Abstract
Premature birth and disruption of the normal maturation process leave the immature ductus arteriosus unable to respond to postnatal cues for closure. Recent strategies that advocate conservative management of the patent ductus arteriosus (PDA) in premature infants are dependent on identification of the symptomatic PDA and understanding the risk factors that predispose to PDA. Exposure of premature infants to unintended vasodilatory stimuli may be one of the risk factors for PDA that is under-recognized. In this paper, we summarize the clinical factors that are associated with PDA and review commonly used neonatal drugs for their vasodilatory properties. Data demonstrating relaxation of the ductus arteriosus by gentamicin and other aminoglycoside antibiotics, by cimetidine and other H2 receptor antagonists, and by heparin are provided as examples of neonatal therapies that have unanticipated effects that may promote PDA.
Introduction
Postnatal closure of the ductus arteriosus is a critical step in circulatory adaptation to newborn life. During the immediate postpartum period, ductus closure is preceded by lung aeration and increased oxygenation, with a subsequent fall in pulmonary vascular resistance, an increase in systemic vascular resistance, closure of the foramen ovale and reversal of shunting across the ductus. These hemodynamic changes coincide with a loss of vasodilatory stimuli and activation of intrinsic contractile mechanisms that facilitate muscular constriction and occlusion of the ductus lumen.
An imbalance in vasodilatory and contractile forces may lead to on-going patency of the ductus arteriosus (PDA) and subsequent physiological compromise. Premature birth interrupts development of ductus contractile mechanisms and the normal maturation process, leaving the immature ductus more susceptible to vasodilatory stimuli. In general, immaturity of the ductus arteriosus is the greatest risk factor for PDA, as reflected by its consistently strong association with lower birth weight and/or earlier gestational age (Table 1). Additional factors that are well-known to be associated with PDA include severity of respiratory distress, sepsis, persistence of blood flow through the ductus lumen, maternal diabetes, and excess fluid administration. Less consistent findings from various studies also suggest that PDA may occur in association with furosemide treatment, breech delivery, IUGR, certain maternal treatments, and other more controversial or less-studied risk factors (Table 1). PDA in full term as well as in preterm infants is also a feature of numerous genetic syndromes, and has a heritable component based on its identification at a higher rate in siblings of affected individuals, the presence of recessive and dominant inheritance patterns in occurrences of familial PDA, and identification of gene regions or susceptibility genes that are associated with PDA (Table 1). Exposure to excess vasodilatory stimuli in the presence of these risk factors may promote ductus relaxation and exacerbate PDA.
Table 1. Factors associated with PDA.
Risk factors for PDA were considered to be well-established if they were identified by studies that sought causative factors for PDA, remained significant after multivariate analysis, or were consistently observed in multiple controlled trials in different patient populations. Other factors that have been shown to have an association with PDA were drawn from single studies, epidemiologic surveys, birth defect registry reports, case reports, or small studies that did not control for confounding variables. PDA at term (T) gestation is regarded as a congenital malformation, but these risk factors may also occur in preterm (PT) infants. Conflicting studies that did not detect an association of PDA with these factors are not presented. Genetic conditions were considered separately. Only a subset of representative citations are shown for risk factors that were consistently identified in numerous studies.
| Established Risk Factors for PDA | Other Factors Associated with PDA | |
|---|---|---|
| Early gestational age 59-64 | IUGR59,65 | Maternal drugs: |
| Low birth weight 63,64,73 | Delay in indocin treatment 62 | Antihistamine 66 |
| RDS59,61,64,73 | Furosemide treatment 7,8 | Magnesium 32,33 |
| Persistence of DA flow 74 | Use of HFOV 75,76 | ACE inhibitors 67,68 |
| Sepsis 5,6 | Race: | Anticonvulsants 69 |
| Excess fluid administration 60,78 | Caucasian (PT) 60,61,66 | Ca channel blockers 34 |
| Antenatal NSAID exposure 13,14 | African American (T) 77 | Cocaine 70 |
| Initial hypotension 66,73 | Gender: | Maternal PKU (T) 71,72 |
| Need for intubation/airway pressure59,66 | Male (PT) 66,79 | |
| Lack of antenatal betamethasone 61 | Female (T) 80,81 | Genetic Conditions (T) 82 |
| Maternal diabetes (T) 79,85 | Prolonged ROM 64 | (trisomy 21, 18, 13, Char, Holt-Oram, DiGeorge, Noonan, CHARGE, TAAD/PDA) |
| Birth at high altitude (T) 86,87 | Twins79,88,89 | |
| Congenital rubella (T) 90 | Perinatal stress 59 | |
| Hypothyroidism (T, PT) 91,92 | Antenatal hemorrhage 60,79 | |
| Breech 66 | ||
| Phototherapy 93 | Genetic Susceptibility 83,84 | |
| Familial PDA 82 | ||
Extensive research efforts have focused on understanding the molecular mechanisms of ductus arteriosus regulation and identifying the clinical factors that are associated with PDA. Alternatively, it would be helpful to know whether medications that are currently used in neonatal practice have an adverse impact on the incidence or severity of PDA. Toxicology studies typically test new drugs for deleterious effects in the fetus and newborn before their clinical use, but these studies are unlikely to identify a causal relationship to ductus arteriosus problems unless they are lethal. In addition, the current slow pace of neonatal pharmacokinetic studies and prospective trials to assess adverse drug effects in ELBW infants, who are most at risk for PDA, prevents the timely a priori identification of potentially harmful effects of new compounds on patency of the ductus. As a result, new drugs or even those that are commonly used in the NICU may have unexpected effects on the ability of the ductus to spontaneously close, resulting in increased risks for PDA. The purpose of this article is to review current treatments that may unwittingly contribute to relaxation of the ductus arteriosus in premature infants.
Drugs or compounds with recognized vasodilatory effects on the ductus arteriosus
Prostaglandins
Prostaglandins or the cyclooxygenase inhibitors that prevent their synthesis are the most widely-known agents that affect patency of the ductus arteriosus. In 1973, Coceani and Olley first reported that E-series prostaglandins induced relaxation of isolated strips of the sheep ductus arteriosus 1. Subsequent studies have demonstrated that PGE1 and PGE2 are the most potent endogenous dilators of the ductus, although PGI2 and its metabolites may also play important vasodilatory roles. Pharmacological agents that specifically stimulate individual PGE receptor subtypes are under development and may soon be available to modulate ductus tone 2,3. Other prostanoids may have roles in relaxation of the human ductus 4, but these have not been clinically demonstrated. An increase in circulating prostaglandins has been implicated as an underlying cause of PDA in infants with sepsis 5,6. In addition, furosemide treatment as part of the clinical management of PDA may stimulate excessive prostaglandin synthesis 7,8. These findings suggest that consideration of inadvertent prostaglandin actions may be warranted under any clinical scenario where ductus patency is enhanced.
