Synopsis
Osteoarthritis (OA) is the most common joint disorder in the United States. Symptomatic knee OA occurs in 10% men and 13% in women aged 60 years or older. The number of people affected with symptomatic OA is likely to increase due to the aging of the population and the obesity epidemic. OA has a multi-factorial etiology and can be considered the product of an interplay between systemic and local factors. Old age, female gender, overweight and obesity, knee injury, repetitive use of joints, bone density, muscle weakness, and joint laxity all play roles in the development of joint osteoarthritis, particularly in the weight-bearing joints. Modifying these factors may reduce the risk of osteoarthritis and prevent subsequent pain and disability.
Osteoarthritis (OA) is the most common joint disorder in the United States (1). Among adults 60 years of age or older the prevalence of symptomatic knee OA is approximately 10% in men and 13% in women (2). The number of people affected with symptomatic OA is likely to increase due to the aging of the population and the obesity epidemic.
Pain from OA is a key symptom in the decision to seek medical care and is an important antecedent to disability (3). Because of its high prevalence and the frequent disability that accompanies disease in major joints such as the knee and hip, OA accounts for more difficulty with climbing stairs and walking than any other disease (4). OA is also the most common reason for total hip and total knee replacement (5). The rapid increase in the prevalence of this already common disease suggests that OA will have a growing impact on health care and public health systems in the future (6).
Defining OA
Epidemiologic principles can be used to describe the distribution of OA in the population and to examine risk factors for its occurrence and progression. For the purpose of epidemiologic investigation, OA can be defined pathologically, radiographically, or clinically. Radiographic OA has long been considered the reference standard, and multiple ways to define radiographic disease have been devised. The most common method for radiographic definition is the Kellgren-Lawrence (K/L) radiographic grading scheme and atlas which has been in use for over four decades. This overall joint scoring system grades OA in five levels from 0 to 4, defining OA by the presence of a definite osteophyte (Grade≥2), and more severe grades by the presumed successive appearance of joint space narrowing, sclerosis, cysts, and deformity (7). Other radiographic metrics including semi-quantitative examination of individual radiographic features, such as osteophytes and joint space narrowing, or the direct measurement of the inter-bone distance as an indicator of the joint space width in the knees and hips are used to investigate progression in epidemiologic studies and clinical trials of disease modifying therapies (8, 9). More sensitive imaging methods using magnetic resonance imaging (MRI) can visualize multiple structures in a joint and are undergoing evaluation for their role in defining OA and for their usefulness in detecting the effects of potential disease-modifying interventions more quickly than possible with conventional radiographs (10, 11).
Studies of OA in people who have joint symptoms may be more clinically relevant, because not all persons who have radiographic OA have clinical disease, and not all persons who have joint symptoms demonstrate radiographic OA (12). Each set of clinical and radiographic criteria may yield slightly different groups of subjects defined as having OA (12).
Prevalence and Incidence of OA
The prevalence of OA varies according to the definition of OA, the specific joint(s) under study, and the characteristics of the study population. The age standardized prevalence of radiographic knee OA in adults age ≥ 45 was 19.2% among the participants in the Framingham Study and 27.8% in the Johnston County Osteoarthritis Project (6). In the third National Health and Nutrition Examination Survey (NHANES III), approximately 37% of participants age >60 years or older had radiographic knee OA (6).
Age-standardized prevalence of radiographic hand OA was 27.2% among the Framingham participants. Radiographic hip OA was less common than hand or knee OA. For example, about 7% of women age ≥65 years in the Study of Osteoporotic Fractures had radiographic hip OA. However, prevalence of hip OA was much higher in Johnston County, with 27% of subjects at least 45 years old demonstrating radiographic evidence of K/L grade 2 or higher OA (6). Potential explanations for the differences between these studies relate to differences in study populations, definitions of OA, distribution of risk factors for disease, and radiographic readers.
Symptomatic OA is generally defined by the presence of pain, aching, or stiffness in a joint with radiographic OA. The age-standardized prevalence of symptomatic hand and knee OA is 6.8% and 4.9%, respectively, in Framingham subjects age ≥26 years. However, prevalence of symptomatic knee OA was 16.7% among subjects age ≥45 in the Johnston County Osteoarthritis Project, much higher than that reported in the Framingham Study. About 9% of subjects in the Johnston County study had symptomatic hip OA (6). There is a paucity of meaningful data on the cumulative incidence of developing OA. Here, the length of time over which the risk of OA is calculated is critical but not always clearly specified or known. Further, because OA is a chronic disease occurring mostly among the elderly, competing risk or death from other diseases makes direct estimation of the cumulative incidence of OA difficult.
Oliveria and colleagues (13) reported the age- and sex-standardized incidence rates of symptomatic hip, knee, and hand OA to be 88, 240 and 100/100,000 person-years, respectively, in participants in a Massachusetts health maintenance organization, and the incidence rates of symptomatic OA of either hand, or knee, or hip increase rapidly around age 50 and then leveled off after age 70 (Figure 1). Murphy and others (14) estimated the lifetime risk of developing symptomatic knee OA to be about 40% in men and 47% in women. Such a risk rises to 60% in subjects with body mass index (BMI) of 30 or higher.
Figure 1.
