Abstract
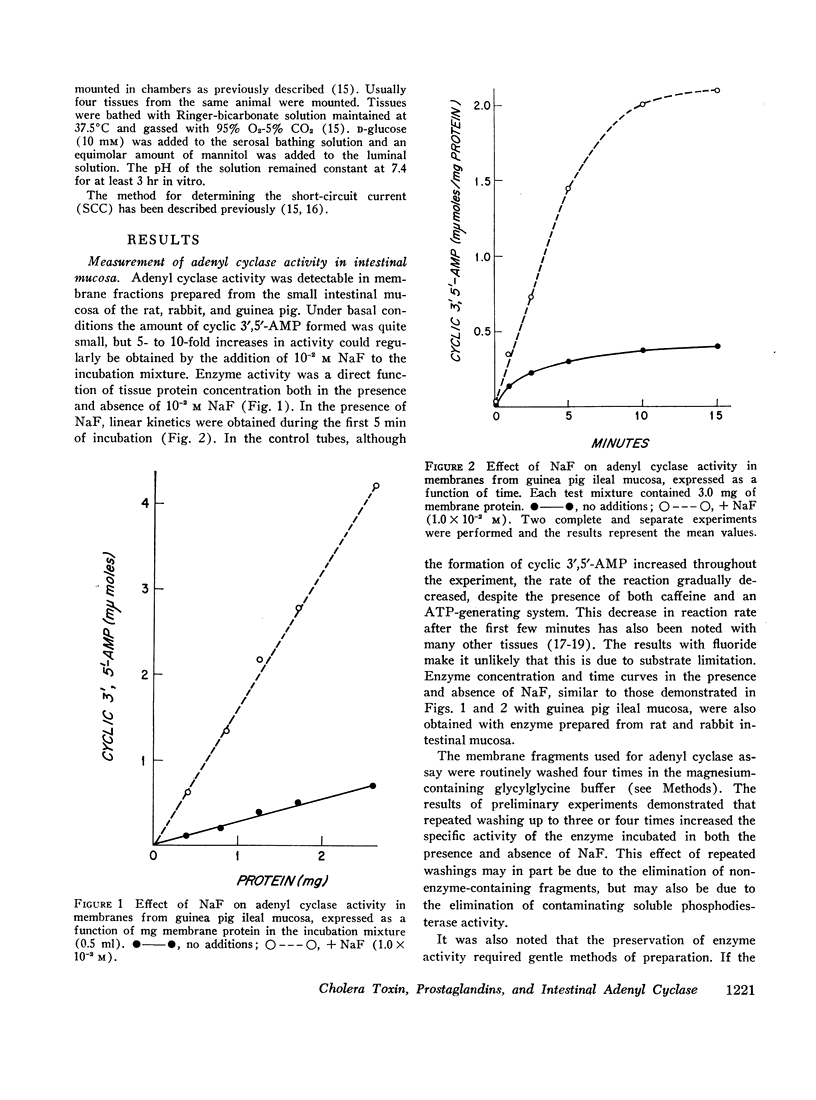
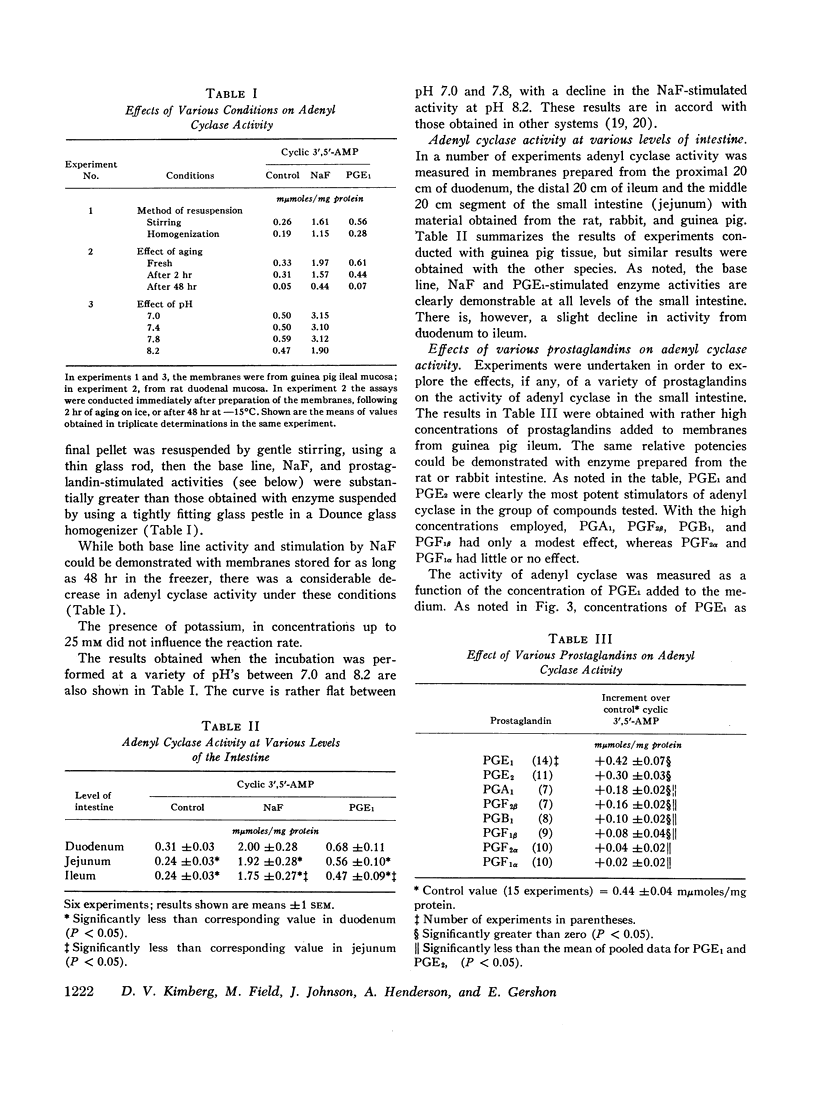
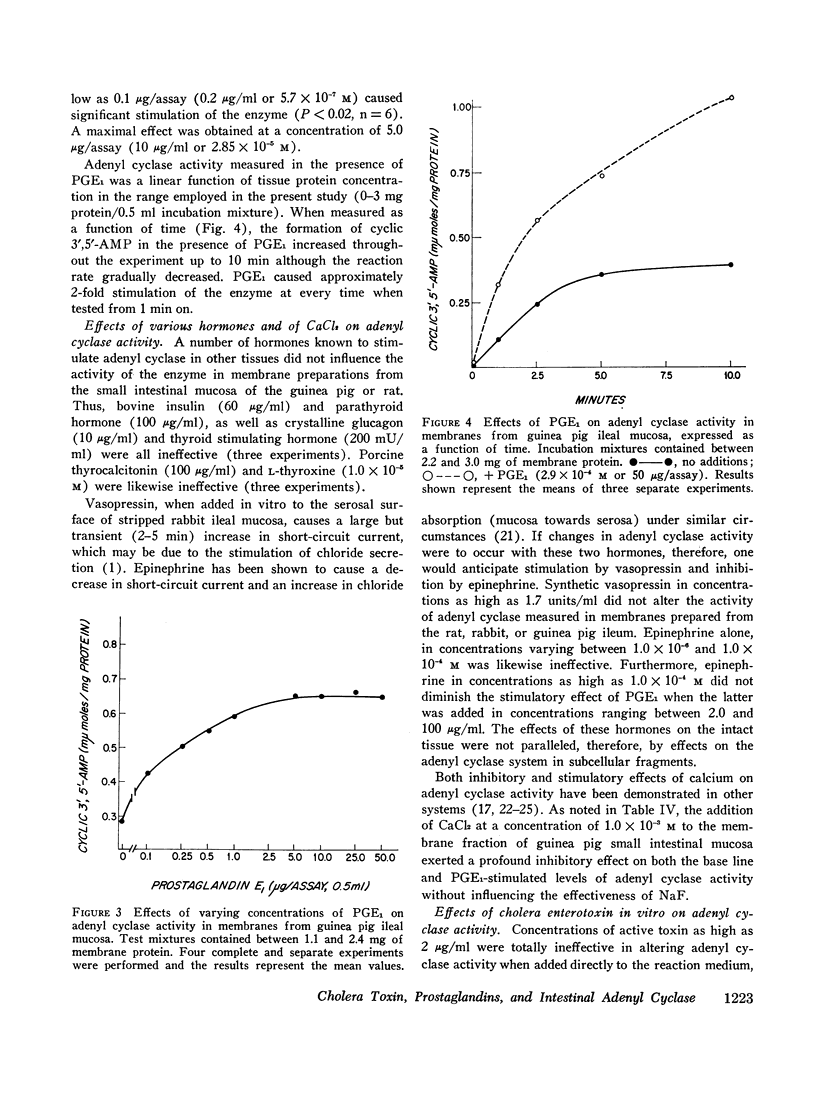
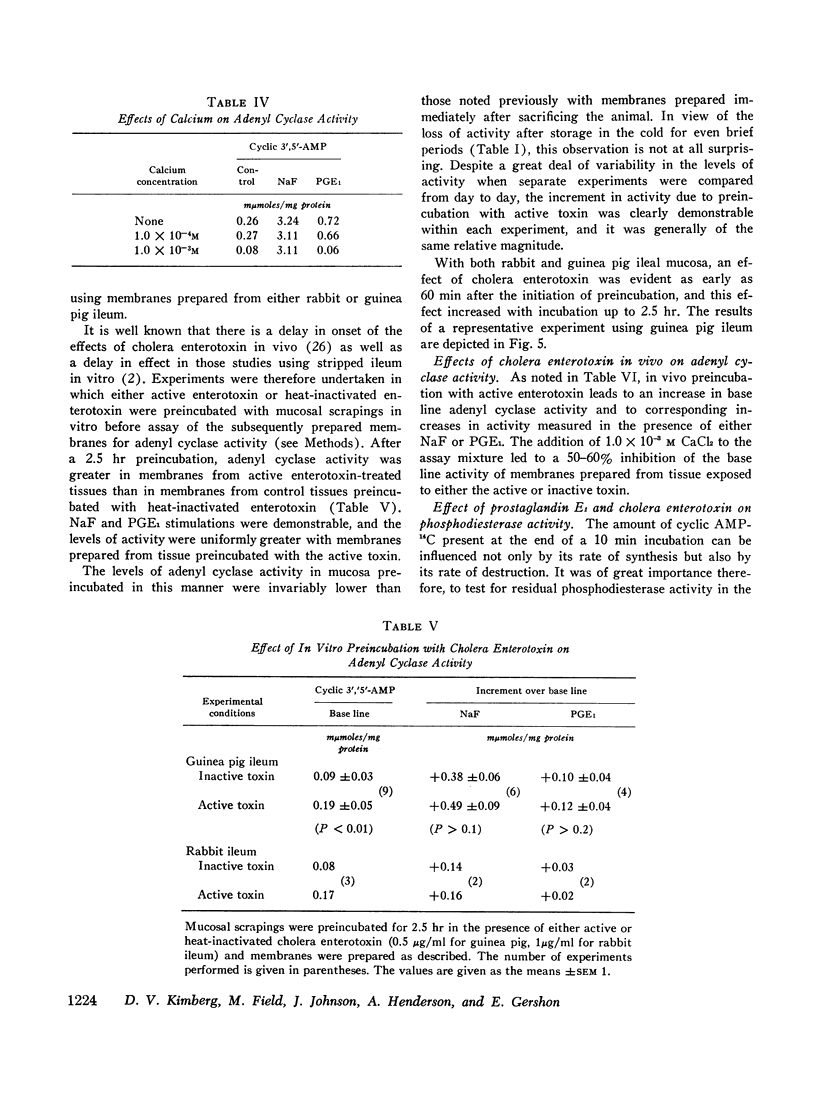
The effects of several prostaglandins (PG) and a highly purified preparation of cholera enterotoxin (CT) on intestinal mucosal adenyl cyclase activity and the effect of CT on intestinal mucosal cyclic 3′,5′-adenosine monophosphate concentration were determined in guinea pig and rabbit small intestine and were correlated with the effects of the same agents on ion transport. Adenyl cyclase activity, measured in a crude membrane fraction of the mucosa, was found at all levels of the small intestine with the highest activity per milligram protein in the duodenum. The prostaglandins, when added directly to the assay, increased adenyl cyclase activity; the greatest effect (2-fold increase) was obtained with PGE1 (maximal effect at 0.03 mM) and PGE2. The prostaglandins also increased short-circuit current (SCC) in isolated guinea pig ileal mucosa, with PGE1 and PGE2 again giving the greatest effects. The prior addition of theophylline (10 mM) reduced the subsequent SCC response to PGE1 and vice versa. It was concluded, therefore, that the SCC response to PGE1, like the response to theophylline, represented active Cl secretion. CT increased adenyl cyclase activity in guinea pig and rabbit ileal mucosa when preincubated with the mucosa from 1 to 2.5 hr in vitro or for 2.5 hr in vivo but not when added directly to the assay. The increments in activity caused by PGE1 and NaF were the same in CT-treated and control mucosa. Cyclic 3′,5′-AMP concentration in rabbit ileal mucosa was increased 