A constrictive effect of non-steroidal anti-inflammatory drugs (NSAIDs) on the ductus arteriosus was first suggested in 1969 by unexplained ductus closure in a newborn infant delivered to a mother who was treated with salicylates for acute polyarthritis 9. Over the next decade, the constrictive effects of indomethacin and ibuprofen were demonstrated on the ductus of various lab animals followed by the first successful trials using indomethacin to close the PDA of premature infants 10,11. Indomethacin freely crosses the placenta and has increasing contractile effects on the fetal ductus with advancing gestation 12, leading to cautionary warnings for the use of NSAID-related compounds during the latter part of pregnancy. Paradoxically, prenatal exposure to NSAIDs is also associated with PDA 13,14. Although some clinical studies fail to detect this association, animal models now suggest that injury to the vessel wall 15, alterations in ductus development 16, or upregulation of other vasodilators 17 may be underlying causes for this effect.
Nitric Oxide
Nitric oxide (NO) is a potent dilator of most vascular beds and has well-described effects on the ductus arteriosus. Animal studies show that the preterm ductus is more dependent on NO signaling than the term gestation ductus to maintain its relaxed state in utero 18,19. Treatment with a combination of NSAIDs and NO synthesis inhibitors produces more potent ductus constriction than inhibition of either cyclooxygenase or NO synthase alone 20, corresponding to the synergistic interaction of NO and prostaglandins in ductus regulation 21. Nitroglycerin, nitroprusside, and other pharmaceutical agents that increase nitrates or act as NO-donors relax ductus tone via stimulation of cGMP and have the potential to dilate the ductus clinically, although this is not therapeutically beneficial due to the non-specific nature of the currently available nitrovasodilators. Although the use of inhaled NO for the treatment of neonatal pulmonary hypertension has the potential to increase PDA rates, this has not been observed clinically 22,23. The introduction of nitro-NSAIDs and similar novel NO-donating compounds into clinical practice will require a focused evaluation to determine whether there are deleterious effects on closure of the ductus arteriosus.
Other DA dilators
Numerous other biological mediators have been identified that have vasodilatory effects on the ductus. Adenosine-induced dilation of the preconstricted fetal sheep ductus 24 suggests its role as an endogenous ductus dilator. Adenosine exerts direct actions via one of four purinergic receptors, but their expression in the ductus has not been fully characterized. Adenosine also participates in cell signaling as a cyclic purine nucleotide. The cyclic nucleotides adenosine 3′,5′-monophosphate (cAMP) and guanosine 3′,5′-monophosphate (cGMP) play a fundamental role as second messengers in cell-signaling and in inducing relaxation of most vascular beds, including the ductus. Atrial natriuretic peptide (ANP) induces vasorelaxation via stimulation of cGMP production, similar to NO. Although ANP did not affect ductus tone in rabbits 25, potent vasodilatory effects were noted in the ductus arteriosus of rats, including prevention of postnatal ductus closure 26. Phosphodiesterase (PDE) inhibitors block cGMP and cAMP catabolism and stimulate vasodilation, including relaxation of the ductus arteriosus 27. Selective inhibitors are only recognized for a few of the 11 different PDE family members. However, the PDE3 inhibitors milrinone and amrinone 26,27 and the PDE5 inhibitor, sildenafil 28, which are currently in use in the NICU, have consistent vasodilatory effects on the ductus, and have been proposed to serve an adjunct role in maintaining ductus patency and extending the bridge to corrective surgery for congenital heart disease. Finally, beta-adrenergic catecholamine receptors also stimulate vascular smooth muscle relaxation and have been detected in the ductus, although there is conflicting information on whether they play a vasodilatory role 29,30.
Therapeutic regimens used in the NICU have the potential to stimulate or block several of these pathways with resulting alterations in ductus tone. For example caffeine and theophylline have antagonistic effects on the A1 and A2a adenosine receptors, but also play an important role as phosphodiesterase inhibitors. Although caffeine is widely used in many NICUs, current evidence suggests that it does not have vasodilatory effects on the ductus 31. Drugs that are used during pregnancy should also be considered. In particular, tocolytic agents used to inhibit uterine smooth muscle contraction during preterm labor may have the unintended consequence of prolonging ductus patency after birth. Most studies on the use of magnesium sulfate in pregnant women suggest that there is no effect on postnatal ductus closure rates. However, a few investigators have found a significant relationship between antenatal magnesium exposure and PDA 32,33. In addition, calcium channel blockers are used as tocolytic agents and may be associated with PDA 34. Antenatal exposure to ACE inhibitors, antihistamines, anticonvulsants and other medications are also associated with increased rates of PDA (Table 1). Concerted efforts to reduce the incidence of PDA in premature infants will require an increased awareness of treatments that may have deleterious effects on postnatal ductus constriction, including maternal medications that cross the placenta.
Drugs with unexpected vasodilatory effects on the ductus arteriosus
Until the mechanisms that regulate the ductus arteriosus are completely defined, the potential exists for drugs and compounds that are used in the NICU to adversely affect ductus patency. One strategy to address this problem is to expose the ductus arteriosus to various vasodilators 25. Another approach is to survey the drugs that are commonly used in the NICU to identify agents that have known vasodilatory effects in other vascular beds. The most frequently used neonatal pharmacopoeias include numerous drugs that have vasodilatory potential (Table 2). Although the vascular relaxation properties of a given compound do not necessarily predict an adverse effect on the ductus, caution may be warranted with these agents due to the early age of exposure in premature neonates and the prolonged nature of certain drug treatments. Unfortunately, the potential for ductus-specific effects cannot be easily identified by a general vasodilatory survey. A more common approach is to exploit the fortuitous discovery of ductus-related drug effects during the course of neonatal studies. Examples of these approaches are provided herein.
Table 2. Vasoactive medications commonly used in the NICU.
