Abstract
In the last two decades, large scale axenic cell cultures of the marine species comprising the family Euplotidae have resulted in the isolation of several new classes of terpenoids with unprecedented carbon skeletons including the (i) euplotins, highly strained acetylated sesquiterpene hemiacetals; (ii) raikovenals, built on the bicyclo[3.2.0]heptane ring system; (iii) rarisetenolides and focardins containing an octahydroazulene moiety; and (iv) vannusals, with a unique C30 backbone. Their complex structures have been elucidated through a combination of nuclear magnetic resonance spectroscopy, mass spectrometry, molecular mechanics and quantum chemical calculations. Despite the limited number of biosynthetic experiments having been performed, the large diversity of ciliate terpenoids has facilitated the proposal of biosynthetic pathways whereby they are produced from classical linear precursors. Herein, the similarities and differences emerging from the comparison of the classical chemotaxonomy approach based on secondary metabolites, with species phylogenesis based on genetic descriptors (SSU-rDNA), will be discussed. Results on the interesting ecological and biological properties of ciliate terpenoids are also reported.
Keywords: marine ciliates, terpenoids, biosynthesis, bioactivity, phylogenetic relationships
1. Introduction
Ciliate is the common name assigned to a protist taxon comprising the phylum Ciliophora. Over 7000 ciliate species have been described from marine, freshwater and terrestrial habitats, where they play a key role in microbial food webs. These eukaryotic microorganisms are characterized by a nuclear dualism and body-covering cilia used for locomotion and feeding. Relying solely on their outwardly visible characteristics as the main discriminating parameters for identifying protistan species, some claim that protists show a poor biodiversity [1,2] and thereby wrongly assume that this is accompanied by poor chemical diversity [3]. However, there is increasing evidence that protists express a biodiversity comparable to other taxa [4,5]. Furthermore, the genetic distance among ciliate taxa of the same taxonomic rank, species included, is far larger than that occurring among many multicellular representatives. The fact that protists violate the first species property (i.e., morphological uniqueness) does not imply that other species-specific properties cannot undergo selective pressures leading to new, separate evolutionary units. The low level of biodiversity found in protists to-date is likely to be a reflection of the lack of workable methods for addressing the species problem in protists. Hence, it may frequently occur that two strains showing the same identifying, morphological features behave quite differently. Molecular genetic methodologies have provided a powerful means to address the protistan systematic-taxonomic problems. The outcomes of these new approaches strongly suggest that establishment of protistan biodiversity is a task that has just commenced [6]. Moreover, in the last two decades detailed investigation of ciliate secondary metabolites from the genus Euplotes has added “a new dimension” to the problem of their species-specific allocation via a “chemotaxonomic” approach, which is able to define protistan taxonomy to the subspecific level [7]. In line with current views of protistan microbiologists, the results obtained by the latter approach have highlighted a rich diversity of ciliate secondary metabolites. Further investigation into the morphological, physiological, and ecological properties of this interesting taxon is therefore essential, especially given the interest in their exploitation as new biotechnological tools.
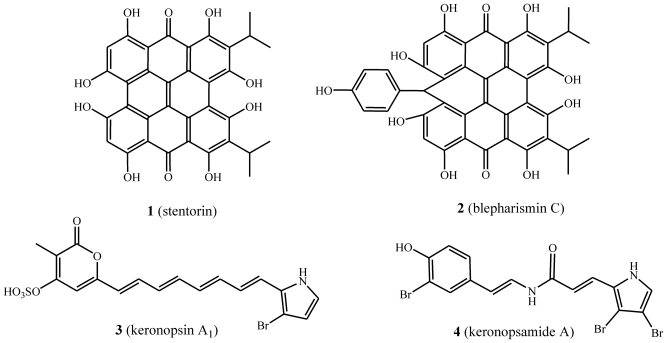
From a natural products point of view, ciliates are microorganisms able to synthesize, inter alia, secondary metabolites belonging to three main biogenetic classes. The first class is represented by pheromone peptides and proteins, of which structural, functional, and evolutionary properties have been described by Luporini and co-workers. These cell-specific signal proteins, isolated from ciliated protozoa belonging to the genus Euplotes, constitutively diffuse into the extracellular environment [8]. Among the several peptide pheromones isolated from marine ciliates, the shortest show sequences of 38–40 amino acids arranged to form a bundle of three α-helices, which have an up-down-up orientation and are maintained in close juxtaposition by three to four disulfide bonds located in conserved positions within the family. From a functional point of view, these peptides have been demonstrated to induce mating of cells via paracrine-like (or heterotypic) interactions and, as a consequence of their autocrine binding, to promote the vegetative (mitotic) proliferation of the same cells from which they are released. The second class of products reported from ciliates is broadly defined as ciliate pigments, which are compounds of mixed biosynthetic origin. In particular, ciliates are known to produce three main families of pigments (Scheme 1). The first contains metabolites built on the hypericin skeleton such as stentorin (1) [9] and blepharismin C (2) [10,11]; the second is represented by compounds wherein a ß-bromine substituted pyrrole is linked to a sulfate pyrone through an extended conjugated chain, such as keronopsin (3) [12]; and the last derives from a peptide-like linked brominated tyramine with a brominated pyrrole carboxylic acid, such as the keronopsamides A (4) [13]. Although the bioactive alkyl-resorcinols isolated from Climacostomum virens [14], the so called “climacostol family”, are not true pigments, they can broadly be thought to belong to this class, being metabolites of mixed biosynthetic origin.
Scheme 1.
Pigments isolated from freshwater and marine ciliates.
From the early 90s, investigation into the natural products from marine ciliates belonging to the genus Euplotes revealed a dominance in the production of terpenoids. At that time, only one study on crude extracts of Tetrahymena pyriformis reported the production of the polycyclic triterpene tetrahymanol, an important metabolite along the squalene cyclization cascade [15]. In 1992, the ability to obtain large mass cultures of the marine morphospecies Euplotes crassus led to the isolation of the highly strained acetylated hemiacetals, the first sesquiterpenoids from marine protists. Since then, the intra- and interspecific diversity of ciliates has been widely investigated.
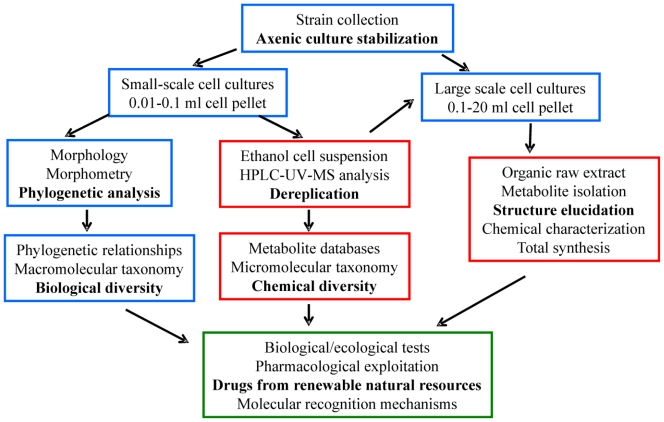
This review summarizes the results of almost two decades of research in the field by the authors, but also, and more importantly, presents a unifying picture of the chemical and biological results, including both reported and previously unpublished data. The review begins with a short description of the approach used to tackle the topic. As shown schematically in Figure 1, a general methodology that relies on strong collaboration between microbiologists, molecular biologists and natural product chemists is essential. For each investigated morphospecies the following is discussed: (a) the chemical structures and reactivity of the isolated terpenoids; (b) the intra-species chemical diversity; (c) putative biosynthetic pathways accounting for the formation of the isolated terpenoids; (d) the intra-species biological diversity through phylogenetic relationships; and (e) the results of bioactivity screening. The evolutionary significance of these metabolites is described in the last section, along with a comparison of the outcomes from the analysis of micromolecular markers (terpenoids) with that of macromolecular descriptors (SSU-rRNA).
