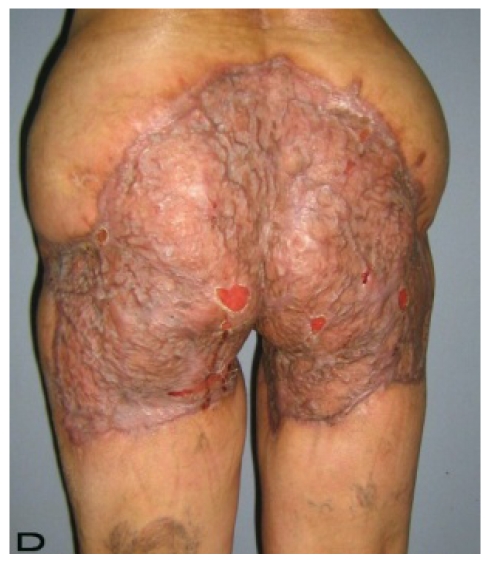
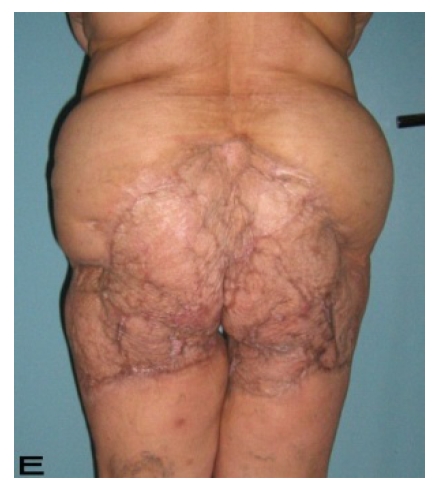
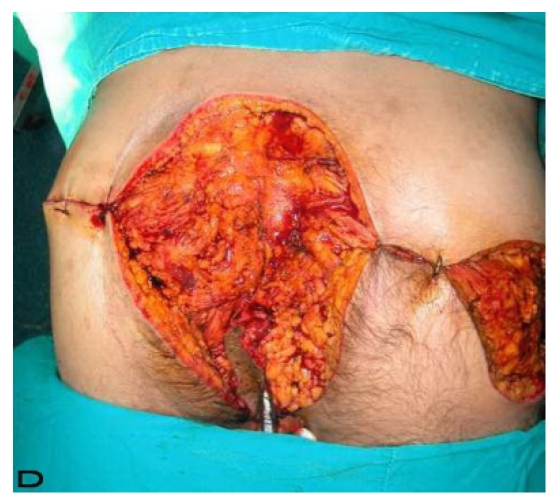
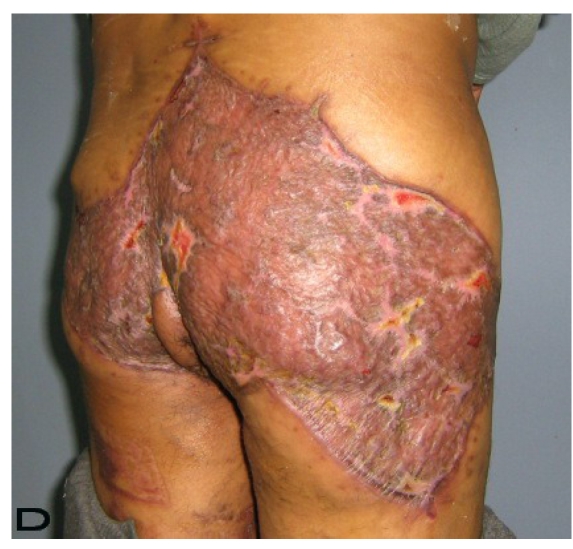
57-year old female patient. 30 years ago a lesion was excised from the coccygeal region but recurred 10 years later and treatment with retinoids was started. However the lesions relapsed and disseminated to a wide area over time (Figure 2). The lesions of this patient were very extensive and drastic which are rare in the literature and especially the effects of the disease on the perineal area was dramatic. Both gluteal areas, perianal, perineal, inguinal and axillary regions were affected. The lesion on right labia majora was enlarged to 10x15 cm and left labia majora was 10x20 cm. Surgical treatment was performed in 3 stages. At the first stage all perineal and gluteal lesions were excised with at least 1 cm margins. The size of the excised material from the gluteal region was 42x40 cm. Split thickness skin grafts from the thigh were used for reconstruction. Excision and primary closure of the axillary lesions were done in second and third stages. We obtained excellent functional results (convenience during urination etc.) and moderate cosmetic results.