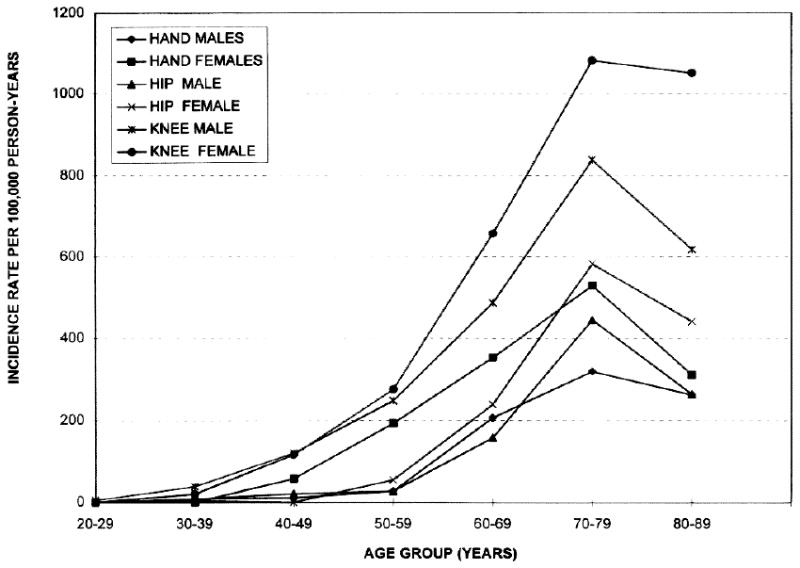
Incidence of OA of the hand, hip, and knee in members of the Fallon Community Health Plan, 1991-1992, by age and sex (From Oliveria SA, Felson DT, Reed JI, et al. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum 1995;38:1139; with permission.)
Risk Factors for OA
OA has a multifactorial etiology, and can be considered the product of an interplay between systemic and local factors as shown in Figure 2 (1). For example, a person may have an inherited predisposition to develop OA but may only develop it if an insult to the joint has occurred. The relative importance of risk factors may vary for different joints, for different stages of the disease, for the development as opposed to the progression of disease, and for radiographic versus symptomatic disease. There is even some evidence suggesting that risk factors may act differently according to individual radiographic features, such as osteophytes and joint space narrowing (1). Whether some of these differences are genuine or are spurious results of different study populations, definition of risk factors and OA, or statistical power, or analytic methods is open to debate.
Figure 2.
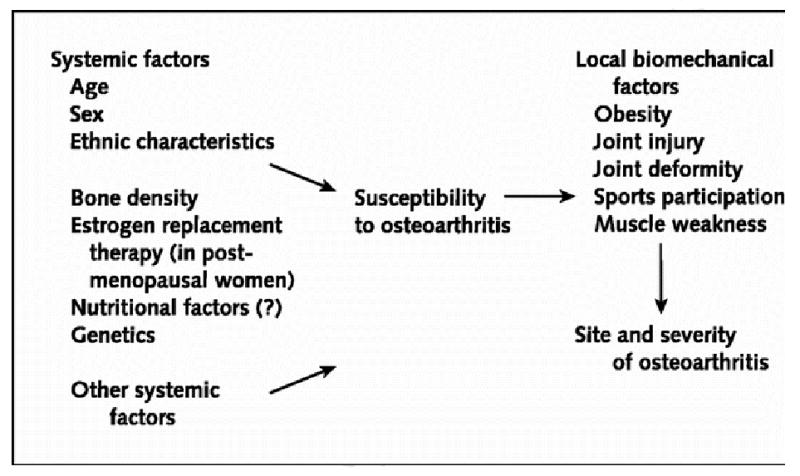
Pathogenesis of OA with Putative Risk Factors (Reprinted with permission from Felson DT, et al. Osteoarthritis: New Insights. Part 1: The disease and Its Risk Factors. Ann Intern Med. 2000; 133:635-646. with permission.)
Systemic Risk Factors for OA
Age
Age is a one of the strongest risk factors for OA of all joints (1, 6, 15). The increase in the prevalence and incidence of OA with age probably is a consequence of cumulative exposure to various risk factors and biologic changes that occur with aging that may make a joint less able to cope with adversity, such as cartilage thinning, weak muscle strength, poor proprioception, and oxidative damage.
Gender and hormones
Women not only are more likely to have OA than men, they also have more severe OA (16). The definite increase in OA in women around the time of menopause has led investigations hypothesize that hormonal factors may play a role in the development of OA. However, results on effect of estrogen, either endogenous or exogenous, on OA from observational studies have been conflicting (17-19). In a randomized clinical trial (the Heart and Estrogen/Progestin Replacement Study) in a group of older postmenopausal women with heart disease, no significant difference was found in the prevalence of knee pain or its associated disability between those taking estrogen plus progestin therapy or those taking placebo (20). Data from the Women's Health Initiative showed that, women on estrogen replacement therapy were 15% less likely to require total knee or hip arthroplasty than those not taking such therapy (hazard ratio 0.86; 95% Confidence Interval, 0.70-1.00), but that estrogen combined with progestin therapy was not associated with the risk of joint replacement (21).