Drugs with the potential to induce vasodilation were identified from available neonatal pharmacopeias. Examples of general vasodilatory effects are shown. The ability of these compounds to induce ductus-specific relaxation remains largely unknown. SVR, systemic vascular resistance; PVR, peripheral vascular resistance; Ach, acetylcholine. Supporting references available upon request.
| Category | Generic Name | Effects |
|---|---|---|
|
Analgesics and Sedatives |
Morphine | Vasorelaxant effects: Fentanyl > Morphine |
| Fentanyl | Vasorelaxant effects | |
| Methadone | Functions as calcium antagonist | |
| Diazepam | Synergistic with cAMP-elevating agents | |
| Midazolam | Vasodilation, esp. prostaglandin pre-contracted vessels | |
| Ketamine | In-vitro vasodilatory effects | |
| Clonidine | Centrally acting sympatholytic: blocks sympathetic activity by binding to and activating α2-adrenoceptors |
|
| Antimicrobials | Penicillin G | Nafcillin-induced vasodilation and decr SVR, PVR |
| Gentamicin | See discussion | |
| Vancomycin | Decr BP due to histamine, myocard depr and vasodilation | |
| Cardiovascular | Dopamine | Mimics sympathetic adrenergics via β-adrenoceptors; dilation of lg conductance and small resistance vessels |
| Dobutamine | Dose-dependent reductions in SVR | |
| Milrinone | See discussion | |
| Sildenafil | See discussion | |
| Bosentan | Vasodilation by non-selective ET-1 receptor antagonism | |
| Captopril, Enalapril | ACE/kininase inhibition; incr tissue concentration of kinins, contributes to vasodilatory effects |
|
| Losartan | Inhibits AT(1)-mediated constrictive and antidiuretic effects of angiotensin; activates vasodilating and diuretic AT(2) receptors |
|
| Phentolamine | α-adrenoceptor antag; endothelium-independent vasodil. | |
| Nifedipine | Reduces SVR and arterial pressure; can lead to reflex cardiac stimulation |
|
| Nitric Oxide (iNO) | See discussion | |
| Sodium nitroprusside | SNP decomposes intravascularly to release NO | |
| Caffeine | Dose-dependent relaxation of various arteries. | |
| Respiratory | Aminophilline | Incr in adenosine contributes to hypox-induced vasodil. |
| Furosemide | See discussion | |
| Diuretics | Phenobarbital | Increased potency of acetylcholine as an endothelium- dependent vasodilator |
|
Anticonvulsants GI Drugs itamins, Minerals, Hormones |
Omeprazole | Induces relaxation of isolated human arteries |
| Cholecalciferol | Ach-induced vasorelaxation is preserved/restored | |
| Vitamin E | Increases production of vasodilator prostanoids | |
| Calcium | High calcium diet improves vasorelaxation | |
| Phophate | Impairs vasorelaxation | |
| Magnesium | Dilates both epicardial and resistance coronary arteries | |
| Ferric Gluconate | Intravenous form has a vasodilatory effect | |
| Insulin | Dose-dependent effects in the cerebral vasculature; Biphasic response: initial vasoconstr, then vasodilation |
|
| Heparin | See discussion |
Gentamicin and other aminoglycosides
A hemodynamically significant PDA has adverse effects on renal perfusion and can alter the volume of distribution of numerous drugs, resulting in concerns for inaccurate gentamicin dosing in premature newborn infants 35. Conversely, there is little or no information available on the effect of gentamicin on the PDA, despite the recognized vasodilatory effects of aminoglycosides in other tissues 36-39.
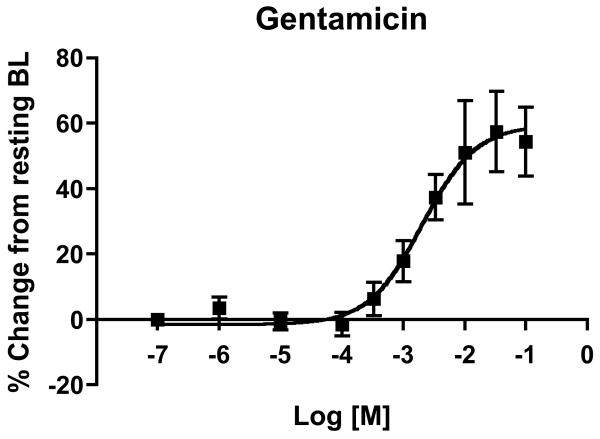
Our laboratory 16,21 and others 40 have developed methods to study the vascular response of the surgically isolated ductus arteriosus of fetal and newborn mice. As part of our efforts to better understand the response of the ductus arteriosus to sepsis and inflammation, we evaluated changes in ductus tone after in vitro exposure to commonly used neonatal treatments for infection. With this approach, the ductus arteriosus was excised, mounted on glass micropipette tips in a microvessel myography chamber, pressurized to physiologic levels, and studied under low oxygen tension, in order to stimulate relaxation of ductus smooth muscle and maintain stable resting diameter 21. Changes in vessel tone were monitored by computer-assisted videotracking of lumen diameter and expressed as percent change from resting baseline. Exposure to increasing concentrations of gentamicin (Figure 1) or tobramycin (not shown) produced a dose-dependent dilation of the cannulated, pressurized ductus arteriosus at term (day 19) gestation. Compared to resting baseline dimensions, the isolated ductus underwent 60% increase in vessel diameter (Figure 1). Vessels that were submaximally preconstricted by pre-incubation with U46619 (a thromboxane mimetic) showed similar results (not shown). These findings suggest that aminoglycosides have vasodilatory properties in the murine ductus.
Figure 1. Response of the isolated ductus arteriosus to gentamicin.
The ductus arteriosus of term gestation fetal mice was excised and mounted in a cannulated vessel myograph. Isolated vessels were pressurized to physiological levels under fetal oxygen conditions and then exposed to increasing doses of gentamicin. Changes in lumen size are expressed as a percent change from the baseline (BL) vessel diameter after equilibration. Compared to pretreatment dimensions, gentamicin induced dose-dependent vasodilation of the ex vivo ductus arteriosus (n = 6).