3.5-fold after a 2 hr preincubation with CT in vitro. Phosphodiesterase activity in the crude membrane fraction of the mucosa was unaffected by either CT or PGE1. A variety of other agents including insulin, glucagon, parathormone, thyroid-stimulating hormone, L-thyroxine, thyrocalcitonin, vasopressin, and epinephrine all failed to change adenyl cyclase activity. It is concluded that CT and certain prostaglandins produce small intestinal fluid secretion by increasing mucosal adenyl cyclase activity, thereby stimulating an active secretory process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E. Effects of prostaglandins on adenosine 3',5'-monophosphate levels in fat and other tissues. J Biol Chem. 1968 Apr 25;243(8):1713–1717. [PubMed] [Google Scholar]
- Bär H. P., Hechter O. Adenyl cyclase and hormone action. 3. Calcium requirement for ACTH stimulation of adenyl cyclase. Biochem Biophys Res Commun. 1969 Jun 6;35(5):681–686. doi: 10.1016/0006-291x(69)90459-8. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase L. R., Fedak S. A., Aurbach G. D. Activation of skeletal adenyl cyclase by parathyroid hormone in vitro. Endocrinology. 1969 Apr;84(4):761–768. doi: 10.1210/endo-84-4-761. [DOI] [PubMed] [Google Scholar]
- Elliott H. L., Carpenter C. C., Sack R. B., Yardley J. H. Small bowel morphology in experimental canine cholera. A light and electron microscopic study. Lab Invest. 1970 Feb;22(2):112–120. [PubMed] [Google Scholar]
- Field M., Plotkin G. R., Silen W. Effects of vasopressin, theophylline and cyclic adenosine monophosphate on short-circuit current across isolated rabbit ileal mucosa. Nature. 1968 Feb 3;217(5127):469–471. doi: 10.1038/217469a0. [DOI] [PubMed] [Google Scholar]