Race/ethnicity
The prevalence of OA and patterns of joints affected by OA vary among racial and ethnic groups. Both hip and hand OA were much less frequent among Chinese in the Beijing Osteoarthritis Study than in whites in the Framingham Study (22, 23), but Chinese women in the Beijing Osteoarthritis Study had significantly higher prevalence of both radiographic and symptomatic knee OA than white women in Framingham Study (24). Results from the Johnston County Osteoarthritis Project have shown that the prevalence of hip OA in African American women (23%) was similar to that in white women (22%), and prevalence was slightly higher in African American men (21%) than that in white men (17%)(25). Interestingly, prevalence of individual radiographic features of hip OA varied between African Americans compared with Whites. For example, superior joint space narrowing and osteophytes at lateral compartment are more common in African Americans than in Whites (25). In addition, African Americans were also more likely to have more severe and tri-compartmental osteophytes than their White counterparts(26). Whether some of these racial/ethnic differences are related to differences in anatomic femoral and acetabular features, shown to be important in radiographic hip OA in whites (27, 28), is worthy of further study.
Genetics
Results from several studies have shown that OA is inherited and may vary by joint site. Twin and family studies have estimated the heritable component of OA to be between 50 and 65% with larger genetic influences for hand and hip OA than for knee OA (29-31). In a genome-wide association study, Kerkhof et al (32) reported that the C allele of rs3815148 on chromosome 7q22 was associated with a 1.14-fold increased prevalence of knee and/or hand OA and also with a 30% increased risk of knee OA progression. Several studies also found that an inverse association between general joint hypermobility, a lone benign trait, with hand and knee OA and serum cartilage oligometric matrix protein levels (33, 34).
Congenital/developmental conditions
A few congenital or developmental abnormalities (i.e., congenital subluxation, Legg-Calvé-Perthes disease, and slipped capital femoral epiphysis) have been associated with occurrence of hip OA in later life (35-37); however, because these developmental deformities are uncommon, they probably only account for a small proportion of the hip OA in the general population. Several studies have examined sub-clinical acetabular dysplasia, a more common, milder developmental abnormality, in relation to hip OA, with conflicting results (27, 38-41). Lane and colleagues (27) reported that abnormal center-edge angle or acetabular dysplasia were each associated with an approximately threefold increased risk of incident hip OA in women (27), suggesting that subclinical acetabular dysplasia may be a significant risk factor for the development of hip OA.
Diet
Dietary factors are the subject of considerable interest in OA, results of studies, however, are conflicting. One of the most promising nutritional factors for OA is vitamin D. Without sufficient vitamin D, bones can become thin, brittle, or misshapen. In the Framingham Study subjects in the lowest (<27 ng/ml) and middle (27.0 – 33.0 ng/ml) tertile of serum 25-hydroxyvitamin D had a 3-fold increased risk for progressive knee OA compared with those in the highest tertile; however, no such effect was observed for risk of incident disease (42). In the Study of Osteoporotic Fractures, women in the middle (23-29 ng/mL) and lowest (8-22 ng/mL) tertiles of serum 25-vitamin D were 3 times as likely to develop incident hip OA, defined by joint space narrowing, as those in the highest tertile (30-72 ng/mL). However, serum vitamin D levels were not associated with the risk of hip OA characterized by osteophytes or with new disease defined according to the summary grade (43). However, results from two cohort studies failed to confirm protective effect of vitamin D on the structural worsening of knee OA (44). A randomized, placebo-controlled clinical trial of vitamin D is currently underway to examine whether this vitamin can affect knee symptoms and cartilage loss measured on MRI in established knee OA.
Low vitamin C dietary intake was associated with an increased risk of progression, but not incidence, of both radiographic and symptomatic knee OA among the participants in the Framingham Study (45). In the Johnston County Osteoarthritis Project subjects with a high ratio of alpha:gamma tocopherol had 50% lower risk in development of radiographic knee OA (46). However, results from a controlled clinical trial of vitamin E failed to ameliorate symptoms in patients who had symptomatic knee OA or to prevent knee OA progression, as measured by cartilage volume by MRI (47).
Animal studies have shown that selenium deficiency is associated with irregular bone formation, decreased bone strength, and abnormalities in type I and II collage in cartilage (48, 49). In areas of China and eastern Asian where selenium levels in the soil is extreme low, the prevalence of Kashin-Beck Disease, an early onset of osteoarthropathy, was also high and food supplement of selenium decreased the incidence of this disease(50, 51). Preliminary results from the Johnston County Osteoarthritis Project have shown that sub-optimal selenium levels, measured in toenails, were associated with worse knee OA (52). However, others have reported that high selenium intake was significantly associated with increased risk of both hip and knee OA (53).
In one study, high levels of serum vitamin K were associated with a low prevalence of radiographic hand OA in one study, particularly for the presence of large osteophytes (54). The relation of serum vitamin K levels to knee radiographic OA is less clear. A recent randomized, placebo-controlled trial of vitamin K supplementation (phylloquinone, 500 μg/day) did not confirm a protective effect of vitamin K on the severity of radiographic hand OA.
Local Risk Factors
Obesity
Obesity and overweight have long been recognized as potent risk factors for OA, especially OA of the knee (1). The results from the Framingham Study demonstrated that women who had lost about 5 kg had a 50% reduction in the risk of development of symptomatic knee OA (55). The same study also found that weight loss was strongly associated with a reduced risk of development of radiographic knee OA. Weight-loss interventions have been shown to decrease pain and disability in established knee OA (56, 57). The Arthritis, Diet, and Activity Promotion Trial showed that weight loss combined with exercise, but neither weight loss nor exercise alone, were effective in decreasing pain and improving function in obese elders who had symptomatic knee OA (56). Results from a meta-analysis concluded that while the effects of weight loss on pain were less consistent weight reduction by about a 5% was associated with an improvement of physical function (57).