Figure 1.
The biological (blue boxes) and chemical (red boxes) steps of an integrated approach, leading to an understanding of the functional roles of secondary metabolites from marine ciliates (green boxes).
2. Generalities on Biological and Chemical Methodologies
2.1. Cell cultures, ecological tests and phylogenetic analysis
All the Euplotes strains collected were established starting from single naturally occurring cells to obtain cellular line clones, which were fed on both microalgae and bacteria. Mass cultures were grown in salt water (32‰ salinity), sterilized, and inoculated with microalgae, with the latter first incubated at 23 ± 1 °C in a 12 h light/dark cycle for at least 10 days using a daylight (Osram Daylight lamp, 36 W/10) and fluorescent (Osram Fluora lamp, 40 W/77) illuminated incubator system. Ciliates thrive in a continuously percolating culture of the autonomously reproducing autotrophic microalgae, representing a renewable source.
Starved cells from mass cultures of the strains were pelletted by successive centrifugation rounds to provide from 0.01–0.1 mL of closely packed ciliates for small-scale cell cultures to 0.1–20 mL for large-scale samples. Small-scale cell cultures were used for the morphological and phylogenetic analyses. Morphological information was obtained from living and fixed specimens, employing differential interference contrast microscopy (DIC) and scanning electron microscopy (SEM). The silver nitrate impregnation protocol devised by Foissner et al. [16] and the standard Feulgen staining procedure were applied after cell fixation in Bouin’s fluid to reveal the nuclear apparatus. In assigning strains to the appropriate morphospecies, the intra- (within and between strains) and inter-population variability was considered. Eleven characters commonly used for morphological distinctness among Euplotes taxa were chosen. The determination of the phenotypic traits was carried out on 10 selected specimens of each preparation at 1000X magnification on a Leica DMR microscope, using the dedicated Leica IM1000 version 1.0 software STATISTICA (StatSoft, Inc., USA). Data produced by this morphometric procedure were analyzed using the multivariate technique of discriminating functions.
To gain insight into the taxonomic and phylogenetic relationships among congeneric species of Euplotes, the nuclear small subunit rRNA (SSU-rRNA) gene was selected to determine the phylogenetic trees. The nuclear SSU-rRNA gene sequences were amplified by polymerase chain reaction (PCR) of DNA prepared from small-scale cell cultures concentrated by centrifugation and incubated in lysis buffer (0.5 M EDTA, 1% SDS, 10 mM Tris-HCl, pH 9.5) containing proteinase K (200 μg/mL), at 55 °C for 12–15 h. The universal eukaryotic forward primer 18S F9 (5′-CTGGTTGATCCTGCCAG-3′) [17] and the 18S R1513 Hypo reverse primer (5′-TGATCCTTCYGCAGGTTC-3′) were used [18]. The PCR products were directly sequenced in both directions with one forward [F783 (5′-GACGAAATCAAAGAAATACCGTC-3′)] and two reverse [R536 (5′-CTGGAATTACCGCCGGCTG-3′) and R1052 (5′-AACCTTAAGGAACCCCCG GCCATGGCAA-3′)] internal primers [18]. The SSU-rRNA gene sequences were aligned using the program ClustalX [19] with default parameter settings. The alignment was adjusted interactively with the program BioEdit Sequence Alignment Editor [20] to optimize the base-pairing scheme of the rRNA gene molecule secondary structure [21]. The phylogenetic analysis was performed in PAUP version 4.0b10 [22], applying a neighbor joining method and using Modeltest 3.06 [23] to select the appropriate model of substitution. The Akaike Information Criterion (AIC) indicated that the Tamura-Nei model [24], which considers unequal base frequencies, was the most appropriate. The reliability of the internal branches of the phylogenetic tree was assessed using the bootstrap method [25].
Biological/ecological tests were carried out on various strains of the different morphospecies. Responses of strains to metabolite treatments were assessed by examining the impact on cell fission rates and cell tolerance. Cleaving cells were isolated from well-fed cultures, thus producing groups of physiologically synchronized single progeny individuals. These fission progeny cells normally underwent at least one fission in ciliates of larger size, or two fissions in ciliates of smaller size. Using a micropipette, they were individually transferred to wells of three-spot hemispheric depression slides for 16 h incubation in salt water, sterilized, and then assessed in the absence (control) or in the presence of metabolite. The cultures were kept under observation by light microscopy. The effects of each experimental treatment, as well as the controls, were scored on a series of nine cells, individually distributed into the depressions. The fission rate of a cell’s progeny was calculated using the formula: n = log2 nx; where n is the fission number performed by a single cell since its isolation into a depression, and nx is the number of cells scored after 16 h. Cells that were unable to swim or creep on the bottom of the depression, or with motionless cirri (or both), were considered dead.
All the terpenoids reported here, including those isolated from E. focardii, have been tested using an unbiased sample of marine interstitial ciliates in order to verify their biological activities.
2.2. Cell extracts, de-replication analysis, isolation and structure elucidation of secondary metabolites
Since secondary metabolites in ciliates are not-dispersed in the culture-medium, cells obtained by centrifugation were extracted in ethanol and stored in the same solvent (at -20 °C) prior to analysis. Rapid identification and quantitative estimates of known compounds were obtained from the ethanol extracts using de-replication techniques based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods. Photo diode array was used for UV-Vis detection, while sample MS ionization used an ESI or APCI ion-source (in either positive- or negative-ion mode) coupled to an ion trap mass analyzer. An aliquot of 5 μL of the ethanol solution of each sample (the supernatant of the cell suspension containing about 0.2 mL of cell-pellet in 1 mL of alcohol) was injected into a C18 column (4.6 × 150 mm, 3.5 μm) with a 7:3 mixture of CH3CN/H2O as the eluent and a flow rate of 0.9 mL/min.
In order to isolate pure metabolites, the ethanol solution obtained through cell pellet filtration was evaporated and the residue partitioned between hexane-ethyl acetate 9:1 (organic part) and methanol-water 9:1 (aqueous part). The organic extract was then subjected to reversed-phase flash chromatography (RP-FC) on a Lichrolut RP18 column with a CH3CN-MeOH gradient elution, collecting 5 fractions of about 2 mL. The first fractions that eluted usually contained the terpenoid metabolites, which could be further purified by RP semi-preparative HPLC (RP18, CH3CN-H2O 7:3, 5 mL/min). The latter FC fractions contained different classes of lipids such as sterols, di- and triacylglycerols, free fatty acids and phospholipids. Structural elucidation of the compounds under investigation was performed using nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS) and infrared spectroscopy (IR), combined with the results from molecular mechanics (MMX and MM3 force fields) and density functional theory (DFT).