The hypotensive and myocardial depressant effects of gentamicin and other aminoglycoside antibiotics are extensively documented in different species 38,39. Alterations in intracellular calcium flux are implicated as an underlying mechanism for their cardiac effects 36-38. Aminoglycosides also inhibit phospholipase C and PLC-induced hydrolysis of inositol phospholipids, preventing the necessary increase in [Ca2+]i to maintain contractility and vascular tone 41. Inhibition of phospholipase D, protein kinase C, and inhibition of calcium channel activity 42 have also been implicated as mechanisms for aminoglycoside-mediated effects. Although we observed a vasodilatory effect of gentamicin on the murine ductus arteriosus in vitro, these results occurred at doses 100-1000 fold higher than target serum levels used in clinical practice, and cannot be extrapolated to human infants until further studies are performed. Nevertheless, the use of bolus dosing strategies and prolonged courses of aminoglycosides may have unintended subclinical vasodilatory effects on the postnatal ductus arteriosus. Aminoglycoside exposure should be considered a factor of interest in future efforts to identify PDA risk factors.
Cimetidine and other H2 antagonists
Cimetidine and more recently developed H2 receptor antagonists have been used in premature infants for management of gastroesophageal reflux, treatment of stress-induced gastritis, and for occasional use as an inhibitor of histamine-mediated inflammatory conditions. However, cimetidine is also recognized as an inhibitor of the cytochrome P450 (CYP) system, where it is known to interact with the heme iron moiety and bind to CYP1A2, CYP2C, CYP2D6, CYP3A4 and other CYP family members 43,44, resulting in competitive and non-competitive enzyme inhibition 45. Certain CYP enzymes are induced by oxygen exposure and may serve as mediators of oxidant injury. CYP enzyme inhibition by cimetidine was found to completely block the severe pulmonary effects of exposure to 95% oxygen in newborn lambs 46, possibly by inhibiting the formation of toxic oxygen metabolites or other free radical species. A follow-up randomized clinical trial was performed to evaluate whether treatment with cimetidine (0.5 mg/kg/d infusion for 10 days, starting within 24h of birth) would reduce lung injury in ELBW preterm infants at high risk for chronic lung disease. Despite therapeutic serum levels, cimetidine did not prevent lung disease 47 but an increase in PDA was noted in treated infants (34% vs 10%; p=0.012) 48. This association remained significant after logistic multivariable regression analysis controlling for the most common predictors of symptomatic PDA.
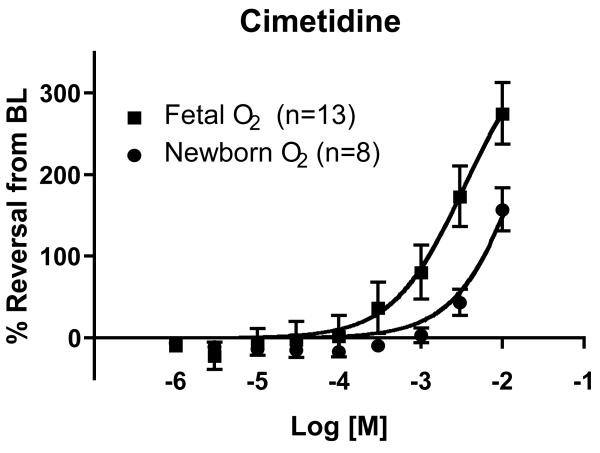
A role for CYP enzyme activity in regulation of the ductus arteriosus has been extensively demonstrated by Coceani and colleagues, where CYP enzymes are postulated to serve as a transducer of oxygen signals and exert their actions via the downstream effector ET-149. Although ABT and other biochemical inhibitors were used to evaluate CYP function in the ductus, clinically relevant CYP inhibitors like cimetidine have not been studied. Using cannulated vessel myography, as described above, we observed dose-dependent dilation of the isolated fetal mouse ductus arteriosus in response to cimetidine exposure (Figure 2). The drug doses that elicited a significant vasodilatory response were several orders of magnitude higher than the therapeutic levels used in clinical practice. However, 100-1000 fold higher cimetidine concentrations are typically required to inhibit drug metabolism in vitro compared with hepatic drug clearance in vivo 45. Ranitidine, which also has H2 antagonistic and CYP inhibitory effects, induced a similar vasodilatory response, whereas famotidine, a selective H2 blocker with little or no CYP inhibitory effects, produced significantly less dilation of the isolated ductus (not shown). We also found that pre-treatment with cimetidine prevented oxygen-induced constriction of the cannulated ductus, while famotidine-exposed and untreated control vessels progressively constricted in response to increasing oxygen tension (not shown).
Figure 2. Response of the isolated ductus arteriosus to cimetidine.
The ductus arteriosus of term gestation fetal mice was excised and mounted in a cannulated vessel myograph. Isolated vessels were pressurized to physiological levels under fetal oxygen conditions and then preconstricted either by treatment with the thromboxane agonist U46619 or by exposure to newborn oxygen conditions. Changes in lumen diameter are expressed as percent reversal from the preconstricted baseline (BL). Cimetidine stimulated dose-dependent vasodilation of the ex vivo ductus arteriosus. Cimetidine-induced ductus relaxation was more prominent under fetal than newborn oxygen conditions. Similar results were observed in the preterm fetal ductus (not shown). Maximum response curves were limited by cimetidine solubility.
Together, these results suggest that “cimetidine-associated PDA”, based on data from a randomized clinical trial to evaluate lung-protective strategies, is a valid clinical entity. The use of antireflux medications is common in premature infants. However, warnings on the use of H2 blockers due to their potential association with NEC and neonatal sepsis have prompted concern for their casual use in the NICU population. Our studies indicate that the CYP inhibitory and PDA-promoting effects of certain H2 blockers should also be taken into account if these drugs are required in preterm infants.
Heparin
Heparin remains a mainstay of neonatal practice due to the ongoing need for vascular access in critically ill infants and well-documented concerns for the thrombogenic nature of umbilical lines and peripherally-inserted central venous catheters (PCVCs). Meta-analysis of randomized clinical trials of heparin use in umbilical arterial catheters (UACs) 50 and PCVCs 51 found that heparinized fluids improve UAC and PCVC patency and are generally not associated with extension of intraventricular hemorrhage, death, or catheter-related sepsis. None of the studies were powered to evaluate changes in the incidence of other adverse events, including PDA. Theoretical concerns for PDA exist, however, since most anticoagulation therapies, including heparin, tissue plasminogen activator, and coumarin have vasodilatory effects.