The relationship between overweight and hip OA is inconsistent and, if it exists, is weaker than that with knee OA (58, 59). There is, however, more consistent evidence that obesity increases the risk of bilateral radiographic as well as symptomatic hip OA (60). In the Nurses' Health Study, higher BMI, especially BMI at age 18, was strongly associated with an increased risk of total hip replacement therapy(61). Increased loading on the joint is probably the main, but not only, mechanism by which obesity causes knee or hip OA. Overloading the knee and hip joints could lead to synovial joint breakdown and failure of ligamentous and other structural support.
Injury/surgery
Numerous studies have shown that knee injury is one of the strongest risk factors for OA. Severe injury to the structures of a joint, particularly a trans-articular fracture, meniscal tear requiring meniscectomy, or anterior cruciate ligament injury, can result in an increased risk of OA development and musculoskeletal symptomatology (62, 63). In the Framingham Study the prevalence of meniscal damage was much higher among subjects with radiographic knee OA (82%) than those without OA (25%). The prevalence of meniscal damage increased with an increase in K/L grade (P<0.001 for trend); among those with moderate (K/L=3) and severe (K/L=4) radiographic knee OA 95% had evidence of meniscal damage (64).
Occupation
Repetitive use of joints at work is associated with an increased risk of OA. Studies have found that farmers have a high prevalence of hip OA (65). The prevalence of Heberden's nodes was much higher in the cotton mill workers, whereas spinal OA was no more common in these workers than in controls (66). Workers whose jobs required repeated pincer grip had more OA at distal interphalangeal joints than did workers whose job required power grip (67).
The risk of development of knee OA was more than two times greater for men whose jobs required both carrying and kneeling or squatting in mid-life had more than for those whose jobs did not require these physical activities (68). And the risks of knee OA associated with kneeling and squatting were much higher among subjects who were overweight or whose job also involved with lifting (69).
Physical activity/sports
Studies examining the relationship between sports activities and subsequent OA have produced conflicting results. There is some evidence that elite long distance runners are at high risk for the development of knee and hip OA (70-72); and elite soccer players are at higher risk of getting knee OA when compared with non-soccer players (71, 73). Surprisingly, the general level of physical activity itself may also increase the risk of OA. For instance, physical activity among elderly subjects in the Framingham Study was generally characterized by leisure time walking and gardening. However, person who engaged in relatively high levels of such activity had a threefold greater risk of developing radiographic knee OA than sedentary persons over 8 years of follow-up (74). Similar findings also were reported in another study in which women who had a high lifetime level of physical activity had a high prevalence of hip OA (75). In contrast, others have shown that, in the absence of acute injury, recreational (moderate) long distance running and jogging did not appear to increase the risk of OA (76, 77).
Mechanical factors
The relationship between muscle strength and OA is complex, may vary by joint site, and is not entirely understood. Muscle weakness and atrophy commonly associated with knee OA had been thought to be the product of disuse resulting from pain-avoidance. One study reported that women who had asymptomatic radiographic knee OA but had no muscle atrophy showed quadriceps muscle weakness (78), suggesting that this might be a risk factor for the development of symptomatic knee OA (78). Baker and colleagues (79) confirmed that persons who had both asymptomatic patellofemoral and tibiofemoral radiographic knee OA had weaker quadriceps strength than those who did not have OA (79). In a follow-up study quadriceps muscle weakness not only resulted from painful knee OA, but also increased the risk of knee structural damage (80, 81). Other studies (82), however, showed that greater quadriceps muscle strength in the setting of malalignment and laxity may actually be associated with an increased risk of knee OA progression.
Using the data from the Genetics of Generalized Osteoarthritis Study, Dominick and colleagues (83) found an inverse cross-sectional associations between grip strength and OA of the carpometacarpal joint and between pinch strength and OA of the metacarpophalangeal joint. On the other hand, greater grip strength was associated with an increased risk of radiographic hand OA among the participants of the Framingham Study: men whose maximal grip strength was in the highest tertile had a threefold increased risk of OA in the proximal interphalangeal, metacarpophalangeal, or thumb base joints, as compared with those with lowest tertile (84). The authors suggested that the maximal force exerted on specific joints might influence development of OA in those joints.
Alignment
Knee alignment (i.e., the hip-knee-ankle angle) is a key determinant of load distribution. Any shift from a neutral or collinear alignment of the hip, knee and ankle affects load distribution at the knee. Therefore, one would speculate that malaligned knees may have a higher risk of developing OA and a higher subsequent risk of progression than knees with neutral alignment.
In a prospective cohort study, Sharma et al (85) demonstrated that in the presence of existing knee OA, abnormal anatomic alignment was strongly associated with accelerated structural deterioration in the compartment under greatest compressive stress (85). Knees with varus alignment at baseline had a fourfold increase in the risk of medial progression of knee OA, and those with valgus alignment at baseline had a nearly fivefold increase in the risk of lateral progression. The same study also found that the impact of varus or valgus malalignment on the risk of OA progression was greater in knees with more severe baseline radiographic disease than knees with mild or moderate disease (86). Another study found that knee malalignment was associated with the size and progression of bone marrow lesions as well as with rapid cartilage loss on MRI (87).