3. Terpenoids from Euplotes crassus
3.1. Structures, chemical reactivity and total synthesis
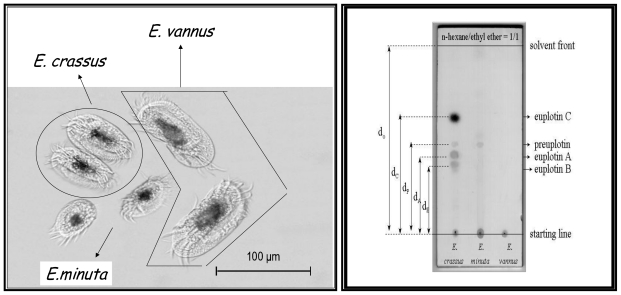
When investigation into marine ciliated protozoa in the subclass Hypotrichida first began, morphological data proved ambiguous in deciding whether Euplotes vannus and Euplotes crassus were separate species or part of a species complex. Indeed, the Euplotes vannus-crassus-minuta group is a complex of closely related, cosmopolitan, marine ciliates, for which the ability to distinguish between them continues to be contentious. Three morphospecies have been identified with E. vannus being the largest form, E. minuta the smallest, and E. crassus presenting intermediate morphological characteristics. In particular, the morpho-variability of E. vannus and E. crassus is low and their microscopic identifying features are not clearly separated (Figure 2, left).
Figure 2.
Left: Transmission electron microscopy (TEM) image of Euplotes crassus, Euplotes minuta and Euplotes vannus ciliates; Right: TLC plate (stationary phase: Merck Si60 PF254, eluent: n-hexane-ethyl ether 1:1, staining reagent: Ce(SO4)2/H2SO4) of crude cell extracts (0.1 mL of cell pellet) from typical strains of E. crassus, E. minuta and E. vannus.
Starting with the most frequently sampled hypotrichid E. crassus, strains collected in different geographical sites have all been found to give the same secondary metabolites, referred to as the euplotins (Scheme 2). These are sometimes also accompanied by their putative acyclic precursor, preuplotin [26]. Thin-layer chromatography (TLC) can be used to distinguish the protozoan E. crassus as it is the only euplotin producer within the E. vannus-crassus-minuta complex of sibling species. As shown in Figure 2, for E. crassus extracts, three UV active spots ascribed to euplotin A, B and preuplotin are detected, along with a dark spot (Retention factor, Rf 0.62) for euplotin C, which appears upon staining. For E. minuta, only preuplotin is detected, while strains of the E. vannus morphospecies contain neither euplotin nor preuplotin.
Scheme 2.
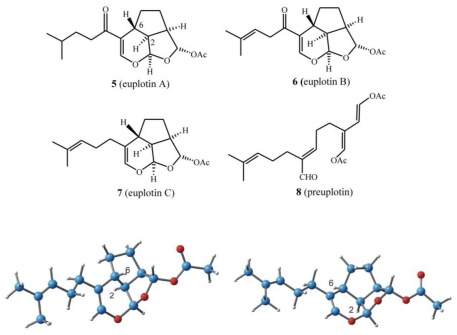
Top: Sesquiterpenoids 5–8 isolated from cell cultures of Euplotes crassus. Bottom: Molecular mechanics minimized structures of euplotin C (left, trans 2–6 ring junction) and of its hypothetical more stable C(6) epimer (right, cis 2–6 ring junction).
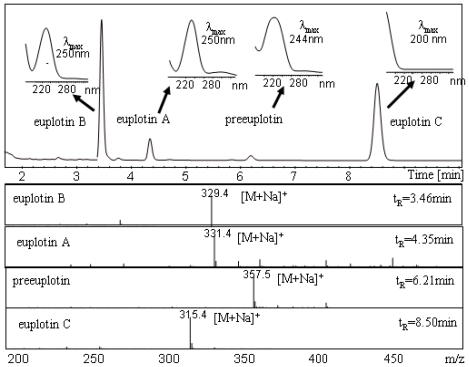
A typical chromatogram obtained from analysis of an E. crassus strain (Pdr1) is reported in Figure 3, showing the chromatographic separation of the euplotins, characterized by peak-selective UV and ESI-MS ion spectra. Depending on the environment of the electrospray ionization chamber, concentration and composition of the eluate, the sodium adduct [M + Na]+ of the molecular ion is usually detected in the positive-ion mode ESI-MS spectrum, followed by potassium adducts [M + K]+ and/or dimeric ion-species [2M + 2Na]2+. This analysis provides both qualitative and quantitative data and is currently employed in the initial de-replication analysis of new strains. With a single HPLC-UV-MS run, three important pieces of molecular information are obtained simultaneously: polarity (retention time, tR), chromophoric groups (UV-Vis spectra), and molecular weight (MS spectra) of every metabolite eluted by the LC system. These data can also be used to track metabolic changes during the life-cycles of the microorganisms.
Figure 3.
LC-UV chromatogram λ 220 nm) of a crude extract of Euplotes crassus (strain Pdr1): for all metabolites UV spectra are reported on the top and ESI(+)-MS spectra at the bottom.
In all investigations on different strains of E. crassus, the acetylated sesquiterpenoid euplotin C (7) was the most abundant metabolite, and can thus be used as a chemotaxonomic marker of this morphospecies. Euplotin C is often followed by at least one of its structural analogs, euplotin A (5) or euplotin B (6), and occasionally by the putative acyclic precursor, preuplotin (8) (Scheme 2) [27].
The euplotane skeleton is a tricyclic ring system, built on a trialdehyde-masked system wherein an unusual 2,6-trans-stereochemistry imposes a high degree of strain on the overall structure. According to ab initio DFT calculations, the 2,6-trans ring junction leads to an increase in the euplotin strain energy of about 12 kcal/mol compared to that of the diastereoisomeric 2,6-cis analog (Scheme 2, bottom). Interestingly, the structural features of the latter are present in udoteatrial hydrate [28], a diterpenoid isolated from the marine alga Udotea flabellum.
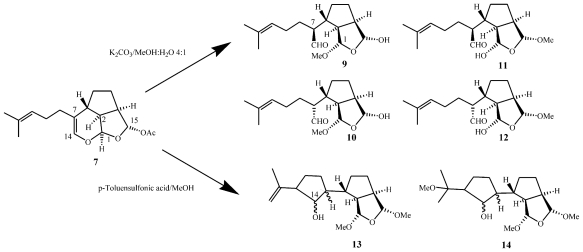
Another feature of the euplotins is their high chemical instability [16]. In mild basic conditions, euplotin C readily undergoes a cascade of irreversible transformations, leading to four major products, as regio- and diastereoisomeric mixtures (9–12 in Scheme 3). Loss of the acetate group at C(15), releases the strain imposed by the trans 2–6 ring junction in the euplotane skeleton, since the incipient C(14) aldehyde in the hypothetical trialdehyde intermediate is far from the C(1) aldehyde group, regardless of the relative stereochemistry. Curiously, while C(1) and C(15) are strongly involved in mutual acetal condensations induced by nucleophilic attack of MeOH, the C(14) aldehyde group does not cause any acetalization. The analysis of the end-products of this reaction confirmed the euplotins structure, and also allowed the establishment of their absolute configuration through the application of the modified Mosher method [30,31] involving MTPA esterification of the hemiacetal groups of the regioisomers 9 and 10 [16].