The vasodilatory properties of heparin were initially described over 60 years ago 52. Heparin has anti-hypertensive properties in animals and humans, and has long been recognized as a cause of hypotension in association with cardiopulmonary bypass procedures. A number of mechanisms have been identified that mediate heparin-induced changes in cardiovascular contractility or tone, including sequestration or alterations in calcium availability 53, increases in histamine 54, inhibition of the renin-angiotensin and aldosterone systems 55, induction of NO synthesis 54, and generation of endothelium-derived hyperpolarizing factor 56. In addition to its biochemical and cell-signaling effects, heparin can also relax vascular tone by interacting with proteins enmeshed in the cell-surface glycocalyx of the endothelium and impair the normal shear-mediated sensing mechanisms that regulate blood flow 57.
Heparin-induced relaxation of the ductus arteriosus has not been directly observed. However, Ojala and Lehtonen recently reported an association between PDA treatment failure and the use of heparin in central lines in preterm infants < 1500g 58. In that study, the need for PDA ligation after indomethacin treatment was significantly higher in infants who received 0.6 U/kg/h continuous heparin exposure via PCVC than in infants that received heparin flushes every 12 hours. Comparison of the 3-month trial period of continuous heparin infusion to combined 1-year observation periods before and after their change in practice revealed a 13-fold increase risk for PDA treatment failure. These results remained significant after logistic multivariable regression analysis. In addition, there was an increase trend in the need for PDA ligation in infants with continuous heparin infusion via umbilical or radial arterial catheters compared to infants without arterial catheters. These findings suggest that continuous heparin exposure may interfere with indomethacin effects or might have a competing vasodilatory stimulus, possibly via one or more of the heparin-associated vasodilatory mechanisms above. Although additional information is required to verify these concerns, the judicious use of heparin in neonatal fluids should be considered. Similarly, inadvertent placement of a UAC near the ductus should be avoided, since significant left-to-right shunt and reversal of diastolic flow in the aorta may cause excess exposure of the ductus to heparin-containing fluids.
Conclusions
A better understanding of the balance between vasodilatory forces and active closure mechanisms might provide new information on causes of PDA and help to identify management strategies to increase the chances of spontaneous closure. Although an ever-increasing list of factors are associated with PDA (Table 1), it is possible that predisposition to PDA is enhanced by prolonged exposure to drugs that promote ductus relaxation. It may be possible to tip the scales in favor of ductus closure by identifying a sufficient number of pharmacological, nonpharmacological, and biological risk factors for PDA so that a preventive approach could be developed 59,60. Additional studies are required to determine the extent to which exposure to heparin, certain H2 blockers, and members of the aminoglycoside family are significant clinical risk factors for PDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coceani F, Olley PM. The response of the ductus arteriosus to prostaglandins. Can J Physiol Pharmacol. 1973;51(3):220–225. doi: 10.1139/y73-031. [DOI] [PubMed] [Google Scholar]
- 2.Kajino H, Taniguchi T, Fujieda K, Ushikubi F, Muramatsu I. An EP4 receptor agonist prevents indomethacin-induced closure of rat ductus arteriosus in vivo. Pediatr Res. 2004;56(4):586–590. doi: 10.1203/01.PDR.0000139409.25014.35. [DOI] [PubMed] [Google Scholar]
- 3.Momma K, Toyoshima K, Takeuchi D, Imamura S, Nakanishi T. In vivo reopening of the neonatal ductus arteriosus by a prostanoid EP4-receptor agonist in the rat. Prostaglandins Other Lipid Mediat. 2005;78(1-4):117–128. doi: 10.1016/j.prostaglandins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Leonhardt A, Glaser A, Wegmann M, et al. Expression of prostanoid receptors in human ductus arteriosus. Br J Pharmacol. 2003;138(4):655–659. doi: 10.1038/sj.bjp.0705092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez A, Sosenko IR, Chandar J, et al. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996;128(4):470–478. doi: 10.1016/s0022-3476(96)70356-6. [DOI] [PubMed] [Google Scholar]
- 6.Rojas MA, Gonzalez A, Bancalari E, et al. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995;126(4):605–610. doi: 10.1016/s0022-3476(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 7.Friedman Z, Demers LM, Marks KH, Uhrmann S, Maisels MJ. Urinary excretion of prostaglandin E following the administration of furosemide and indomethacin to sick low-birth-weight infants. J Pediatr. 1978;93(3):512–515. doi: 10.1016/s0022-3476(78)81182-2. [DOI] [PubMed] [Google Scholar]
- 8.Green TP, Thompson TR, Johnson DE, Lock JE. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory-distress syndrome. N Engl J Med. 1983;308(13):743–748. doi: 10.1056/NEJM198303313081303. [DOI] [PubMed] [Google Scholar]
- 9.Arcilla RA, Thilenius OG, Ranniger K. Congestive heart failure from suspected ductal closure in utero. J Pediatr. 1969;75(1):74–78. doi: 10.1016/s0022-3476(69)80103-4. [DOI] [PubMed] [Google Scholar]
- 10.Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med. 1976;295(10):526–529. doi: 10.1056/NEJM197609022951003. [DOI] [PubMed] [Google Scholar]
- 11.Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med. 1976;295(10):530–533. doi: 10.1056/NEJM197609022951004. [DOI] [PubMed] [Google Scholar]
- 12.Moise KJ., Jr Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993;168(5):1350–1353. doi: 10.1016/s0002-9378(11)90763-7. [DOI] [PubMed] [Google Scholar]
- 13.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329(22):1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 14.Hammerman C, Glaser J, Kaplan M, et al. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics. 1998;102(5):E56. doi: 10.1542/peds.102.5.e56. [DOI] [PubMed] [Google Scholar]