The association between malalignment and risk of incident knee OA is less clear, however. The results from the Rotterdam study found that among knees with K/L grade 0 and 1, knees with valgus alignment had a 54% increased risk and knees with varus alignment had a twofold increased risk for the development of radiographic knee OA, compared with normal aligned knees (88). In the Framingham Study, however, Hunter and colleagues using four measures of knee joint alignment (i.e., the anatomic axis, the condylar angle, the tibial plateau angle, and the condylar tibial plateau angle) found none of these measures to be associated with an increased risk of incident radiographic knee OA. The authors speculated that malalignment may not be a primary risk factor for the occurrence of radiographic knee OA but rather a marker of disease severity and/or its progression (89).
Laxity
Knee laxity is another potential risk factor for knee OA. Varus-valgus knee laxity is greater in the non-arthritic knees of patients who have idiopathic disease than in the knees of controls, suggesting that a portion of the increased laxity of knee OA precedes disease development and may predispose to disease (90). And sagittal plane or anterior-posterior laxity may be increased in persons who have mild OA, and anterior-posterior laxity seems to decline with increasing severity of knee OA (91, 92). Because knee laxity may be altered by the disease itself, longitudinal data will be helpful to confirm these relationships.
Another factor that can alter biomechanics at the knee and hip is limb length inequality (LLI). In the Johnston County Osteoarthritis Project persons with a LLI of at least 2 cm were almost twice as likely to have radiographic knee OA and 40% more likely to have knee symptoms (93). The results from the Multicenter Osteoarthritis Study also demonstrated that leg length inequality ≥ 1 cm was not only associated with a higher prevalence of prevalent radiographic knee OA, but also increased the risk of incident symptomatic and progressive knee OA(94). These results point to LLI as a potentially modifiable risk factor for knee OA.
Risk Factors for Symptomatic OA
Although symptomatic knee OA is common, causes substantial disability and consumes tremendous medical costs, most previous studies have focused on risk factors for radiographic OA(1). Not all risk factors for radiographic OA are strong predictors of joint symptoms(1, 15). Women with radiographic knee OA were more likely to have symptoms than men (95), and African Americans generally reported more knee and hip symptoms than Whites (96). Strenuous physical activity, especially activities requiring kneeling, knee-bending, squatting, prolonged standing (74, 97) as well as knee injury and trauma (98) have also been linked to a high prevalence of symptomatic knee OA. People who have severe radiographic OA are more likely to report joint pain than those with milder radiographic abnormalities (9). Results from two observational studies (99) have demonstrated that there was a strong dose-response relation of the severity of radiographic knee OA to the prevalence of frequent knee pain, consistent frequent knee pain and pain severity. Furthermore, joint space narrowing was more strongly associated with each pain measure than were osteophytes.
A few studies have demonstrated that several morphological and pathological changes detected by MRI, i.e., bone marrow lesions (100, 101), synovitis (102, 103), effusion (102), or periarticluar lesions (104) in the knee were associated with knee pain, but others have failed to confirm the association between bone marrow lesions and knee pain (105).
Studying risk factors for symptomatic OA is challenging. While pain in OA has long been considered chronic, it is not necessarily constant. Clinically, physicians often notice that patients with OA experience episodes of recurrent pain or pain exacerbation over the course of the disease. The pain from OA often worsens by using the involved joints, and lessens or is relieved with rest. Such pain patterns are also observed in epidemiologic studies. Gooberman-Hill and colleagues (106) reported that joint pain among subjects with knee or hip OA is often intermittent and variable, and adaptation and avoidance strategies modify the experience of pain. Because of methodological and logistical difficulties, however, few studies have been conducted to examine the dynamic relationship between risk factors and pain fluctuation. One such study utilized weekly telephone interviews over 12 weeks to assess the impact of fluctuations in knee or hip pain. Fluctuations were frequent, observed in about 49% of individuals, and decreases in pain were associated with improved function, decreased work absenteeism, sleep disruption, and health care resource use (107).
Conclusions
Evolving definitions of OA and improvement in risk factor measurement, by utilizing advanced imaging, systemic and local biomarkers, and improved methods for measuring symptoms and their impact, can help to elucidate mechanisms and identify potential areas for intervention or prevention. The application of these new sources of knowledge about the OA process holds promise for the development of new, potentially disease modifying pharmaceuticals and non-pharmacologic therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuqing Zhang, Email: yuqing@bu.edu.
Joanne M. Jordan, Email: joanne_jordan@med.unc.edu.
References
- 1.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Burden of Musculoskeletal Diseases Bone and Joint Decade).