Scheme 3.
Hydrolysis of euplotin C (7) under either mildly basic or acidic conditions.
Different results were obtained when euplotin C (7) was subjected to mildly acidic conditions (e.g., p-toluensulfonic acid in methanol). NMR analysis of the reaction products indicated complete acetalization at C(1) and C(15) (no hemiacetals present) and, more importantly, that the incipient C(14) aldehyde group was trapped by the sp2 C(12) in a carbonyl-ene type reaction, affording the expected hydroxy-cyclopentane ring [as an epimeric mixture at C(14)] as in the derivatives 13 and 14. The latter derivative, 14, is derived from acid-catalyzed electrophilic attack of MeOH on the iso-propylidene moiety of 13. As it will become clearer further on, the possibility of acid-catalyzed carbonyl-ene processes is of paramount importance since this kind of intramolecular cyclization seems to play a dominant role in the biosynthesis of the majority of terpenoids isolated from marine ciliates.
Euplotins are also amphiphilic molecules, with a “water-like” polar part defined by an unusual sequence of electron-withdrawing oxygen atoms, as well as a hydrophobic tail. It is therefore not surprising that they give rise to micellar aggregates in water, at least above their critical concentration (CMC). Although the CMC value of euplotins in aqueous solution or the size of their aggregates has not been measured, evidence of micellar phases can be obtained from line-width analysis of 1H-NMR spectra taken on aqueous samples containing euplotins at different concentrations. In particular, NMR experiments carried out on euplotin C (7) supported the formation of large molecular aggregates by the appearance of strong signal broadening in aqueous 1H-NMR spectra due to significant decreasing of their transversal relaxation times T2. These aggregates are only partially destroyed by extensive sonication treatment, as evidenced by NMR spectra signal re-sharpening.
Euplotins A–C (5–7) are even unstable in water, but the end-products are different from those discussed above in methanol solutions. In general terms, the increase of the reactant concentration in the micellar phase is expected to lead to a considerable acceleration of the hydrolysis reaction due to the well known micellar catalysis effect [31]. With euplotins as reactants, however, it could be demonstrated through LC-UV-MS techniques and deuterium incorporation experiments that three consecutive chemical processes occur. First, there is an initial fast process leading to hydrolytic cleavage of the C(15) acetate. Second, there is an electrophilic addition of water to the activated C(7)=C(14) vinyl ether double bond. Third, there is an electrophilic addition of water, catalyzed by acetic acid (deriving from process 1), to the C(10)=C(11) trisubstituted double bond of the prenyl side-chain. While the first two reactions proceed with similar rate constants, the last is much slower and becomes significant only after complete disappearance of the starting reactant, euplotin C (7). Free aldehydes are expected to be labile intermediates playing an equilibration role among the hemiacetal intermediates, but in these experiments they were undetectable.
Only one report has so far been published on the total synthesis of euplotins. Euplotin A (5, Scheme 2) has been synthesized [33] by exploiting the thermal reactivity of suitable heterodienes (2-acylalkenals) that undergo cycloaddition reactions giving the key 5-acyldihydropyran intermediate with the correct euplotane trans 2–6 stereochemistry. Racemic euplotin A has been obtained by functional group transformations.
3.2. Inclusion complexes
Since euplotins are rather hydrophobic compounds, their low water solubility represents a serious limitation to the reliability of any bioassay method. In order to overcome these difficulties, inclusion complexes of euplotin C with cyclodextrins (CD) were prepared [34]. After various trials with different CD derivatives, heptakis(2,6-di-O-methyl)-β-cyclodextrin (DIMEB) was finally chosen because of its binding capability and enhanced water solubility. The complex stoichiometry, binding constants and three dimensional structure of the 1:1 inclusion complex of euplotin C(7)-DIMEB were determined using 1D/2D-NMR techniques and LC-ESI tandem MS measurements. The data suggests that, in aqueous solution, 7-DIMEB has a 1:1 stoichiometry, although a 1:2 ratio cannot be ruled out at least at high DIMEB/7 molar ratio. By using LC(PDA)-MS/MS as the probe technique, kinetic investigations of the hydrolysis of the 7-DIMEB complex demonstrated that complexed-7 gives rise to the same end-products as free-7 alone, although every step of the overall hydrolytic process occurs at a lower rate. Thus, the increased solubility and the lower hydrolysis rate of complexed-7 with respect to free-7, suggested that these inclusion complexes can be used in biological tests as reliable sources of euplotin C (7), avoiding the use of organic solvents such as DMSO as the carrier agent.
3.3. Biogenetic considerations
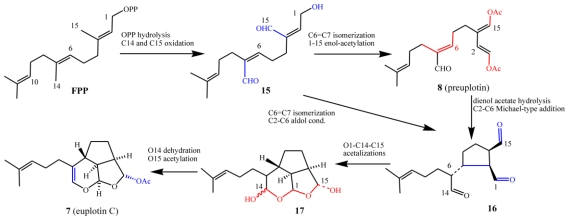
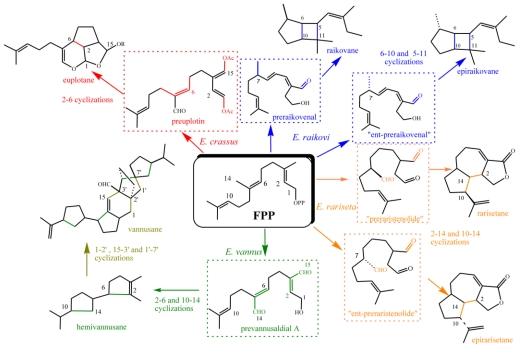
A putative biosynthetic route to the euplotins from farnesyl pyrophosphate (FPP) via preuplotin (8) as a key intermediate is shown in Scheme 4. After C1 pyrophosphate hydrolysis, it is proposed that intermediate 15 could undergo C6=C7 double bond isomerization, along with acetylation of the OH groups at C(1) and C(15), to give the 1,4-dienol acetate moiety of preuplotin (8). Hydrolysis of 8 followed by nucleophilic addition of the electron rich C(2) to the electron poor C(6) would then afford the trialdehyde terpenoid 16. Thereafter nucleophilic attack by water at C(14), followed by a cascade of internal acetalizations, would lead to euplotin C (7) via ß-elimination of water and O-C(15) acetylation of the hemiacetal diastereoisomeric mixture 17. Biosynthetic experiments with labeled isotopic precursors are planned by the authors of this review, in order to support the proposed biosynthetic route described above and to establish whether or not terpenoid biosynthesis in marine ciliates follows the classical mevalonate pathway [35]. It should be noted, however, that no biogenetic data on the euplotins is currently available.
Scheme 4.
Biogenetic proposal of Euplotes crassus sesquiterpenoids.