- 15.Clyman RI, Chen YQ, Chemtob S, et al. In utero remodeling of the fetal lamb ductus arteriosus: the role of antenatal indomethacin and avascular zone thickness on vasa vasorum proliferation, neointima formation, and cell death. Circulation. 2001;103(13):1806–1812. doi: 10.1161/01.cir.103.13.1806. [DOI] [PubMed] [Google Scholar]
- 16.Reese J, Waleh N, Poole SD, et al. Chronic in utero cyclooxygenase inhibition alters PGE2-regulated ductus arteriosus contractile pathways and prevents postnatal closure. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181aa07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sodini D, Baragatti B, Barogi S, Laubach VE, Coceani F. Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br J Pharmacol. 2008;153(8):1631–1640. doi: 10.1038/bjp.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Momma K, Toyono M. The role of nitric oxide in dilating the fetal ductus arteriosus in rats. Pediatr Res. 1999;46(3):311–315. doi: 10.1203/00006450-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Richard C, Gao J, LaFleur B, et al. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R652–660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 20.Keller RL, Tacy TA, Fields S, et al. Combined treatment with a nonselective nitric oxide synthase inhibitor (l-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatr Res. 2005;58(6):1216–1221. doi: 10.1203/01.pdr.0000183659.20335.12. [DOI] [PubMed] [Google Scholar]
- 21.Reese J, O'Mara PW, Poole SD, et al. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 2009;88(3-4):89–96. doi: 10.1016/j.prostaglandins.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinsella JP, Walsh WF, Bose CL, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354(9184):1061–1065. doi: 10.1016/s0140-6736(99)03558-8. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber MD, Gin-Mestan K, Marks JD, et al. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349(22):2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 24.Mentzer RM, Jr., Ely SW, Lasley RD, et al. Hormonal role of adenosine in maintaining patency of the ductus arteriosus in fetal lambs. Annals of surgery. 1985;202(2):223–230. doi: 10.1097/00000658-198508000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GC, McGrath JC. Characterisation of the effect of oxygen tension on response of fetal rabbit ductus arteriosus to vasodilators. Cardiovasc Res. 1993;27(12):2205–2211. doi: 10.1093/cvr/27.12.2205. [DOI] [PubMed] [Google Scholar]
- 26.Toyoshima K, Momma K, Imamura S, Nakanishi T. In vivo dilatation of the postnatal ductus arteriosus by atrial natriuretic peptide in the rat. Neonatology. 2007;92(2):139–144. doi: 10.1159/000101526. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Manganiello V, Waleh N, Clyman RI. Expression, activity, and function of phosphodiesterases in the mature and immature ductus arteriosus. Pediatr Res. 2008;64(5):477–481. doi: 10.1203/PDR.0b013e3181827c2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thebaud B, Michelakis E, Wu XC, et al. Sildenafil reverses O2 constriction of the rabbit ductus arteriosus by inhibiting type 5 phosphodiesterase and activating BK(Ca) channels. Pediatr Res. 2002;52(1):19–24. doi: 10.1203/00006450-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Arishima K, Takizawa T, Oda T, et al. Propranolol inhibits the spontaneous closure of the ductus arteriosus in newborn rats. J Vet Med Sci. 1995;57(5):943–944. doi: 10.1292/jvms.57.943. [DOI] [PubMed] [Google Scholar]
- 30.Friedman WF, Printz MP, Kirkpatrick SE, Hoskins EJ. The vasoactivity of the fetal lamb ductus arteriosus studied in utero. Pediatr Res. 1983;17(5):331–337. doi: 10.1203/00006450-198305000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Clyman RI, Roman C. The effects of caffeine on the preterm sheep ductus arteriosus. Pediatr Res. 2007;62(2):167–169. doi: 10.1203/PDR.0b013e3180a725b1. [DOI] [PubMed] [Google Scholar]
- 32.del moral T, Gonzalez-Quintero VH, Claure N, Vanbuskirk S, Bancalari E. Antenatal exposure to magnesium sulfate and the incidence of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2007;27(3):154–157. doi: 10.1038/sj.jp.7211663. [DOI] [PubMed] [Google Scholar]
- 33.Stigson L, Kjellmer I. Serum levels of magnesium at birth related to complications of immaturity. Acta Paediatr. 1997;86(9):991–994. doi: 10.1111/j.1651-2227.1997.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 34.McGuirl J, Arzuaga B, Lee B. Increased risk for patent ductus arteriosus with antenatal calcium channel blocker exposure in extremely low birth weight infants. Annual Meeting of the Pediatric Academic Societies. 2009;E-PAS2009:461. 2009. abstr. [Google Scholar]
- 35.Watterberg KL, Kelly HW, Johnson JD, Aldrich M, Angelus P. Effect of patent ductus arteriosus on gentamicin pharmacokinetics in very low birth weight (less than 1,500 g) babies. Developmental pharmacology and therapeutics. 1987;10(2):107–117. doi: 10.1159/000457735. [DOI] [PubMed] [Google Scholar]
- 36.Adams HR, Goodman FR, Weiss GB. Alteration of contractile function and calcium ion movements in vascular smooth muscle by gentamicin and other aminoglycoside antibiotics. Antimicrobial agents and chemotherapy. 1974;5(6):640–646. doi: 10.1128/aac.5.6.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belus A, White E. Effects of antibiotics on the contractility and Ca2+ transients of rat cardiac myocytes. Eur J Pharmacol. 2001;412(2):121–126. doi: 10.1016/s0014-2999(01)00717-8. [DOI] [PubMed] [Google Scholar]
- 38.Descotes J, Evreux JC. Cardiac depressant effects of some recent aminoglycoside antibiotics. The Journal of antimicrobial chemotherapy. 1981;7(2):197–200. doi: 10.1093/jac/7.2.197. [DOI] [PubMed] [Google Scholar]
- 39.Gotanda K, Yanagisawa T, Satoh K, Taira N. Are the cardiovascular effects of gentamicin similar to those of calcium antagonists? Japanese journal of pharmacology. 1988;47(3):217–227. doi: 10.1254/jjp.47.217. [DOI] [PubMed] [Google Scholar]
- 40.Coceani F, Liu Y, Seidlitz E, et al. Endothelin A receptor is necessary for O(2) constriction but not closure of ductus arteriosus. Am J Physiol. 1999;277(4 Pt 2):H1521–1531. doi: 10.1152/ajpheart.1999.277.4.H1521. [DOI] [PubMed] [Google Scholar]