Figure 2.
Patient 10. A 57-year-old female patient with very severe widespread Hidradenitis suppurativa. (A,B) Preoperative view of inguinal,perineal and gluteal areas. (C,D) Postoperative results after 6 month. (E) View of postoperative 3 year.
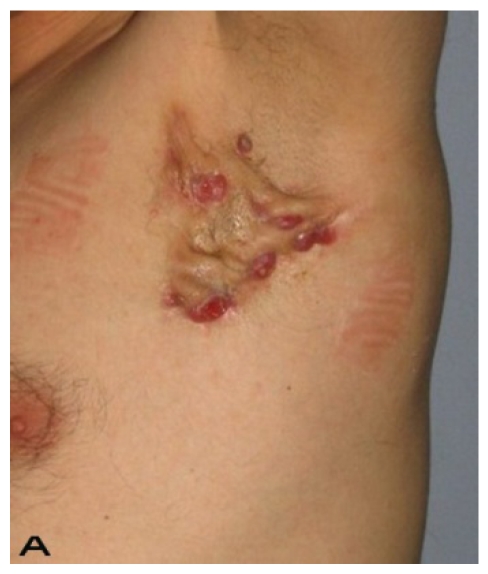
Patient 14
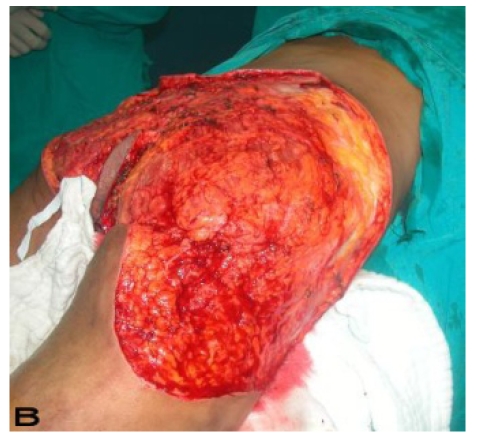
24-year old male patient had bilateral axillary hidradenitis suppurativa for the last 6 years. Both axillae were treated in the same stage. Excised area was 20x15 cm on the right side and 15x15 cm on the left side. Thoracodorsal perforator based fasciocutaneous flaps were used for reconstruction. (See Figure 3).
Figure 3.
Patient 14. A 24-year-old male. (A,B) Preoperative view of axillary region. (C,D) The axillary defect is covered with toracodorsal perforator based fasciocutaneous flaps. (Intraoperative view)
Patient 19
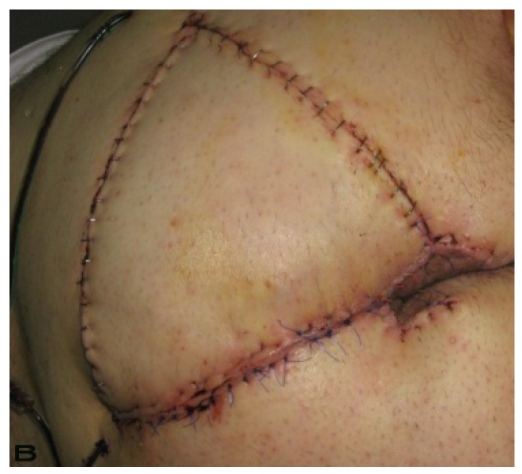
58-year old male patient had draining lesions in his intergluteal and perianal regions for the last 20 years. Abscess drainage was performed at least 3-4 times every year and he had frequent use of various oral antibiotics. We performed total excision of a 15x20 cm lesion with 1 cm surgical margins down to the muscle fascia. The external anal sphincter was protected. A right sided gluteal V-Y advancement flap was used for the closure of the defect. (Figure 4)
Figure 4.
Patient 19. A 58-year-old male patient with severe hidradenitis suppurativa of the gluteal and perianal area. (A) Preoperative view with fistulas in intergluteal area.(B) After excision of the inflammatory region and closed with V-Y advancement fasciacutaneous flap.
