Abstract
Introduction:
Platelet concentrate (PC) remains one of the most important support measures in thrombocytopenic patients. An efficient cell separator is a prerequisite for an optimally functioning apheresis setup. Donor blood count may undergo a temporary reduction after the procedure.
Aim:
The aim was to find the extent of reduction in donor blood count (hemoglobin, hematocrit, white blood cell, and platelet) after plateletpheresis and to evaluate the cell separator for collection efficiency, processing time, and leukoreduction.
Study Design and Methods:
Two hundred and thirty seven procedures performed on the Amicus (N = 121), Fenwal CS-3000 Plus (N = 50) and Cobe spectra (N = 66) in a one year period were evaluated. The procedures performed on the continuous flow centrifugation (CFC) cell separators and donor blood counts (pre and post donation) done were included in the study.
Results:
The percent reduction in hemoglobin (HB), hematocrit (HCT), white blood cell (WBC) and platelet count ((PLT ct) was 2.9, 3.1, 9, 30.7 (Mean, N = 237) respectively after the procedure. The post donation PLT ct reduced to < 100×109/L (range 80-100) in five donors (N = 5/237, Amicus). The pre donation PLT ct in them was 150-200×109/L. Collection efficiency (percent) of Amicus (79.3) was better as compared to the other two machines (CS: 62.5, Cobe: 57.5). PC collected on Cobe spectra had <1×106 WBC. The donor pre donation PLT levels had a positive correlation to the product PLT yield (r = 0.30, P = 0.000).
Conclusion:
Monitoring donor blood counts helps to avoid pheresis induced adverse events. A cautious approach is necessary in donors whose pre donation PLT ct is 150-200×109/L. The main variable in PLT yield is donor PLT ct (pre donation). High collection efficiency is a direct measure of an optimally functioning cell separator.
Keywords: Continuous flow cell separator, donor blood count, plateletpheresis, platelet yield, Blood donor, apheresis
Introduction
The demand for platelet concentrate (PC) is very high in a cancer specialty hospital. Eligible blood donors donate single donor PC on a cell separator. The cell separator functions on the intermittent flow centrifugation (IFC) or the continuous flow centrifugation (CFC) principle. Transient but significant decreases in blood counts have been reported to occur in donors undergoing single and serial short-term plateletpheresis collections. The objective of this study was to investigate the extent of reduction in the blood counts of our platelet donors and to assess how efficient our cell separators were in collecting PC.
Materials and Methods
Donors
PLT donors, especially first time donors, come to the setup with a lot of apprehension. Few hospitals in the vicinity have ventured into apheresis, thus exposure to a cell separator is often a rare phenomenon. The present study had first time (N = 2037) and repeat (N = 111) donors. The repeat donors donated the PC after a complete recovery of their blood counts. We tested the pre-donation sample, at every visit, to confirm this. In-house donation is encouraged as staff are well equipped to attend to any eventualities. A detailed medical history and blood sample testing for counts and serology decides eligibility for the donation. The detailed screening criteria were as per Transfusion Medicine Technical Manual, 2003, Directorate General Health Services, Government of India, New Delhi. If the donor was eligible, he gave the consent for the procedure. The staff answered any queries that the donor had, related to the procedure. Continuous monitoring of the procedure, along with an analysis of the blood cell counts, helped to assure an uneventful procedure with minimal adverse events.
Study Design
We performed two thousand one hundred and forty-eight (N = 2148) plateletpheresis procedures in a one-year period on CFC and IFC cell separators installed in our institute. As per Food and Drug Administration (FDA) guidelines, at least 1% of the procedures performed should undergo quality control tests. Thus, not all donors undergo the post donation blood sample testing. We subjected two hundred and thirty-seven (N = 237) procedures to quality control tests (viz., Donor: pre and post donation blood ct, Procedure: collection efficiency, processing time, Product: visual inspection, pH, PLT yield, WBC and RBC contamination). We did a random sampling to avoid a selection bias, as the results of these tests are a measure of the quality of the overall process.
Inclusion criteria: Procedures performed on the continuous flow cell separator, donor blood samples (pre and post) and product samples tested, and all procedure details documented were included in the study. Procedures performed on IFC separators and omission of the quality control tests resulted in exclusion from the study.