Three main aspects of the biosynthetic proposal must be highlighted because they are atypical of classical terpenoid biosynthesis. The first is that intramolecular cyclizations are not usually driven by nucleophilic additions but by carbocation-mediated, electrophilic reactions [36,37]. In fact, these cyclizations are typically initiated by a regiochemical addition of an electrophile to a sp2 hybridized carbon generating a carbocation, which in turn undergoes subsequent ring forming reactions. In marine organisms this process is often initiated by bromine induced electrophilic attack of double bonds leading to a plethora of haloterpenoids [38]. The second point is that in terpenoid biosynthesis, C(1) usually plays a pivotal role as the electrophilic site of the first intermediate after allylic diphosphate hydrolysis, whilst the C(14) and C(15) methyl groups are rarely used in ring forming processes. The opposite is proposed to occur in the E. crassus metabolism, where the key biogenetic step would be the stereoselective C(2)–C(6) bond formation, possibly deriving from conjugate addition of electron rich C(2) to the electrophilic site at C(6). In fact, the oxidation of C(14) to -CHO inverts the polarity of the C(6)=C(7) double bond making the β-carbon at C(6) particularly electron-poor. At the same time, the hydrolysis/oxidation of C(1) to -CHO makes the α-carbon C(2) an electron-rich, nucleophilic site. Finally, in classical terpenoid biogenesis, oxidations are usually thought of as final processes (sometimes even occurring as isolation artefacts), whereby the end-products (or late carbocationic intermediates) are ultimately quenched by the capture of an external nucleophile such as water.
Enzymes such as terpene cyclases that would be responsible for converting the sesquiterpene precursor FPP into species-specific secondary metabolites in ciliates are expected to be structurally and functionally different from classical cyclases involved in terpenoid biosynthesis of other phyla. This is because they are expected to recognize oxidized FPPs as substrates. Marine ciliates could, however, share the enzymatic pool leading from mevalonic acid (MVA), or from 1-deoxyxylulose-5-phosphate (DXP) [37] to isopentenyl diphosphate (IPP) and FPP.
3.4. Intra-morphospecies phylogenetic analysis
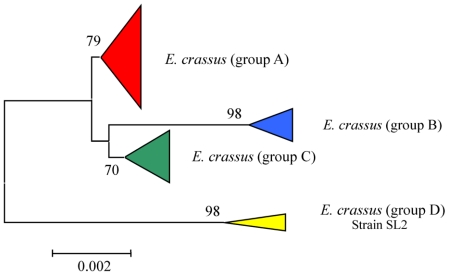
Euplotes crassus is a cosmopolitan and highly diffused marine morphospecies, with an ability to cope with changing environmental conditions [4,39,40]. This may play an important role in ecological significance of this morphospecies, while the production of the euplotins could be another element in their survival strategy [27]. The branching of the phylogenetic tree denotes, within the morphospecies analyzed, the presence of four distinct groups, all including representatives of cosmopolitan origin (Figure 4). Inter-population variation in genetic determinants following geographical separation would be expected to occur, and would result in the production of distinct secondary metabolites.
Figure 4.
Schematic representation of the phylogenetic tree, based on SSU-rRNA gene sequence comparisons, of different strains of Euplotes crassus. The numbers at the nodes are bootstrap percentages from 1000 replicates. The scale bars correspond to a distance of 2 substitutions per 1000 nucleotide positions.
Although most strains of E. crassus produce the same secondary metabolites, there are some exceptions. For example, several strains do not produce preuplotin (8), while other strains do not produce euplotin A (5). Some strains produce euplotin C (7) only in very low quantities accompanied by trace amounts of preuplotin (8). Furthermore, the population SL represents an exception within E. crassus being the only strain which does not produce any euplotins at all. From an evolutionary point of view, euplotins are the result of a metabolic pathway, which appears useful in natural selection, regardless of the environmental conditions. This may explain its ubiquity and larger distribution with respect to other morphospecies that share the same habitats, like E. vannus and E. minuta.
3.5. Ecological role and biological activities
At the onset of the investigations, there were two hypotheses regarding the way in which E. crassus exchanges chemical information: (i) by the release of substances into the extracellular medium, or (ii) by direct cell-to-cell contacts. Reported studies, carried out in vivo, suggested that euplotins are utilized to inhibit populations of competitors via cell-to-cell contact, a good strategy to colonize new habitats or defend settlements [16,27]. Evidence that the toxic effect is a result of euplotins action comes from separate experiments carried out in vitro where a non-producing strain of E. vannus (TB6) was treated with varying concentrations of pure 7 and a 7-DIMEB complex at 10 μg/mL. In both cases a 100% reduction on the viable protozoa was observed within one hour. These results confirm that the complexation process does not influence the activity of 7, while control experiments verified that the effect of dimethylsulfoxide (DMSO) or DIMEB was negligible. At the functional level, euplotin C kills non-producing Euplotes cells, whereas at sublethal levels it alters cell cycle, ciliary motility and cell shape [27]. These findings suggest that euplotin C may play an ecological role, representing an adjuvant factor of the multicomponent strategy pursued by E. crassus in broadening its niche size.
These results, in conjunction with the fact that many natural products from marine resources show pharmaceutical and medical relevance, prompted the investigation into the potential in vitro bioactivity of euplotin C (7) against the non-marine pathogenic protozoa Leishmania major and Leishmania infantum, the opportunistic yeast Candida albicans and several prokaryotic microorganisms [40]. The toxicity of 7 against the macrophage-like cell line J774 was used as a mammalian host cell control. DMSO at a non-toxic concentration and DIMEB as a solubilizing agent in water for 7 were also used in the assays. Both free-7 and complexed-7 (i.e., DIMEB-7 1:1 inclusion complex) demonstrated good antileishmanicidal activity at 20 μg/mL, causing irreversible fatal damage. After 24 h, LD50 values of 4.6 ± 0.5 and 8.1 ± 1.8 μg/mL for free-7 and complexed-7 respectively, were recorded. By contrast, the LD50 value was >200 μg/mL for the control J774 cell line. Subsequent studies on the activity of E. crassus (a euplotin-producer) and E. vannus (a non-producer) towards both L. major and L. infantum were in agreement with the data from the isolated compound, and found that cellular contact with E. crassus SSt22 strain causes irreversibly damage to parasitic cells, while the E. vannus TB6 strain does not induce any effect. Although the required amount of euplotin C is too high to be interesting for drug applications, the absence of any cytotoxicity towards the macrophage cells is quite promising.
Further bioactivity results revealed that at high concentrations of >100 μg/mL, 7 showed an inhibitory effect on the yeast Candida albicans and on several strains of the bacteria Streptococcus and Burkholderia spp., whilst at lower concentrations only marginal antimicrobial activity was observed [40]. Interestingly, the complexed-7 form was noted to be more active in longer test times than free-7, possibly due to the higher chemical stability of the former. An inhibitory effect has also been observed for euplotin C on food vacuole formation and fluid phase endocytosis in Paramecium primaurelia, a process that might be mediated by an altered function of cell membranes as well as by modification of the microtubule network [42,43].
The underlying cellular and molecular basis of the effects of euplotin C were recently elucidated using mouse and rat tumor-derived cell lines, followed by the analysis of the nuclear and cytosolic changes usually associated with apoptosis in specific cell lines [29,45]. The apoptotic process is an auto-programmed mode of cell death that is dependent upon energy, as it requires ATP to transmit the death signal from the cytoplasm to the nucleus. It also causes well-defined morphological and biochemical changes in the cell such as cell shrinkage, chromatin condensation, nucleosomal degradation and changes in intracellular cation concentration such as Ca2+. The cytotoxicity of 7 was tested on the murine AtT-20 line derived from anterior pituitary tumor cells and the PC12 line originating from a rat chromaffin cell tumor, which expresses properties common to both neurons and neurosecretory cells. It was found that treatment of mouse and rat tumor cells with euplotin C resulted in a dose-dependent cytotoxic effect and in the induction of apoptotic cell death by cellular stress that triggered the activation of the caspase cascade.