Results
A total number of 54 operative procedures were performed during the study period with 42,6% (23 sites) involving the axilla, 20,3% (11 sites) involving the gluteal area, %24,2 (13 sites) involving the perineal area and 12,9% (7 sites) involving the inguinal region. Hurley's clinical staging was used for the classification of patients (Table 2). Excision and primary closure was used only for mild and moderate (Hurley stage I) axillary and inguinal disease, whereas wide local excision and split-thickness skin grafting or fasciocutaneous flap was the mainstay of treatment in patients with diffuse disease (Hurley stage II and III).
Table 2.
Hurley Staging System.
| Stage | Characteristics |
|---|---|
| I | Solitary or multiple isolated abscess formation without scarring or sinus tracts. |
| II | Recurrent abscesses, single or multiple widely separated lesions, with sinus tract formation. |
| III | Diffuse or broad involvement across a regional area with multiple interconnected sinus tracts and abscesses. |
Surgical margins were at least 0.5-1 cm in the axillary region and 1-1.5 cm in the gluteal region and down to the muscle fascia. Affected labia majora and scrotal skin was also excised widely. In the perianal region lesions were excised by protecting the external anal sphincter and none of the patients required endoanal excision, Colostomy was not performed for any patient.
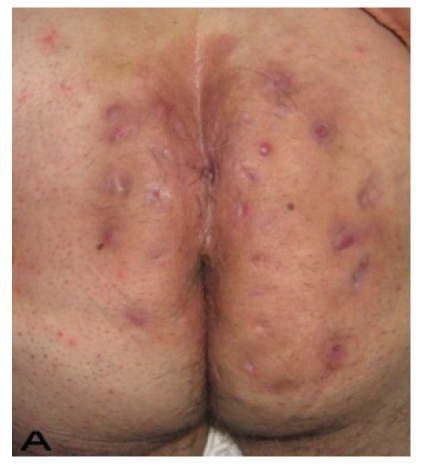
Treatment was performed in 2 stages in three patients and 3 stages in one patient. For the reconstruction of the glutal region Split thickness skin graft (STSG) was used in 9 patients (Figure 5,6), fasciocutaneous V-Y advancement flap in one patient and transposition flap in 1 patient. Primary closure was used in 13 axillary regions and 10 fasciocutaneous flaps from the parascapular region were used. 4 flaps were thoracodorsal perforator based. In 2 patients with bilateral axillary disease, bilateral excision and toracodorsal perforator based flap reconstructions were performed at a single stage. To preserve the shoulder movements and to prevent contactures, skin grafts were not used for the reconstruction of the wide axillary defects. Five of the perineal defects were closed primarily, 4 were by skin grafting, 3 by fasciocutanous transposition flaps and one perineal defect was closed by gracilis myocutaneous flap. Inguinal region reconstruction was done by primary closure in 6 patients, and fasciocutaneous transposition flap was used for 1 patient.
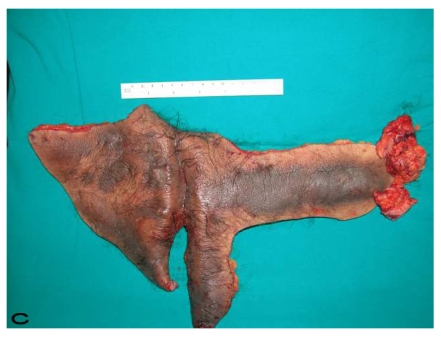
Figure 5.
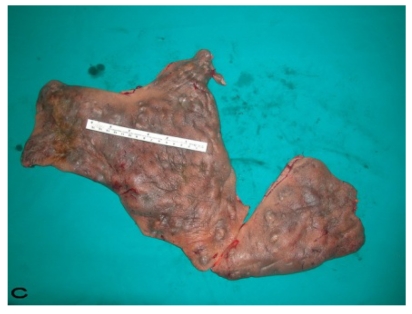
Patient 9. A 47-year-old male (A,B) Gluteal and perianal region before treatment, showing multiple confluent suppurative and inflammatory nodules. Extraordinary widespread of HS in the right gluteal area. (C) Excision material of lesion. (D) View of defect after excision.
Figure 6.
Patient 12. A 51-year-old male patient with spread and severe gluteal and perianal HS. (A) Preoperative view with multiple fistulas in the gluteal area.(B) After excision of the inflamatory region. (C) View of excision material. (D) Postoperatif result after 6 month.
The duration time of hospitalization was related with the extent and severity of the lesions and the mean hospitalization was 6 days (3-41days). All patients were followed with daily dressing changes. All flaps survived totally except for minor distal marginal flap necrosis in 2 parascapular flaps which were treated by simple suturation.