- 3.Hadler NM. Knee pain is the malady--not osteoarthritis. Ann Intern Med. 1992;116(7):598–9. doi: 10.7326/0003-4819-116-7-598. [DOI] [PubMed] [Google Scholar]
- 4.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFrances CJ, Podgornik MN. 2004 National Hospital Discharge Survey. Adv Data. 2006;371:1–19. [PubMed] [Google Scholar]
- 6.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellgren J, Lawrence J. Atlas of standard radiographs. The epidemiology of chronic rheumatism. Vol. 2. Oxford: Blackwell Scientific Publications; 1963. [Google Scholar]
- 8.Altman RD, Bloch DA, Dougados M, et al. Measurement of structural progression in osteoarthritis of the hip: the Barcelona consensus group. Osteoarthritis Cartilage. 2004;12(7):515–24. doi: 10.1016/j.joca.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Brandt KD, Mazzuca SA, Conrozier T, et al. Which is the best radiographic protocol for a clinical trial of a structure modifying drug in patients with knee osteoarthritis? J Rheumatol. 2002;29(6):1308–20. [PubMed] [Google Scholar]
- 10.Hernborg JS, Nilsson BE. The natural course of untreated osteoarthritis of the knee. Clin Orthop. 1977;123:130–7. [PubMed] [Google Scholar]
- 11.Ahlback S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh) 1968 277:7–72. [PubMed] [Google Scholar]
- 12.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–7. [PubMed] [Google Scholar]
- 13.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 14.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343–55. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Wluka AE, Cicuttini FM, Spector TD. Menopause, oestrogens and arthritis. Maturitas. 2000;35(3):183–99. doi: 10.1016/s0378-5122(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 18.Hannan MT, Felson DT, Anderson JJ, Naimark A, Kannel WB. Estrogen use and radiographic osteoarthritis of the knee in women. The Framingham Osteoarthritis Study. Arthritis Rheum. 1990;33(4):525–32. doi: 10.1002/art.1780330410. [DOI] [PubMed] [Google Scholar]
- 19.Nevitt MC, Cummings SR, Lane NE, et al. Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1996;156(18):2073–80. [PubMed] [Google Scholar]
- 20.Nevitt MC, Felson DT, Williams EN, Grady D. The effect of estrogen plus progestin on knee symptoms and related disability in postmenopausal women: The Heart and Estrogen/Progestin Replacement Study, a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2001;44(4):811–8. doi: 10.1002/1529-0131(200104)44:4<811::AID-ANR137>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo DJ, Wallace RB, Wu L, Yood RA. Effect of hormone therapy on risk of hip and knee joint replacement in the Women's Health Initiative. Arthritis Rheum. 2006;54(10):3194–204. doi: 10.1002/art.22138. [DOI] [PubMed] [Google Scholar]
- 22.Nevitt MC, Xu L, Zhang YQ, et al. Very low prevalence of hip osteoarthritis among Chinese elderly in Beijing compared to Caucasians in the U.S.: the Beijing Osteoarthritis Study. Arthritis Rheum. 2002 doi: 10.1002/art.10332. in press. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YQ, Xu L, Nevitt MC, et al. Chinese have a much lower prevaelnce of radiographic osteoarthritis of the hand than Caucasians in the U.S. Arthritis Rheum. 2001;44(9):s225. [Google Scholar]
- 24.Zhang Y, Xu L, Nevitt MC, et al. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: The Beijing Osteoarthritis Study. Arthritis Rheum. 2001;44(9):2065–71. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Nelson E, Braga L, Benner J, et al. Characterization of individual radiographic features of hip osteoarthritis in African American and White women and men: the Johnston County Osteoarthritis Project. Arthritis Care & Research. 2010;62(2):190–197. doi: 10.1002/acr.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga L, Renner JB, Schwartz TA, et al. Differences in radiographic features of knee osteoarthritis in African-Americans and Caucasians: the Johnston county osteoarthritis project. Osteoarthritis Cartilage. 2009;17(12):1554–61. doi: 10.1016/j.joca.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane NE, Lin P, Christiansen L, et al. Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis Rheum. 2000;43(2):400–4. doi: 10.1002/1529-0131(200002)43:2<400::AID-ANR21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Lynch JA, Parimi N, Chaganti RK, Nevitt MC, Lane NE. The association of proximal femoral shape and incident radiographic hip OA in elderly women. Osteoarthritis Cartilage. 2009;17(10):1313–8. doi: 10.1016/j.joca.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. Bmj. 1996;312(7036):940–3. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palotie A, Vaisanen P, Ott J, et al. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989;1(8644):924–7. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41(6):1064–71. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Kerkhof HJ, Lories RJ, Meulenbelt I, et al. A genome-wide association study identifies an osteoarthritis susceptibility locus on chromosome 7q22. Arthritis Rheum. 62(2):499–510. doi: 10.1002/art.27184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolan AL, Hart DJ, Doyle DV, Grahame R, Spector TD. The relationship of joint hypermobility, bone mineral density, and osteoarthritis in the general population: the Chingford Study. J Rheumatol. 2003;30(4):799–803. [PubMed] [Google Scholar]
- 34.Chen HC, Shah SH, Li YJ, Stabler TV, Jordan JM, Kraus VB. Inverse association of general joint hypermobility with hand and knee osteoarthritis and serum cartilage oligomeric matrix protein levels. Arthritis Rheum. 2008;58(12):3854–64. doi: 10.1002/art.24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810–24. doi: 10.1259/0007-1285-38-455-810. [DOI] [PubMed] [Google Scholar]
- 36.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calve-Perthes disease. J Bone Joint Surg Am. 1981;63(7):1095–108. [PubMed] [Google Scholar]