Finally, cellular responses to euplotin C in E. vannus have recently been investigated by analyzing the cytosolic changes likely to be associated with cytotoxicity and apoptosis, i.e., intracellular Ca2+ concentration, organelle components and related caspases [46]. The most important and general outcome of this study is that the chemical signal carried by euplotin is transduced in a rapid impairing of the membrane electrical properties. This causes motility disorders which in turn hinders the ciliate’s ability to escape the external threat. After some minutes the fate of non producers is sealed because the ion channel pumps are completely impaired [43]. These results suggest that euplotin C exerts a marked disruption of homeostatic mechanisms, which play a determinant role in cell-environment interactions.
4. Terpenoids from Euplotes raikovi
4.1. Structures, chemical reactivity and synthesis
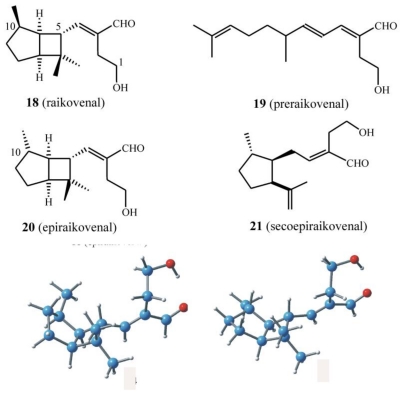
While euplotins are built on an unsaturated dioxa-tricyclic system, the structural feature of E. raikovi metabolites is the bicyclo[3.2.0]heptane ring system [47]. However, different populations of E. raikovi produce C(10)-epimeric metabolites, providing evidence for intra-species variability. In particular, the strain Mor1 collected on the Casablanca coast contains raikovenal (14) and its putative biogenetic precursor preraikovenal (15), while all other strains so far examined [48] (GA8, SB8, 39W, LPSA5) produce the C(10) epimer of raikovenal (epiraikovenal, 16) and its seco-analog (secoepiraikovenal, 17) (Scheme 5).
Scheme 5.
Top: Sesquiterpenoids isolated from cell cultures of Euplotes raikovi. Bottom: MM minimized structures of raikovenal 18 [left, ß-Me-C(10)] and epiraikovenal 20 [right, α-Me-C(10)].
The chemical and spectroscopic properties of epimeric sesquiterpenoids 18 and 20 are, as expected, quite similar. Although this close structural similarity complicates the de-replication process, the two epimers can easily be distinguished by NMR spectroscopy since the 13C resonance of Me-C(10) in 20 is almost 6 ppm downfield with respect to the same carbon resonance in 18. The opposite trend is observed for the proton resonance of Me-C(10), which results in an upfield shift of 0.05 ppm in 20 with respect to 18. This turns out to be very useful in the de-replication process, since this signal can easily be detected, even in 1H-NMR spectra performed on crude cell extracts.
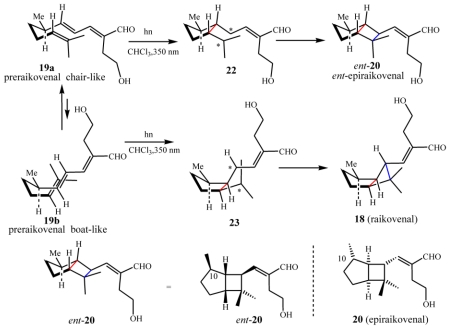
While the structure elucidation of these metabolites was in progress, interesting investigations began into the photochemical conversions among xenicane diterpenes isolated from a brown alga of the genus Dictyota [49,50]. Motivated by the success of these studies, it was hypothesized that the bicyclo ring system embedded in raikovenal could be obtained by direct photo-irradiation of its putative precursor, preraikovenal (19) (Scheme 6). Since [2 + 2]-cycloadditions are photochemically allowed processes, it was decided to sacrifice the small amount of the natural isolated compound 19, in an attempt to obtain an intramolecular photochemical cyclization leading to 18 and/or to 20. Irradiation at α 350 nm of a chloroform solution of preraikovenal produced raikovenal as a minor product, while the major product had a 1H-NMR spectrum that was superimposable to that of epiraikovenal (Scheme 6). According to the proposal [48], the photochemically produced raikovenal 18 could derive from a [2 + 2] concerted reaction (passing through the diradical intermediate 23) from the boat-like conformation of preraikovenal (19b). On the other hand, the photochemically produced epiraikovenal 20 can be explained only by assuming 19 adopts a chair-like conformation (19a), and passes through intermediate 22. However, if one looks at Scheme 6, the expected stereoisomer from the intramolecular addition of the reactive chair-like conformation is not 20, but its enantiomer ent-20. Indeed, it was demonstrated that the major product (epiraikovenal 20) obtained after purification of the photoaddition product mixture showed a circular dichroic (CD) spectrum opposite to that of natural epiraikovenal 20, thus indicating the compound to be ent-20. Although the absolute configuration at the chiral center at C(7) of natural 19 has not yet established, the outcome of this experiment clearly indicates not only that the photoaddition is a strongly stereocontrolled process but also that the inversion of chirality at C(7) is required in order to obtain natural epiraikovenal 20.
Scheme 6.
Photochemical conversion of preraikovenal (19) into raikovenal (18) and ent-epiraikovenal (ent-20).
Two independent and different strategies for the total synthesis of racemic 18 and 20 have been reported. The first synthesis described exploits the photochemical methodology in the key step of the bicyclo[3.2.0]heptane ring construction [51]. In the same year (1997), Rosini et al. [52], relying on a completely different approach, reported another interesting total synthesis of racemic raikovenal.
4.2. Biogenetic considerations
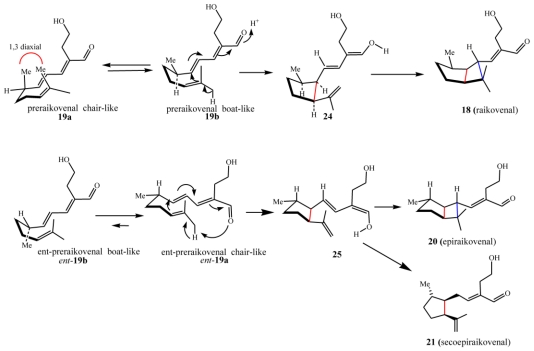
According to the results discussed above, if it is assumed that preraikovenal (19) is the putative biogenetic precursor of natural raikovenal (18), this would suggest that the enzymes involved in the bicyclic ring formation preferentially accommodate and recognize substrate 19 in a boat-like conformation (19b). This is supported by MM calculations, which show this conformation to be relatively stable due to the release of the repulsive 1,3-diaxial interaction of the methyl groups in the relevant transition state between preraikovenal and the intermediate 25 (Scheme 7). In order to explain the production of 20 and 21, an enantiomeric chair-like preraikovenal form, ent-19a, would be required. Since the photocyclization of preraikovenal (19) gave mainly ent-20, the conformer ent-19a is proposed herein to be the biogenetic precursor of epiraikovenal (20).