Cell separators
IFC works in cycles, taking blood from the donor, spinning/processing it in the machine and then giving the unwanted component back to the donor. CFC requires two venipunctures as the “continuous” means that the blood is collected, spun, and returned simultaneously. Newer systems can use a single venipuncture. One of the advantages of CFC, is the low extracorporeal volume used in the procedure. Extracorporeal volume is defined as the quantity of donor blood remaining outside his body at any given time during the procedure. Also, the donor turnaround time is less, hence there is more compliance from donors for repeat donations.
The three CFC separators used were Fenwal Amicus V2.52 (Baxter, USA, N = 121), Fenwal CS 3000 plus (Baxter, USA, N = 50) and Cobe spectra LRS Turbo version 7 (Gambro, N = 66).
Fenwal Amicus
One Fenwal Amicus, software version 2.52 (Baxter Biotech Corp., Fenwal Division, and Roundlake, IL) used for the study. The instrument according to donor size determined inlet rates. The anticoagulant infusion rate was 1.25 mg/Kg/min. The blood to anticoagulant ratio was 9:1. The interface setting was 0.40.
CS-3000 Plus
One Fenwal CS-3000+ (Baxter Healthcare Corp, Fenwal Division, Deerfield, IL), was used for the study. The inlet rate was constant at 50 ml/min. Anticoagulant to whole blood ratio was 10:1 throughout the procedure. The operator enters the PLT ct and the machine calculates the donor blood volume to be processed.
Cobe spectra Version 7 Turbo LRS
One Cobe spectra with version 7 software program and leukoreduction system (Gambro BCT, Inc, Lakewood, CO) formed part of the study. The instrument according to donor size determined the inlet rates. The anticoagulant infusion rate was 1.1 ml/min/liter total body volume. The blood to anticoagulant ratio was 8:1 for 10 minutes, 10:1 for 5 minutes and 12:1 for the remainder of the procedure. The yield-scaling factor was 0.97. The Collection Concentration Monitor (CCM) scaling factor was 0.9.
All three-cell separators work on the CFC principle; however, they vary in weight, centrifuge diameter, number of sensors and valves. The cell separator parameters are as per Table 1.
Table 1.
Cell separator parameters
| Amicus | CS 3000 | Cobe Spectra | |
|---|---|---|---|
| Physical Size (cu ft) | 15 | 23 | 22.8 |
| Weight (pounds) | 350 | 700 | 389 |
| Centrifuge diameter (inches) | 6.75 | 12.8 | 12 |
| Pressure Sensors | 12 | 3 | 3 |
| Valves (nos.) | 34 | 7 | 5 |
| Inlet line gauge (G) | 17 | 17 | 17 |
| Return line gauge (G) | 18 | 17 | 17 |
| Inlet flow rate (ml/min) | 50-70 | 50-60 | 50-70 |
| Target platelet yield (x1011/unit) | 4 | 4 | 4 |
| Leukodepletion | No | No | Yes |
Processing time, total blood volume processed by the machine, amount of anticoagulant (ACD: Acid Citrate Dextrose) used for the entire procedure, i.e. priming and the actual collection was documented for every procedure.
Calculation of collection efficiency
PLT yield = product volume (ml) × product count (platelet/µl × Conversion factor (1000)
Total PLT processed=Pre + Post count ÷ 2 X TBV processed* × Conversion factor(1000)
* Total blood volume processed (TBV) = Blood volume processed(ml) - anticoagulant (ml)
The cell separator calculates a) the blood volume from the donor sex, height, weight,
b) Donor blood volume (to be processed) from the target PLT yield, and c) the processing time from the inlet rate.
Sampling and Counting
The laboratory tests on the donor blood sample viz., HB, HCT, PLT and WBC count were determined using cell counter (Coulter Act, Coulter, USA). We tested the pre donation samples within twenty-four hours before the actual donation, and the post donation samples within one hour after the procedure. We tested the product sample for PLT, WBC and RBC ct on the cell counter, within one hour. The PLT product was placed on a PLT agitator, after a hold period of one hour on the laminar airflow. Manual counting of WBC on a Nageotte counting chamber (Hauser scientific, Germany) was done on PC collected on Cobe. A 1:5 dilution of the representative sample was prepared in Turk’s solution with a stand time for ten minutes and the Nageotte chamber was loaded with the sample using a 100-µl pipette. Two full grids were counted for the presence of WBC.