Early postoperative rehabilitatiton to preserve shoulder motility was started in all patients with axillary disease. Skin grafts were succesful generally, minor graft loss areas were treated by local wound care, and none of the patients required additional grafting. Only 2 recurrences were observed in this patient series. Both were inguinal lesions treated by primary closure. The recurrences were treated by wide excision and defect closure by fasciocutaneous flaps.
Patients have been followed for a mean of 19.7 months (range 6-48 mo). Some patients who underwent HS surgery were not willing come to clinic for checking up previous operated areas if they have no problem. Developing HS on the new areas gave chance to us for long term follow up on these patients.
Discussion
HS remains a challenging disease for both the patient and the physician. It is a chronic debilitating disease whose aetiology is still controversial.1
Because HS rarely is seen before puberty or after menopause, and recurrence of acute disease has appeared after hormone administration, several investigators have suggested that the condition may be caused by an endocrine abnormality or, more specifically, may be androgen dependent.4,5
Because of the varying clinical manifestations and sites involved by the disease, patients with HS present to, or are referred to many different specialties, including gynecology, surgery, medicine, dermatology, plastic surgery, immunology and infection control. Unfortunately, HS is commonly mismanaged owing to a failure of early diagnosis and once established, chronicity and progression ensue. No specific test for diagnosis exists. Inflammatory abscess-like swelling(s) in apocrine gland bearing skin should be regarded as possible HS.
The clinician should also bear in mind the other possible sites of HS, which may have no or little apocrine component. In order of frequency of involvement, the following sites are recognized: axillaries, inguinal, perianal and perineal, mammary and inframammary regions, buttock, pubic region, chest, scalp, retroauricular, and eyelid skin.5
Conservative treatment and incision and/or surgical removal of the abscesses and fistulas are usually futile.1,14 The success of medical therapies, however, often is limited because of the indolent and recurrent nature of the disease. Operative excision of the involved follicles and inflammatory process is the only curative treatment.4,6
Various surgical methods for the treatment of hidradenitis suppurativa have been described previously. Wide local excision with skin grafting, skin flap transfer, and primary closure has been common. However, with the popularization of surgical methods using fasciocutaneous or musculocutaneous flaps in the field of plastic surgery, these flaps have been applied positively for the treatment of Hidradenitis suppurativa.6
In this study we are presenting a case series includes with extensive and severe hidradenitis suppurativa in which some cases are very rare in literature (patient 10). We believe that excision with wide margins and adequate depth and reconstruction with appropriate methods leads to good results in controlling the disease and preventing the recurrences.
Our observations from this group of patients is that lack of personal hygiene and utilization of inadequate treatment modalities result in severe and widely disseminated lesions that affects the quality of life and general health status of the patients.
Although skin grafts may result in contractures and extensive scarring, this can be acceptable especially in the gluteal regions. In this area, skin graft contraction does not cause functional problems and scars are covered easily. Patients generally do not complain about aesthetic results. Reconstruction of the defects with flaps may prevent contractures and bad scarring but local flaps might posses the risk of carrying the same affected skin and lead to recurrences. Hence local or regional flaps can only be used if a wide and adequate excision with sure margins is performed. We used only two flaps in 11 severe HS cases with gluteal lesions. Primary closure was used for mild and moderate (Hurley stage I) HS defects in axillary, inguinal, and perineal regions after wide excision of the diseased areas and elevation and advancement of the skin flaps. Primary closure is primarily preferred by non-plastic surgeons and inadequate limited excision leads to recurrences and dissemination of the disease to wider areas.
Perianal lesions are especially difficult to treat. Despite its relatively common occurrence, perianal hidradenitis suppurativa is infrequently diagnosed correctly and recurs in many patients despite appropriate surgical treatment, making the disease a source of frustration for surgeon and patient alike.16 Preservation of the anal sphincter is important. If endoanal lesions are present, a colostomy might be required to perform adequate excision of such lesions. None of the patients in this series had such lesions, and therefore colostomy was not needed.
In this study, 10 parascapular fasciocutaneous flaps and, 13 primary closures were performed in the axillary region. Preserving shoulder movement and preventing the formation of contractures are important in the axillary region.7,9 The goals of surgical management are to completely excise all the involved tissue, preserve function, avoiding development of axillary contracture and obtain satisfactory aesthetic results.7 Therefore, skin grafts were not used for the reconstruction of the axillary of fasciocutaneous flaps from the parascapular region allows a thin and pliable reconstruction that is suitable for axillary repair. Thoracodorsal perforator flaps were used in especially wide defects.