Scheme 7.
Biogenetic proposal for Euplotes raikovi sesquiterpenoids.
4.3. Intra-morphospecies phylogenetic analysis
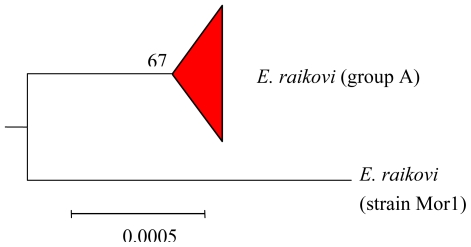
Euplotes raikovi appears as a group with intra-morphospecific heterogeneity, characterized by the synthesis of one or two related terpenes. In particular, the strain Mor1 contains raikovenal and its putative biogenetic precursor preraikovenal, while all other strains so far examined produce the C(10) epimer of raikovenal (epiraikovenal) and its seco-analog (secoepiraikovenal). Considering that one metabolite and its epimer require different biogenetic pathways, it was hypothesized that the production of functional alternatives embedded in the same bicyclo[3.2.0]heptane skeleton is an ecological strategy of adaptation that could also be seen by phylogenetic studies. The phylogenetic tree obtained for E. raikovi shows the evolutionary split between the strain Mor1 and the fairly homogeneous group comprised of representatives of other strains with cosmopolitan distribution (Figure 5). This is in fair agreement with the natural product divergence discussed above. As with the euplotins for E. crassus, the production of raikovenal/epiraikovenal structures by all strains enables their use as taxonomic markers at the morphospecies level.
Figure 5.
Schematic representation of the phylogenetic tree, based on SSU-rRNA gene sequence comparisons, of different strains of Euplotes raikovi. The number at the node is the bootstrap percentage from 1000 replicates. The scale bars correspond to a distance of 5 substitutions per 10000 nucleotide positions.
4.4. Ecological role and biological activities
The terpenoids from E. raikovi are less active than euplotins against other ciliates belonging to the genus Euplotes. Greater activity was observed [48] against Litonotus strains, ciliates with a characteristic predacious life-style which are killed by 18 at concentration as low as 10 μg/mL, and by 19–21 at higher concentrations. It is worth to note that euplotin C (7) was ineffective against the same predator suggesting the possibility of fine-tuned recognition phenomena in ciliated cells. Biological assays on raikovenal and related molecules demonstrated no killing activity against other ciliates even at concentrations above 20 μg/mL. Two particular features were noted in these experiments. First, epiraikovenal was able to depress the fission rate in E. vannus and E. rariseta strains. However, this effect does not seem to be specific, as these strains are sensitive to various aldehyde-terpenoids. Second, it appears that certain E. raikovi strains use a combination of preraikovenal-raikovenal and epiraikovenal-secoepiraikovenal as a defensive strategy. These compounds appear to be effective only in synergy against predators such as Litonotus, accounting for the non-palatability of the strains [48].
5. Terpenoids from Euplotes vannus
When attention was focused on the secondary metabolites of the morphospecies Euplotes vannus, another member of the marine Euplotes vannus-crassus-minuta species complex, it soon became apparent that the greater biodiversity of the E. vannus morphospecies is mirrored by a greater diversity of secondary metabolites. The number of E. vannus strains analyzed so far is fewer than for E. crassus, reflecting the lower probability in isolating these organisms from sand samples. However, these analyses suggest that several E. vannus subgroups differ in terms of their metabolic productions. This scenario is likely to result from a divergence from a common ancestor and/or intra-morphospecies variability.
5.1. Structures, chemical reactivity and synthesis
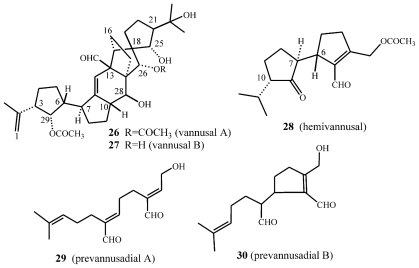
As summarized in Scheme 8, from tropical strains (Sil21, BUN3) of E. vannus morphospecies, two unusual compounds with a novel C30 backbone, named vannusal A (26) and vannusal B (27) were isolated [53]. On the other hand, investigation of two different strains (TB6 and CM1) led to the isolation of new regular sesquiterpenoids such as hemivannusal (28) and prevannusadial A (29) and B (30) [54].
Scheme 8.
Terpenoids isolated from cell cultures of Euplotes vannus.
The structure elucidation of vannusals was extremely challenging [53]. Although the molecular formula suggested mono- and di-acetylated triterpenoid structures, it soon became evident that these were not regular triterpenes deriving from squalene or squalene-oxide internal cyclizations, and in fact cannot be considered triterpenoids at all. There is high structural complexity in the central part of these structures, wherein five- and six-membered rings are interlaced in fused, bridged and spiro forms. A further difficulty arose from the violation of the “isoprenic rule” in these metabolites in the central and more intricate part of the molecules. Natural products chemists’ penchant for this rule is well known since it poses strong constrains on the overall skeleton, thereby minimizing the number of candidate structures compatible with the spectral data. Much assistance in the structure determination was obtained by functional group transformation of 26 into a series of derivatives [53], which ultimately revealed the intricate nature of the vannusal A structure.
The relative stereochemistry of several chiral centers in the vannusals has recently been revised (Scheme 8 and Figure 6) [54–61]. The stereoselective total synthesis of vannusal A and B (26 and 27) carried out by Nicolaou et al. confirms the overall structure and main stereochemical architecture of vannusals. In fact, if the relative stereochemistry at C(25) is not considered, the only difference in terms of the relative configurations between the proposed [53] and the revised structures [54,56,60,61] of vannusals derive from the mis-assigned relative stereochemistry at C(6)–C(7). This led to the assignment of the wrong relative stereochemistry of the chiral centers in the “eastern” domain of the molecule with respect to those in “western” one. The brilliant syntheses designed and executed in recent years in the Nicolaou laboratory has finally solved this intricate matter through the total synthesis of eight diastereomeric vannusal B structures [60,61].
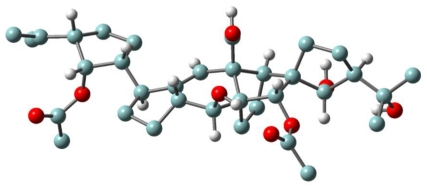
Figure 6.
Minimized structure of vannusal A (26). Only -H atoms on chiral centers are represented.
Even the structural elucidation of hemivannusal 28 [54] was not a simple task. In particular, the assignment of the relative stereochemistry of its 3 chiral centers, which requires correlation between the stereochemistry of the chiral centers on the rings linked together via a carbon-carbon single bond (C6–C7), around which free rotation is expected to occur. Thus, both the relevant vicinal coupling (3J6,7) and the dipolar mutual effects (NOE) between the same protons H(6) and H(7) represent averaged values among several C6–C7 rotamers. Assistance in resolving this problem was obtained through an extended conformational search performed within a molecular mechanics approach and refined by ab initio quantum chemical calculations.