Calculation of Product Leukocytes:
Leukocytes / ml (B) = A × 103
Leukocytes / bag = B × volume (ml) of bag
Statistical Analysis
All the data was analyzed using SPSS (version 11.1) software for windows. We grouped the data according to the three cell separators and the various donor, procedure and PLT product parameters. Paired sample T-Test applied to compare the various parameters of each machine. A P value <0.05 was considered significant. Correlation between the following variables was analyzed with Pearson’s correlation coefficient (r) and a P value <0.05 considered significant. a) Donor PLT count (pre donation) and the product PLT yield b) Percent reduction in donor PLT ct and pre donation PLT ct, age, weight (PLT yield constant at 4.8 in a subset of donors, N = 10).
Results
The mean age, weight and height of the donors taken on the three cell separators is as per Table 2. All donors were males. The incidence of females donating PC in our setup is low. The hemoglobin level (<12.5 g/dl) in Indian women is one of the major causes for deferral for blood and PLT donation. Blood ct reduced in donors taken on all the three separators [Table 3].
Table 2.
Donor parameters: Mean +/- standard deviation (Range)
| Donor Parameters | Amicus | CS 3000 | Cobe Spectra |
|---|---|---|---|
| Age (years) | 32 ± 8 | 32 ± 7 | 30 ± 7 |
| (18-54) | (21-53) | (19-48) | |
| Weight (kilograms) | 71 ± 9.3 | 74.5 ± 12.5 | 72.8 ± 11 |
| (55-103) | (55-103) | (55-98) | |
| Height (centimeters) | 170.5 ± 5.3 | 169.8 ± 5 | 171.4 ± 5 |
| (154-182) | (158-182) | (160-190) |
Table 3.
Post donation reduction in donor blood counts: Mean ± standard deviation (Range)
| Donor Parameters | Amicus (N = 121) | CS 3000 (N = 50) | Cobe Spectra (N = 66) | Total (N = 237) | |
|---|---|---|---|---|---|
| HB | : Pre | 13.7 ± 1 | 13.6 ± 1 | 14.1 ± 0.9 | 13.8±1.1 |
| (g/dL) | (12.5-17.3) | (12.5-16.4) | (12.5-16.6) | (12.5-17.3) | |
| : Post | 13.4 ± 1.1 | 12.9 ± 1.1 | 13.7 ± 1.1 | 13.4 ±1.1 | |
| (8.9-16.3) | (9.5-15.6) | (9-16.3) | (8.9-16.6) | ||
| P = 0.001 | P = 0.000 | P= 0.001 | |||
| HCT | : Pre | 41.6 ± 3.5 | 41.4 ± 2.8 | 43 ± 2.6 | 41.9 ± 3.2 |
| (%) | (34.0-54.2) | (35.4-50.3) | (34.9-49.3) | (34.0-54.2) | |
| : Post | 40.6 ± 3.3 | 39.2 ± 3 | 41.7 ± 3.1 | 40.6 ± 3.3 | |
| (26.3-49.7) | (28.1-44.3) | (28.5-49.2) | (26.3-49.7) | ||
| P= 0.000 | P= 0.000 | P= 0.001 | |||
| WBC count | : Pre | 6.5 ± 1.5 | 6.9 ± 1.48 | 6.6 ± 1.3 | 6.6 ± 1.5 |
| (x109/L) | (3.3-11.3) | (3.8-12.1) | (4.3-11) | (3.3-12.1) | |
| : Post | 5.9 ± 1.48 | 6.3 ± 1.4 | 5.9 ± 1.3 | 6.0 ± 1.4 | |
| (2.9-10.2) | (3.7-10.8) | (2.7-10.1) | (2.7-10.8) | ||
| P= 0.000 | P= 0.000 | P= 0.000 | |||
| PLT count | : Pre | 241.9 ± 50.5 | 276 ± 59.2 | 263.9 ± 55.6 | 255.2 ± 55.5 |
| (x109/L) | (150-389) | (154-424) | (150-438) | (150-438) | |
| : Post | 164.5 ± 42.4 | 200.6 ± 56.6 | 181.5 ± 46.1 | 176.9 ± 48.6 | |
| (80-334) | (109-385) | (106-343) | (80-385) | ||
| P= 0.000 | P= 0.000 | P= 0.000 | |||
Paired T Test, P < 0.05
The procedure parameters like total blood volume processed (ml), processing time (minutes), anticoagulant (ml), collection efficiency (percentage) are as per Table 4. The product parameters such as PLT yield, WBC content, RBC content and volume are as per Table 5. A Pearson’s correlation test showed a positive correlation between the donor PLT count (pre) and the product PLT yield [Table 6, Figure 1].