- Grayer D. T., Serebro H. A., Iber F. L., Hendrix T. R. Effect of cycloheximide on unidirectional sodium fluxes in the jejunum after cholera exotoxin exposure. Gastroenterology. 1970 Jun;58(6):815–819. [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa S., Davis J. W., Sutherland E. W. Determination of guanosine 3',5'-monophosphate in tissues and of guanyl cyclase in rat intestine. J Biol Chem. 1969 Dec 10;244(23):6371–6376. [PubMed] [Google Scholar]
- KOWLESSAR O. D., LAW D. H., SLEISENGER M. H. Malabsorption syndrome associated with metastatic carcinoid tumor. Am J Med. 1959 Oct;27:673–677. doi: 10.1016/0002-9343(59)90051-8. [DOI] [PubMed] [Google Scholar]
- Kraft A. R., Tompkins R. K., Zollinger R. M. Recognition and management of the diarrheal syndrome caused by nonbeta islet cell tumors of the pancreas. Am J Surg. 1970 Feb;119(2):163–170. doi: 10.1016/0002-9610(70)90028-0. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marinetti G. V., Ray T. K., Tomasi V. Glucagon and epinephrine stimulation of adenyl cyclase in isolated rat liver plasma membranes. Biochem Biophys Res Commun. 1969 Jul 23;36(2):185–193. doi: 10.1016/0006-291x(69)90313-1. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Engstrom G. W., Baetz A. L. Response of the rabbit ileal loop to cell-free products from Escherichia coli enteropathogenic for swine. J Infect Dis. 1970 Feb;121(2):182–187. doi: 10.1093/infdis/121.2.182. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958 Jun;232(2):1077–1091. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Sandler M., Karim S. M., Williams E. D. Prostaglandins in amine-peptide-secreting tumours. Lancet. 