- 41.Gergawy M, Vollrath B, Cook D. The mechanism by which aminoglycoside antibiotics cause vasodilation of canine cerebral arteries. Br J Pharmacol. 1998;125(6):1150–1157. doi: 10.1038/sj.bjp.0702180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keith RA, Mangano TJ, DeFeo PA, Horn MB, Salama AI. Actions of neomycin on neuronal L-, N-, and non-L/non-N-type voltage-sensitive calcium channel responses. J Mol Neurosci. 1992;3(3):147–154. doi: 10.1007/BF02919406. [DOI] [PubMed] [Google Scholar]
- 43.Martinez C, Albet C, Agundez JA, et al. Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clinical pharmacology and therapeutics. 1999;65(4):369–376. doi: 10.1016/S0009-9236(99)70129-3. [DOI] [PubMed] [Google Scholar]
- 44.Rendic S, Kajfez F, Ruf HH. Characterization of cimetidine, ranitidine, and related structures' interaction with cytochrome P-450. Drug metabolism and disposition: the biological fate of chemicals. 1983;11(2):137–142. [PubMed] [Google Scholar]
- 45.Levine M, Bellward GD. Effect of cimetidine on hepatic cytochrome P450: evidence for formation of a metabolite-intermediate complex. Drug metabolism and disposition: the biological fate of chemicals. 1995;23(12):1407–1411. [PubMed] [Google Scholar]
- 46.Hazinski TA, France M, Kennedy KA, Hansen TN. Cimetidine reduces hyperoxic lung injury in lambs. J Appl Physiol. 1989;67(6):2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- 47.Cotton RB, Hazinski TA, Morrow JD, et al. Cimetidine does not prevent lung injury in newborn premature infants. Pediatr Res. 2006;59(6):795–800. doi: 10.1203/01.pdr.0000219397.35473.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cotton RB. J. R. Symptomatic patent ductus arteriosus is significantly associated with cimetidine treatment in premature newborn infants at risk for chronic lung disease. Pediatr Res. 1999 E-PAS2009:5511.178 (abstr) [Google Scholar]
- 49.Coceani F. Cytochrome P450 in the contractile tone of the ductus arteriosus: regulatory and effector mechanisms. In: Weir EK, Archer SL, Reeves JT, editors. The Fetal and Neonatal Pulmonary Circulations. Futura Publishing Company, Inc.; Armonk, N.Y.: 1999. pp. 331–341. [Google Scholar]
- 50.Barrington KJ. Umbilical artery catheters in the newborn: effects of heparin. Cochrane database of systematic reviews (Online) 2000;(2):CD000507. doi: 10.1002/14651858.CD000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah PS, Shah VS. Continuous heparin infusion to prevent thrombosis and catheter occlusion in neonates with peripherally placed percutaneous central venous catheters. Cochrane database of systematic reviews (Online) 2008;(2):CD002772. doi: 10.1002/14651858.CD002772.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert NC, Nalefski LA. The effect of heparin and dicumarol in increasing the coronary flow volume. The Journal of laboratory and clinical medicine. 1949;34(6):797–805. [PubMed] [Google Scholar]
- 53.Urban P, Scheidegger D, Buchmann B, Skarvan K. The hemodynamic effects of heparin and their relation to ionized calcium levels. The Journal of thoracic and cardiovascular surgery. 1986;91(2):303–306. [PubMed] [Google Scholar]
- 54.Tangphao O, Chalon S, Moreno HJ, Jr., et al. Heparin-induced vasodilation in human hand veins. Clinical pharmacology and therapeutics. 1999;66(3):232–238. doi: 10.1016/S0009-9236(99)70030-5. [DOI] [PubMed] [Google Scholar]
- 55.Susic D, Mandal AK, Jovovic D, et al. Antihypertensive action of heparin: role of the renin-angiotensin aldosterone system and prostaglandins. Journal of clinical pharmacology. 1993;33(4):342–347. doi: 10.1002/j.1552-4604.1993.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 56.Tasatargil A, Golbasi I, Sadan G, Karasu E. Unfractioned heparin produces vasodilatory action on human internal mammary artery by endothelium-dependent mechanisms. J Cardiovasc Pharmacol. 2005;45(2):114–119. doi: 10.1097/01.fjc.0000151897.65260.8e. [DOI] [PubMed] [Google Scholar]
- 57.VanTeeffelen JW, Brands J, Jansen C, Spaan JA, Vink H. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension. 2007;50(1):261–267. doi: 10.1161/HYPERTENSIONAHA.107.089250. [DOI] [PubMed] [Google Scholar]
- 58.Ojala TH, Lehtonen L. A preliminary report--heparin counteracts indomethacin effect on ductus arteriosus in very low birthweight infants. Pediatr Crit Care Med. 2007;8(3):258–260. doi: 10.1097/01.pcc.0000270203.07648.8e. [DOI] [PubMed] [Google Scholar]
- 59.Cotton RB, Lindstrom DP, Stahlman MT. Early prediction of symptomatic patent ductus arteriosus from perinatal risk factors: a discriminant analysis model. Acta paediatrica Scandinavica. 1981;70(5):723–727. doi: 10.1111/j.1651-2227.1981.tb05775.x. [DOI] [PubMed] [Google Scholar]
- 60.Furzan JA, Reisch J, Tyson JE, Laird P, Rosenfeld CR. Incidence and risk factors for symptomatic patent ductus arteriosus among inborn very-low-birth-weight infants. Early Hum Dev. 1985;12(1):39–48. doi: 10.1016/0378-3782(85)90135-5. [DOI] [PubMed] [Google Scholar]
- 61.Chorne N, Jegatheesan P, Lin E, Shi R, Clyman RI. Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr. 2007;151(6):629–634. doi: 10.1016/j.jpeds.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Itabashi K, Ohno T, Nishida H. Indomethacin responsiveness of patent ductus arteriosus and renal abnormalities in preterm infants treated with indomethacin. J Pediatr. 2003;143(2):203–207. doi: 10.1067/S0022-3476(03)00303-2. [DOI] [PubMed] [Google Scholar]
- 63.Siassi B, Blanco C, Cabal LA, Coran AG. Incidence and clinical features of patent ductus arteriosus in low-birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics. 1976;57(3):347–351. [PubMed] [Google Scholar]
- 64.van de Bor M, Verloove-Vanhorick SP, Brand R, Ruys JH. Patent ductus arteriosus in a cohort of 1338 preterm infants: a collaborative study. Paediatric and perinatal epidemiology. 1988;2(4):328–336. doi: 10.1111/j.1365-3016.1988.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 65.Robel-Tillig E, Knupfer M, Vogtmann C. Cardiac adaptation in small for gestational age neonates after prenatal hemodynamic disturbances. Early Hum Dev. 2003;72(2):123–129. doi: 10.1016/s0378-3782(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham MD, Ellison RC, Zierler S, et al. Perinatal risk assessment for patent ductus arteriosus in premature infants. Obstet Gynecol. 1986;68(1):41–45. [PubMed] [Google Scholar]