Table 4.
Procedure parameters (Mean ± S.D)
| Amicus | CS 3000 | Cobe Spectra | |
|---|---|---|---|
| Total Blood Volume processed (ml) | 3338.8 ± 653.2 | 3424.9 ± 546.7 | 3530 ± 502.7 |
| Processing time (min) | 59.81 ± 13.5 | 79.5 ± 13.5 | 69.26 ± 10.2 |
| Anticoagulant (ml) | 417.58 ± 71.36 | 343.52 ± 53.59 | 342.88 ± 39.65 |
| Collection Efficiency (%) | 79.31 ± 17.8 | 62.56 ± 11.7 | 57.5 ± 9.7 |
Table 5.
Platelet Product (Mean +/- S.D)
| Amicus | CS 3000 | Cobe Spectra | |
|---|---|---|---|
| PLT yield (×1011/unit) | 4.54 ± 0.82 | 4.42 ± 0.95 | 3.98 ± 0.73 |
| Product WBC (×109 /unit) | 0.09 ± 0.05 | 0.10 ± 0.06 | 0.00009 ± 0.0002 |
| Product RBC (×109 /unit) | 3.7 ± 1.86 | 2.46 ± 1.07 | 3.33 ± 1.61 |
| Product volume (ml) | 298.43 ± 9.26 | 229.54 ± 15.82 | 273.69 ± 35.4 |
Table 6.
Correlation between donor PLT CT (pre donation) and product PLT yield
| Donor PLT pre-count (×10e9 /L) | Product PLT yield (Mean ± S.D (×1011 /unit)) |
|---|---|
| =150 (N = 4) | 3.65 ± 0.58 |
| 151-250 (N = 117) | 4.14 ± 0.72 |
| 251-350 (N = 103) | 4.59 ± 0.87 |
| >350 (N = 13) | 4.68 ± 1.23 |
| Pearson’s correlation coefficient | r = 0.30 (P = 0.000) |
Figure 1.
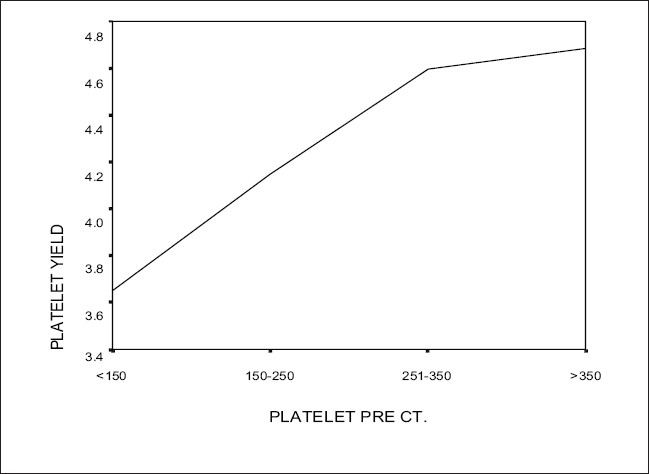
Correlation graph between donor platelet count (pre) and product platelet yield
Discussion
A cancer specialty hospital has many immunocompromised patients with low blood counts who need a reinforced transfusion set up, which provides quality blood products, mainly PC. It is prepared from whole blood by differential centrifugation (Buffy coat derived platelets - random donor platelets, RDP) or by plateletpheresis (Single donor platelets, SDP). SDP offers major advantages over RDP, particularly when improved patient care is given primary emphasis.[1] However, SDP is more expensive than RDP, which is a deterrent for economically challenged patients. Apheresis technology has progressed rapidly in the last two decades, thus helping healthcare setups to overcome PLT shortages. Our institute has installation of IFC and CFC cell separators. Most new cell separators are CFC based, and are more advantageous than the IFC. As per the Food and Drug Administration (FDA), each apheresis system and each type of product needs a separate validation. Parameters to be validated vary, but usually PLT yield and WBC content (if product is labeled ‘Leukoreduced’) are monitored.[2] Although the collection of a quality SDP is easy with the new cell separators, donor related factors, both clinical and laboratory might influence the PLT yield.[3] Transient but significant decreases in complete blood counts may occur in donors undergoing plateletpheresis.[4,5]
In this study, the donor testing was done one day prior to the donation, to assess his eligibility for pheresis. In addition, collection of blood sample was done immediately after the donation, to assess the reduction in HB, HCT, WBC and PLT counts. We tested the post donation sample within one hour after collection. The mean percent reduction in the blood counts was 2.9, 3.1, 9, 30.7 (N = 237) for HB, HCT, WBC, PLT respectively. Beyan et al.[5] found the drop in HB and HCT was significantly less on Cobe Spectra, as compared to Fenwal CS. The present study had the following mean reductions {CS vs. Cobe, HB (g/dl):0.7 vs. 0.4, HCT (%):2.2 vs. 1.3} [Table 3]. The mean percent reduction in PLT ct on Amicus, CS and Cobe was 31.9, 27.3 and 31.2% respectively as compared to Tenorio et al.[6] where it was 24, 30, and 32%. The drop in blood count was not high enough to cause any clinical problems for the donors. However, repeat PLT donors should be assessed critically for the reductions. Lazarus et al.