Conclusion
Hidradenitis suppurativa is a disease which has many different treatment modalities. Surgical options have wide variabilities according to affected areas. We believe that it is very important to choose appropriate surgical operation to diseased area to avoid contractures, recurrens and bad aesthetic results. We have not noted any scar contracture preventing functional movements in our patients. All patients were very satisfied with the aesthetic result.
References
- 1.Ather S, Chan DSY, Leaper DJ, Harding KG. Surgical treatment of hidradenitis suppurativa: case series and review of the literature. Int Wound J. 2006;3:159–169. doi: 10.1111/j.1742-481X.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard JH, Mudge M, Hughes L. Recurrence after surgical treatment of hidradenitis suppurativa. British Medical Journal. 1987;294:487–489. doi: 10.1136/bmj.294.6570.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balik E, Eren T, Bulut T, Büyükuncu Y, Bugra D, Yamaner S. Surgical Approach to Extensive Hidradenitis Suppurativa in the Perineal/Perianal and Gluteal Regions. World J Surg. 2009;33:481–487. doi: 10.1007/s00268-008-9845-9. [DOI] [PubMed] [Google Scholar]
- 4.Kagan RJ, Yakuboff KP, Warner P, Warden GD. Surgical treatment of hidradenitis suppurativa: A 10-year experience. Surgery. 2005;138:734–41. doi: 10.1016/j.surg.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 5.Slade DEM, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. The British Association of Plastic Surgeons. 2003;56:451–461. doi: 10.1016/s0007-1226(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka A, Hatoko M, Tada H, Kuwahara M, Mashiba K, Yurugi S. Experience with surgical treatment of hidradenitis suppurativa. Ann Plast Surg. 2001;47:636–642. doi: 10.1097/00000637-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sharma RK, Kapoor KM, Singh G. Reconstruction in extensive axillary Hidradenitis suppurativa with local fasciocutaneous V-Y advancement flaps. Indian J Plast Surg. 2006;39(1):18–21. [Google Scholar]
- 8.Mortimer PS, Lunniss PJ. Hidradenitis Suppurotiva. Journal of the Royal Society of Medicine. 2000;93:420–422. doi: 10.1177/014107680009300808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes PJ, Earley MJ, Lawlor D. Split-thickness skin grafts and negative-pressure dressings in the treatment of axillary hidradenitis suppurativa. British Journal of Plastic Surgery. 2002;55:507–509. doi: 10.1054/bjps.2002.3899. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig LB, Brett AS, Lefaivre JF, Vandersteenhoven JJ. Hidradenitis Suppurativa Complicated by Squamous Cell Carcinoma and Paraneoplastic Neuropathy. The American Journal Of The Medical Sciences. 2005;329(3):150–152. doi: 10.1097/00000441-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis Suppurativa. British Journal of Dermatology. 2006;154:726–729. doi: 10.1111/j.1365-2133.2005.07067.x. [DOI] [PubMed] [Google Scholar]
- 12.Thielen AM, Barde C, Saurat JH. Long-term infliximab for severe hidradenitis Suppurativa. British Journal of Dermatology. 2006;154:1074–1208. doi: 10.1111/j.1365-2133.2006.07528.x. [DOI] [PubMed] [Google Scholar]
- 13.Morgan Wp, Leicester G. The role of depilation and deodorants in hidradenitis suppurativa. Arch Dermatol. 1982;118(2):101–2. [PubMed] [Google Scholar]
- 14.Altmann S, Fansa H, Schneider W. Axillary Hidradenitis Suppurativa: A Further Option for Surgical Treatment. J Cutan Med Surg. 2004:6–10. doi: 10.1007/s10227-003-0162-5. [DOI] [PubMed] [Google Scholar]
- 15.König A, Lehmann C, Rompel R, Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198(3):261–4. doi: 10.1159/000018126. [DOI] [PubMed] [Google Scholar]
- 16.Wiltz O, Schoetz DJ Jr, Murray JJ, Roberts PL, Coller JA, Veidenheimer MC. Perianalhidradenitis suppurativa.The Lahey Clinic experience. Dis Colon Rectum. 1990 Sep;33(9):731–4. doi: 10.1007/BF02052316. [DOI] [PubMed] [Google Scholar]