- 67.Kreft-Jais C, Plouin PF, Tchobroutsky C, Boutroy MJ. Angiotensin-converting enzyme inhibitors during pregnancy: a survey of 22 patients given captopril and nine given enalapril. British journal of obstetrics and gynaecology. 1988;95(4):420–422. doi: 10.1111/j.1471-0528.1988.tb06619.x. [DOI] [PubMed] [Google Scholar]
- 68.Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. The American journal of medicine. 1994;96(5):451–456. doi: 10.1016/0002-9343(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 69.Thomas SV, Ajaykumar B, Sindhu K, et al. Cardiac malformations are increased in infants of mothers with epilepsy. Pediatr Cardiol. 2008;29(3):604–608. doi: 10.1007/s00246-007-9161-4. [DOI] [PubMed] [Google Scholar]
- 70.Dusick AM, Covert RF, Schreiber MD, et al. Risk of intracranial hemorrhage and other adverse outcomes after cocaine exposure in a cohort of 323 very low birth weight infants. J Pediatr. 1993;122(3):438–445. doi: 10.1016/s0022-3476(05)83438-9. [DOI] [PubMed] [Google Scholar]
- 71.Levy HL, Guldberg P, Guttler F, et al. Congenital heart disease in maternal phenylketonuria: report from the Maternal PKU Collaborative Study. Pediatr Res. 2001;49(5):636–642. doi: 10.1203/00006450-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Rouse B, Matalon R, Koch R, et al. Maternal phenylketonuria syndrome: congenital heart defects, microcephaly, and developmental outcomes. J Pediatr. 2000;136(1):57–61. doi: 10.1016/s0022-3476(00)90050-7. [DOI] [PubMed] [Google Scholar]
- 73.Treszl A, Szabo M, Dunai G, et al. Angiotensin II type 1 receptor A1166C polymorphism and prophylactic indomethacin treatment induced ductus arteriosus closure in very low birth weight neonates. Pediatr Res. 2003;54(5):753–755. doi: 10.1203/01.PDR.0000088016.67117.39. [DOI] [PubMed] [Google Scholar]
- 74.Keller RL, Clyman RI. Persistent Doppler flow predicts lack of response to multiple courses of indomethacin in premature infants with recurrent patent ductus arteriosus. Pediatrics. 2003;112(3 Pt 1):583–587. doi: 10.1542/peds.112.3.583. [DOI] [PubMed] [Google Scholar]
- 75.Cambonie G, Guillaumont S, Luc F, et al. Haemodynamic features during high-frequency oscillatory ventilation in preterms. Acta Paediatr. 2003;92(9):1068–1073. doi: 10.1080/08035250310004856. [DOI] [PubMed] [Google Scholar]
- 76.Van Overmeire B, Smets K, Lecoutere D, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343(10):674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 77.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107(3):E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 78.Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane database of systematic reviews (Online) 2008;(1):CD000503. doi: 10.1002/14651858.CD000503.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Hammoud MS, Elsori HA, Hanafi EA, et al. Incidence and risk factors associated with the patency of ductus arteriosus in preterm infants with respiratory distress syndrome in Kuwait. Saudi medical journal. 2003;24(9):982–985. [PubMed] [Google Scholar]
- 80.Rothman KJ, Fyler DC. Sex, birth order, and maternal age characteristics of infants with congenital heart defects. American journal of epidemiology. 1976;104(5):527–534. doi: 10.1093/oxfordjournals.aje.a112326. [DOI] [PubMed] [Google Scholar]
- 81.Samanek M, Voriskova M. Congenital heart disease among 815,569 children born between 1980 and 1990 and their 15-year survival: a prospective Bohemia survival study. Pediatr Cardiol. 1999;20(6):411–417. doi: 10.1007/s002469900502. [DOI] [PubMed] [Google Scholar]
- 82.Forsey JT, Elmasry OA, Martin RP. Patent arterial duct. Orphanet journal of rare diseases. 2009;4:17. doi: 10.1186/1750-1172-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhandari V, Zhou G, Bizzarro MJ, et al. Genetic contribution to patent ductus arteriosus in the premature newborn. Pediatrics. 2009;123(2):669–673. doi: 10.1542/peds.2008-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dagle JM, Lepp NT, Cooper ME, et al. Determination of genetic predisposition to patent ductus arteriosus in preterm infants. Pediatrics. 2009;123(4):1116–1123. doi: 10.1542/peds.2008-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seppanen MP, Ojanpera OS, Kaapa PO, Kero PO. Delayed postnatal adaptation of pulmonary hemodynamics in infants of diabetic mothers. J Pediatr. 1997;131(4):545–548. doi: 10.1016/s0022-3476(97)70059-3. [DOI] [PubMed] [Google Scholar]
- 86.Alzamora-Castro V, Battilana G, Abugattas R, Sialer S. Patent ductus arteriosus and high altitude. The American journal of cardiology. 1960;5:761–763. doi: 10.1016/0002-9149(60)90052-7. [DOI] [PubMed] [Google Scholar]
- 87.Miao CY, Zuberbuhler JS, Zuberbuhler JR. Prevalence of congenital cardiac anomalies at high altitude. J Am Coll Cardiol. 1988;12(1):224–228. doi: 10.1016/0735-1097(88)90378-6. [DOI] [PubMed] [Google Scholar]
- 88.Doyle PE, Beral V, Botting B, Wale CJ. Congenital malformations in twins in England and Wales. Journal of epidemiology and community health. 1991;45(1):43–48. doi: 10.1136/jech.45.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Layde PM, Erickson JD, Falek A, McCarthy BJ. Congenital malformation in twins. Am J Hum Genet. 1980;32(1):69–78. [PMC free article] [PubMed] [Google Scholar]
- 90.Gibson S, Lewis KC. Congenital heart disease following maternal rubella during pregnancy. AMA. 1952;83(3):317–319. doi: 10.1001/archpedi.1952.02040070063007. [DOI] [PubMed] [Google Scholar]
- 91.Nakagawa T. Delayed closure of ductus arteriosus in premature infants with transient hypothyroidism. Lancet. 1993;341(8848):839. doi: 10.1016/0140-6736(93)90623-o. [DOI] [PubMed] [Google Scholar]
- 92.Williams FL, Ogston SA, van Toor H, Visser TJ, Hume R. Serum thyroid hormones in preterm infants: associations with postnatal illnesses and drug usage. The Journal of clinical endocrinology and metabolism. 2005;90(11):5954–5963. doi: 10.1210/jc.2005-1049. [DOI] [PubMed] [Google Scholar]
- 93.Barefield ES, Dwyer MD, Cassady G. Association of patent ductus arteriosus and phototherapy in infants weighing less than 1000 grams. J Perinatol. 1993;13(5):376–380. [PubMed] [Google Scholar]