1968 Nov 16;2(7577):1053–1054. doi: 10.1016/s0140-6736(68)91528-6. [DOI] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebro H. A., Iber F. L., Yardley J. H., Hendrix T. R. Inhibition of cholera toxin action in the rabbit by cycloheximide. Gastroenterology. 1969 Mar;56(3):506–511. [PubMed] [Google Scholar]
- Streeto J. M., Reddy W. J. An assay for adenyl cyclase. Anal Biochem. 1967 Dec;21(3):416–426. doi: 10.1016/0003-2697(67)90316-8. [DOI] [PubMed] [Google Scholar]
- Streeto J. M. Renal cortical adenyl cyclase: effect of parathyroid hormone and calcium. Metabolism. 1969 Nov;18(11):968–973. doi: 10.1016/0026-0495(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]
- Williams E. D., Karim S. M., Sandler M. Prostaglandin secretion by medullary carcinoma of the thyroid. A possible cause of the associated idarrhoea. Lancet. 1968 Jan 6;1(7532):22–23. doi: 10.1016/s0140-6736(68)90010-x. [DOI] [PubMed] [Google Scholar]
- Williams R. H., Walsh S. A., Ensinck J. W. Effect of metals upon the conversion of adenosine triphosphate to adenosine 3',5'monophosphate in lipocytes. Proc Soc Exp Biol Med. 1968 May;128(1):279–283. doi: 10.3181/00379727-128-32997. [DOI] [PubMed] [Google Scholar]
- Zollinger R. M., Moore F. T. Zollinger-Ellison syndrome comes of age. Recognition of the complete clinical spectrum and its management. JAMA. 1968 Apr 29;204(5):361–365. [PubMed] [Google Scholar]
- Zor U., Kaneko T., Lowe I. P., Bloom G., Field J. B. Effect of thyroid-stimulating hormone and prostaglandins on thyroid adenyl cyclase activation and cyclic adenosine 3',5',-monophosphate. J Biol Chem. 1969 Oct 10;244(19):5189–5195. [PubMed] [Google Scholar]