- 37.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 1986;213:20–33. [PubMed] [Google Scholar]
- 38.Croft P, Cooper C, Wickham C, Coggon D. Osteoarthritis of the hip and acetabular dysplasia. Ann Rheum Dis. 1991;50(5):308–10. doi: 10.1136/ard.50.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RW, Egger P, Coggon D, Cawley MI, Cooper C. Osteoarthritis of the hip joint and acetabular dysplasia in women. Ann Rheum Dis. 1995;54(3):179–81. doi: 10.1136/ard.54.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau EM, Lin F, Lam D, Silman A, Croft P. Hip osteoarthritis and dysplasia in Chinese men. Ann Rheum Dis. 1995;54(12):965–9. doi: 10.1136/ard.54.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane NE, Nevitt MC, Cooper C, Pressman A, Gore R, Hochberg M. Acetabular dysplasia and osteoarthritis of the hip in elderly white women. Ann Rheum Dis. 1997;56(10):627–30. doi: 10.1136/ard.56.10.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125(5):353–9. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 43.Lane NE, Gore LR, Cummings SR, et al. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42(5):854–60. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 44.Felson DT, Niu J, Clancy M, et al. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56(1):129–36. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 45.McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648–56. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 46.Jordan JM, De Roos AJ, Renner JB, et al. A case-control study of serum tocopherol levels and the alpha- to gamma-tocopherol ratio in radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Am J Epidemiol. 2004;159(10):968–77. doi: 10.1093/aje/kwh133. [DOI] [PubMed] [Google Scholar]
- 47.Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29(12):2585–91. [PubMed] [Google Scholar]
- 48.Sasaki S, Iwata H, Ishiguro N, Habuchi O, Miura T. Low-selenium diet, bone, and articular cartilage in rats. Nutrition. 1994;10(6):538–43. [PubMed] [Google Scholar]
- 49.Turan B, Balcik C, Akkas N. Effect of dietary selenium and vitamin E on the biomechanical properties of rabbit bones. Clin Rheumatol. 1997;16(5):441–9. doi: 10.1007/BF02238935. [DOI] [PubMed] [Google Scholar]
- 50.Fang W, Wu P, Hu R, Huang Z. Environmental Se-Mo-B deficiency and its possible effects on crops and Keshan-Beck disease (KBD) in the Chousang area, Yao County, Shaanxi Province, China. Environ Geochem Health. 2003;25(2):267–80. doi: 10.1023/a:1023271403310. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Reyes R, Mathieu F, Boelaert M, et al. Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am J Clin Nutr. 2003;78(1):137–44. doi: 10.1093/ajcn/78.1.137. [DOI] [PubMed] [Google Scholar]
- 52.Jordan JM, Fang F, Arab L, et al. Low selenium levels are associated with increased risk for osteoarthritis of the knee. Arthritis Rheum. 2005;52:s455. [Google Scholar]
- 53.Engstrom G, De Verdier MG, Nilsson PM, et al. Incidence of severe knee and hip osteoarthritis in relation to dietary intake of antioxidants beta-carotene, vitamin C, vitamin E and selenium: a population-based prospective cohort study. Arthritis Rheum. 2009;60:s235–s236. [Google Scholar]
- 54.Neogi T, Booth SL, Zhang YQ, et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54(4):1255–61. doi: 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 55.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116(7):535–9. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 56.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 57.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433–9. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: data from the First National Health and Nutrition Examination Survey (NHANES-I) Am J Epidemiol. 1993;137(10):1081–8. doi: 10.1093/oxfordjournals.aje.a116611. [DOI] [PubMed] [Google Scholar]
- 59.van Saase JL, Vandenbroucke JP, van Romunde LK, Valkenburg HA. Osteoarthritis and obesity in the general population. A relationship calling for an explanation. J Rheumatol. 1988;15(7):1152–8. [PubMed] [Google Scholar]
- 60.Heliovaara M, Makela M, Impivaara O, Knekt P, Aromaa A, Sievers K. Association of overweight, trauma and workload with coxarthrosis. A health survey of 7,217 persons. Acta Orthop Scand. 1993;64(5):513–8. doi: 10.3109/17453679308993681. [DOI] [PubMed] [Google Scholar]
- 61.Karlson EW, Mandl LA, Aweh GN, Sangha O, Liang MH, Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114(2):93–8. doi: 10.1016/s0002-9343(02)01447-x. [DOI] [PubMed] [Google Scholar]
- 62.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 63.Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9(4):316–24. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 64.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Croft P, Cooper C, Wickham C, Coggon D. Osteoarthritis of the hip and occupational activity. Scand J Work Environ Health. 1992;18(1):59–63. doi: 10.5271/sjweh.1608. [DOI] [PubMed] [Google Scholar]
- 66.Lawrence JS. Rheumatism in cotton opertatives. Br J Industr Med. 1961;18:270–276. doi: 10.1136/oem.18.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadler NM, Gillings DB, Imbus HR, et al. Hand structure and function in an industrial setting. Arthritis Rheum. 1978;21(2):210–20. doi: 10.1002/art.1780210206. [DOI] [PubMed] [Google Scholar]
- 68.Felson DT, Hannan MT, Naimark A, et al. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham Study. J Rheumatol. 1991;18(10):1587–92. [PubMed] [Google Scholar]