[4] studied the effect of plateletpheresis on long-term regular donors. Even though a sustained reduction in PLT count was seen, clinically significant thrombocytopenia was unusual. Kalish et al. found a 29.4% decrease in PLT count after donation, which was not clinically significant.[7] In the present study, five out of 237 donors (N = 5, Amicus) showed a post donation PLT ct of <100×109/L (Range 80-100). All five donors had a pre donation PLT ct between 150-200×109/L. A cautious approach is recommended in these groups of donors. The Cobe spectra have an inbuilt alarm, to warn the operator, if there is a likelihood of donor PLT ct reducing below 100×109/L.
A Pearson’s correlation test done to assess the correlation between the donor PLT ct (pre donation) and the product PLT yield and a P value <0.05 was considered significant. A positive correlation was found i.e., higher the pre donation PLT ct, higher is the product PLT yield, correlation coefficient, r = 0.30 (P = 0.000) [Table 6, Figure 1]. Guerrero-Rivera et al.[8] found a direct relation between the two parameters, and in addition, they report an inverse relationship between HB and PLT yield. Lasky[9] found that PLT ct was the main predictor of PLT yield. He also found in his study that gender also influences PLT yield and women had higher yields, possibly because there is a higher prevalence of iron deficiency among women with consequent increase in PLT ct, and due to hormonal influence. The main variable in PLT yield is donor PLT ct (pre donation) and this variable is so important that some authors have used thrombopoietic factor to increase the pre donation PLT ct.[10]
Keeping the PLT yield (4.8×1011/unit) as a constant parameter in a subset of donors (N = 10), Pearson’s correlation test showed a 1)a positive correlation of the donor’s percent reduction in platelet count with pre donation platelet count (r=0.384,P=0.274) and also with age (r=0.282,P=0.43) 2)a negative correlation of the donor’s percent reduction in platelet count with weight (r=0.358,P=0.31) As the number of donors in this subset is limited, the P value >0.05. Kalish et al. established the above correlations in a study of 607 donors with P values <0.01.[11]
PC contain donor leukocytes, however white cell reduced PC are possible on the newer generation of blood cell separators. These devices achieve a high degree of separation between donor platelets and leukocytes because of several design principles. Flow path geometry, counter flow centrifugation, elutriation and separation of fluid particle bed are used to separate PLT and leukocytes on the basis of difference in cell mass.[12] In the Cobe spectra, initially PLT harvesting is done from the Buffy coat and they enter the LRS (leukoreduction system) chamber to form a fluidized particle bed. As the saturated platelet bed forms, it becomes virtually impenetrable by WBC. When WBC find their way into the LRS chamber, they are effectively and continuously trapped in the lower section of the chamber and are also prevented from exiting the LRS chamber due to the centrifugal force applied. Platelets continue to advance until they converge in the narrow upper region of the LRS chamber. In the present study, Cobe PC had 0.09×106 WBC / unit [Table 5], and thus met the standard norms for leukoreduction (<1×106 WBC / unit). However, process control is required, as sudden changes in inlet rate, paused collections that disturb the centrifugal separation or characteristics of the donor blood that interfere with optical sensors on the device, may result in failure to give a leukoreduced component.
The extracorporeal volume is the least on Cobe spectra (131 ml), as compared to the other two machines (250 ml). Cobe needed the least amount of anticoagulant (342.88 ±39.65), hence fewer chances of donor reactions. This is similar to the findings of Benjamin et al.[13] where Amicus used significantly more ACD, as compared to Cobe spectra. However, we inform the donors of the symptoms of citrate toxicity at their first attendance itself. The blood volume processed in the three cell separators was between 3.3 to 3.5 liters, to achieve a target PLT yield of 4×1011/unit [Table 4]. The time needed to process this blood volume was between 60-80 minutes [Table 4]. CS (79.5 ± 13.5) needed the maximum time, whereas Amicus (59.81 ± 13.5) was the quickest. The product volumes of PC collected on the three cell separators were within the recommended limits [Table 5]. As per guidelines, the volume is 150-300 ml for PC prepared by apheresis.[2] Optimizing the PLT yield by increasing the collection efficiencies provides several benefits (viz. increased dose in one SDP, SDP split into two, reduced donation time).[13] In the present study, all the three separators gave acceptable platelet yields (Mean 3.9-4.5×1011/unit) for a set target of 4×1011/unit. Amicus gave a mean yield of 4.5±0.82 with corresponding collection efficiency of mean 79.31±17.8, which was the best amongst the three machines. Burgstaler et al. had obtained 73% collection efficiency on Amicus as opposed to 53% on the Cobe spectra.[14]
To conclude, new generation cell separators are capable of giving optimum PLT yield in a short duration. Technological developments in cell separators have resulted in cleaner separation between platelets and white cells, thus reducing cross contamination.[15] Monitoring donors for changes in blood counts ensures a robust quality assurance system for donor safety.
Figure 2.
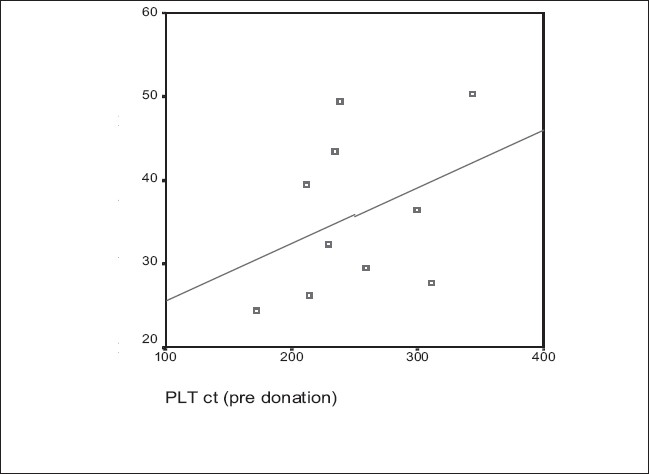
Percent reduction in platelet count and Pre procedure platelet count (Platelet yield constant: 4.8×1011/unit, N = 10)
Acknowledgments
The authors acknowledge Mrs. Rohini Havaldar and Ms. Anagha Kakade - Clinical Research Secretariat, Tata Memorial Hospital, Mumbai for their contribution towards statistical analysis of the data, Mr. Nair for secretarial assistance.