- 69.Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43(7):1443–9. doi: 10.1002/1529-0131(200007)43:7<1443::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 70.Puranen J, Ala-Ketola L, Peltokallio P, Saarela J. Running and primary osteoarthritis of the hip. Br Med J. 1975;02(5968):424–5. doi: 10.1136/bmj.2.5968.424-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kujala UM, Kettunen J, Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38(4):539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 72.Spector TD, Harris PA, Hart DJ, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39(6):988–95. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- 73.Roos H, Lindberg H, Gardsell P, Lohmander LS, Wingstrand H. The prevalence of gonarthrosis and its relation to meniscectomy in former soccer players. Am J Sports Med. 1994;22(2):219–22. doi: 10.1177/036354659402200211. [DOI] [PubMed] [Google Scholar]
- 74.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106(2):151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 75.Lane NE, Hochberg MC, Pressman A, Scott JC, Nevitt MC. Recreational physical activity and the risk of osteoarthritis of the hip in elderly women. J Rheumatol. 1999;26(4):849–54. [PubMed] [Google Scholar]
- 76.Lane NE, Michel B, Bjorkengren A, et al. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. J Rheumatol. 1993;20(3):461–8. [PubMed] [Google Scholar]
- 77.Newton PM, Mow VC, Gardner TR, Buckwalter JA, Albright JP. Winner of the 1996 Cabaud Award. The effect of lifelong exercise on canine articular cartilage. Am J Sports Med. 1997;25(3):282–7. doi: 10.1177/036354659702500302. [DOI] [PubMed] [Google Scholar]
- 78.Slemenda C, Brandt KD, Heilman DK, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127(2):97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 79.Baker KR, Xu L, Zhang Y, et al. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese: the Beijing osteoarthritis study. Arthritis Rheum. 2004;50(6):1815–21. doi: 10.1002/art.20261. [DOI] [PubMed] [Google Scholar]
- 80.Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41(11):1951–9. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 81.Brandt KD, Heilman DK, Slemenda C, et al. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26(11):2431–7. [PubMed] [Google Scholar]
- 82.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138(8):613–9. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 83.Dominick KL, Jordan JM, Renner JB, Kraus VB. Relationship of radiographic and clinical variables to pinch and grip strength among individuals with osteoarthritis. Arthritis Rheum. 2005;52(5):1424–30. doi: 10.1002/art.21035. [DOI] [PubMed] [Google Scholar]
- 84.Chaisson CE, Zhang Y, Sharma L, Kannel W, Felson DT. Grip strength and the risk of developing radiographic hand osteoarthritis: results from the Framingham Study. Arthritis Rheum. 1999;42(1):33–8. doi: 10.1002/1529-0131(199901)42:1<33::AID-ANR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 85.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286(2):188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 86.Cerejo R, Dunlop DD, Cahue S, Channin D, Song J, Sharma L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46(10):2632–6. doi: 10.1002/art.10530. [DOI] [PubMed] [Google Scholar]
- 87.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 88.Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56(4):1204–11. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 89.Hunter DJ, Niu J, Felson DT, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007;56(4):1212–8. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 90.Sharma L, Lou C, Felson DT, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42(5):861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 91.Wada M, Imura S, Baba H, Shimada S. Knee laxity in patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1996;35(6):560–3. doi: 10.1093/rheumatology/35.6.560. [DOI] [PubMed] [Google Scholar]
- 92.Brage ME, Draganich LF, Pottenger LA, Curran JJ. Knee laxity in symptomatic osteoarthritis. Clin Orthop. 1994;304:184–9. [PubMed] [Google Scholar]
- 93.Golightly YM, Allen KD, Renner JB, Helmick CG, Salazar A, Jordan JM. Relationship of limb length inequality with radiographic knee and hip osteoarthritis. Osteoarthritis Cartilage. 2007;15(7):824–9. doi: 10.1016/j.joca.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harvey WF, Yang M, Cooke TDV, et al. Association of Leg-Length Inequality With Knee Osteoarthritis: A Cohort Study. Ann Intern Med. 2010;152:287–295. doi: 10.1059/0003-4819-152-5-201003020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 96.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 97.Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure--a systematic overview of the evidence. J Rheumatol. 1997;24(8):1599–607. [PubMed] [Google Scholar]
- 98.Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130(2):278–88. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- 99.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. Bmj. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 101.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 102.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–7. [PubMed] [Google Scholar]
- 103.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hill CL, Gale DR, Chaisson CE, et al. Periarticular lesions detected on magnetic resonance imaging: prevalence in knees with and without symptoms. Arthritis Rheum. 2003;48(10):2836–44. doi: 10.1002/art.11254. [DOI] [PubMed] [Google Scholar]
- 105.Link TM, Steinbach LS, Ghosh S, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 106.Gooberman-Hill R, Woolhead G, Mackichan F, Ayis S, Williams S, Dieppe P. Assessing chronic joint pain: lessons from a focus group study. Arthritis Rheum. 2007;57(4):666–71. doi: 10.1002/art.22681. [DOI] [PubMed] [Google Scholar]
- 107.Hutchings A, Calloway M, Choy E, et al. The Longitudinal Examination of Arthritis Pain (LEAP) study: relationships between weekly fluctuations in patient-rated joint pain and other health outcomes. J Rheumatol. 2007;34(11):2291–300. [PubMed] [Google Scholar]