Footnotes
Source of Support:Nil
Conflict of Interest: None declared.
References
- 1.Ness PM, Campbell-Lee SA. Single donor versus pooled random donor platelet concentrates. Curr Opin Hematol. 2001;8:392–6. doi: 10.1097/00062752-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 2.British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10–23. doi: 10.1111/j.1365-2141.2010.08444.x. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary R, Das SS, Khetan D, Sinha P. Effect of donor variables on yield in single donor plateletpheresis by continuous flow cell separator. Transfus Apher Sci. 2006;34:156–61. doi: 10.1016/j.transci.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus EF, Browning J, Norman J, Oblitas J, Leitman SF. Sustained decreases in platelet count associated with multiple, regular plateletpheresis donations. Transfusion. 2001;41:756–61. doi: 10.1046/j.1537-2995.2001.41060756.x. [DOI] [PubMed] [Google Scholar]
- 5.Beyan C, Cetin T, Kaptan K, Nevruz O. Effect of plateletpheresis on complete blood count values using three different cell separator systems in healthy donors. Transfus Apher Sci. 2003;29:45–7. doi: 10.1016/S1473-0502(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 6.Tenorio GC, Strauss RG, Wieland MJ, Behlke TA, Ludwig GA. A randomized comparison of plateletpheresis with the same donors using four blood separators at a single blood center. J Clin Apher. 2002;17:170–6. doi: 10.1002/jca.10036. [DOI] [PubMed] [Google Scholar]
- 7.Kalish RI, Chambers LA, Linden JV. The effect of plateletpheresis on the Fenwal CS-3000 on donor platelet counts. J Clin Apher. 1987;3:230–4. doi: 10.1002/jca.2920030408. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero-Rivera S, Gutiérrez-Espíndola G, Talavera JO, Meillón-García LA, Pedraza-Echevarría M, Pizzuto-Chávez J. Hemoglobin and platelet count effect on platelet yields in plateletpheresis. Arch Med Res. 2003;34:120–3. doi: 10.1016/S0188-4409(02)00453-8. [DOI] [PubMed] [Google Scholar]
- 9.Lasky LC, Lin A, Kahn RA, McCullough J. Donor platelet response and product quality assurance in plateletpheresis. Transfusion. 1981;21:247–60. doi: 10.1046/j.1537-2995.1981.21381201794.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodnough L, Kuter J, McCollough J, Slichter S, DiPersio J, Romo J, et al. Prophylactic platelet transfusions from healthy apheresis platelet donors undergoing treatment with thrombopoietin. Blood. 2001;98:1346–51. doi: 10.1182/blood.v98.5.1346. [DOI] [PubMed] [Google Scholar]
- 11.Kalish RI, Chambers LA, Linden JV. The effect of plateletpheresis on the fenwal CS3000 on donor platelet counts. J Clin Apher. 1987;3:230–4. doi: 10.1002/jca.2920030408. [DOI] [PubMed] [Google Scholar]
- 12.Simon TL, Dzik WH, Snyder EL, Stowell CP, Strauss RG. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2002. Rossi’s Principles of Transfusion Medicine; p. 273. [Google Scholar]
- 13.Benjamin RJ, Rojas P, Christmas S, Neal J, Broughton S, Burgio C, et al. Plateletpheresis efficiency: A comparison of the spectra LRS and Amicus separators. Transfusion. 1999;39:895–9. doi: 10.1046/j.1537-2995.1999.39080895.x. [DOI] [PubMed] [Google Scholar]
- 14.Burgstaler EA, Pineda AA, Bryant SC. Prospective comparison of plateletpheresis using four apheresis systems on the same donors. J Clin Apher. 1999;14:163–70. doi: 10.1002/(sici)1098-1101(1999)14:4<163::aid-jca2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Lewis SL, Kutvirt SG, Bonner PN, Simon TL. Effect of long term platelet donation on lymphocyte subsets and plasma protein concentrations. Transfus Sci. 1997;18:205–13. doi: 10.1016/s0955-3886(97)00011-8. [DOI] [PubMed] [Google Scholar]