The minor metabolites of the CM1 strain, prevannusadial A (29) and prevannusadial B (30), were isolated in very low amounts. Their structures were established [54] by direct comparison of their 1H-NMR spectra with those reported in the literature for the “diterpenoid 9” [62] isolated by Paul and Fenical from a Caribbean Udotea species (U. flabellum) and for petiodial isolated by Fattorusso et al. [63] from the Mediterranean U. petiolata. Prevannusadial B represents the deprenyl analog of these Udotea diterpenoids. This situation has a strong resemblance with that already discussed of the linear sesquiterpenoid preuplotin (8), with udoteal isolated from the tropical green alga U. flabellum [28].
5.2. Biogenetic considerations
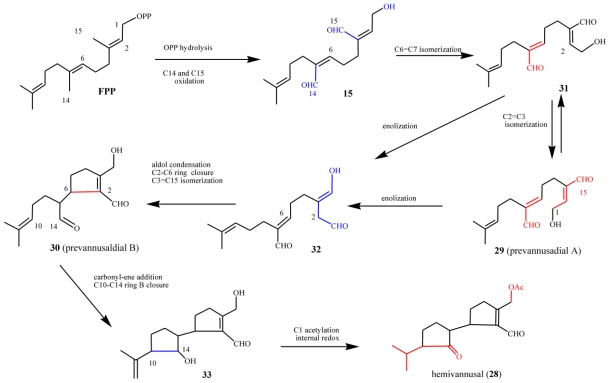
The possible biosynthetic origin of E. vannus metabolites is proposed in Scheme 9 along similar lines to that of the E. crassus terpenoids. The biosynthesis of hemivannusal 28 may derive from FPP and prevannusadial A (29) precursors through three enzymatic processes. After C1 pyrophosphate hydrolysis, C(14) and C(15) methyl group oxidation would afford the putative intermediate 15 (see also Scheme 4) bearing two α,β-unsaturated aldehyde functions with Z stereochemistry. Following Z→E isomerization of the C6,7 double bond, the intermediate 31 could then lead i) to 29, after Z→E isomerization of the C2,3 double bond and ii) to the intermediate 32, after oxidation of the allylic hydroxyl group of 29 and enolization of the C(15) aldehyde group. The intermediate 32 can be converted via intramolecular addition of the activated nucleophilic center C(2) to C(6), an electrophilic center due to its α position in the conjugated unsaturated system, leading to prevannusadial B (30). The latter could readily react through an intramolecular C(14)–C(10) carbonyl-ene process affording the bicyclic intermediate 33. The main metabolite of the strain TB6, hemivannusal (28), can be obtained after i) acetylation of the free primary –OH group and ii) internal redox reaction taking place in the saturated cyclopentane ring, a process having precedent in literature reports on carbonyl-ene synthetic procedures [63].
Scheme 9.
Biogenetic proposal for Euplotes vannus sesquiterpenoids.
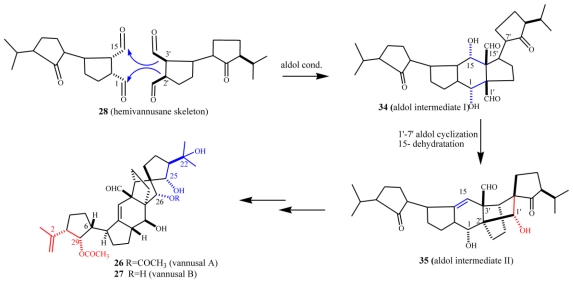
As already outlined in the proposed euplotin biosynthesis (Scheme 4), the unusual route involving methyl oxidation and nucleophilic additions seems to be a feature shared by strains belonging to the morphospecies E. vannus. Divergence among secondary metabolites of the two species occurs at the level of the putative common intermediate 15. Furthermore, it relies on the strong preference for acetalization processes in E. crassus leading to the highly strained tricyclic skeleton of euplotins, compared to carbonyl-ene reactions in E. vannus strains leading to the hemivannusane skeleton. Further evidence of this is given by the C30 terpenoids vannusal A and B (26 and 27). Although first thought to arise from a deviation of the squalene pathway to triterpenes, they are now considered as end-products of the nucleophilic condensation of two molecules possessing the regular hemivannusane sesquiterpenoid skeleton (28), via the hypothetical aldol-derived intermediates 34 and 35 (Scheme 10). The C30 terpenoids are possibly derived from a concerted condensation of the “peripheral” C1 and C15 electron-poor carbonyl groups of the first hemivannusane intermediate with the “internal” C2′ and C3′ electron-rich centers of the second intermediate. This biosynthetic proposal was exploited by Nicolaou et al. in their recent stereoselective total synthesis of the vannusals 26 and 27 [60,61].
Scheme 10.
Biogenetic proposal for Euplotes vannus C30 terpenoids (vannusals).
5.3. Intra-morphospecies phylogenetic analysis
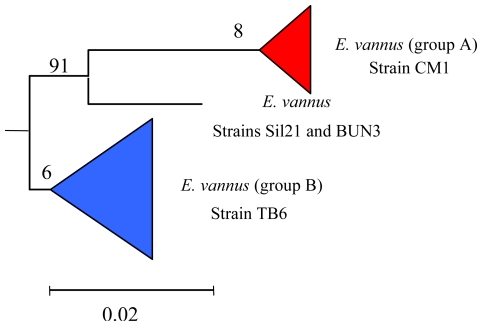
In the morphospecies E. vannus the chemodiversity of its secondary metabolites mirrors its genetic diversity, as revealed by the phylogenetic analysis. This significant intra-morphospecific variability in the secondary metabolite production is also reflected in the phylogenetic tree. E. vannus branches in at least two distinct genetic groups, with each group comprising representatives producing only one of the different classes of secondary metabolites isolated (Figure 7).
Figure 7.
Schematic representation of the phylogenetic tree, based on SSU-rRNA gene sequence comparisons, of different strains of Euplotes vannus. The numbers at the nodes are bootstrap percentages from 1000 replicates. The scale bars correspond to a distance of 2 substitutions per 100 nucleotide positions.
Phylogenetic analysis suggests that all the strains examined to-date constitute a monophyletic clade. From a natural products viewpoint this is advantageous. It means that the evolution of E. vannus utilizes different secondary molecular characters, not only useful as chemical biomarkers to distinguish different populations of the same morphospecies, but also as important representative of new chemical structures that could be exploited as new bioactive molecules.
6. Terpenoids from Euplotes rariseta
6.1. Structures, chemical reactivity and synthesis
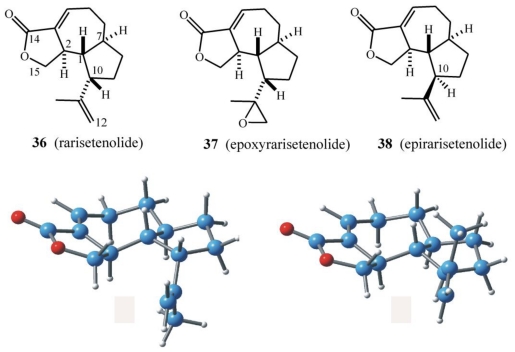
Analysis of the sesquiterpenoids isolated from E. rariseta (Scheme 11) suggests a scenario similar to that already discussed for E. raikovi. For example, different strains of the same morphospecies collected in different geographical areas produce two epimeric sesquiterpenoids, rarisetenolide (36) (and its epoxide 37) and the corresponding C(10) epimer, epirarisetenolide (38) [65], built on a novel octahydroazulene ring system. So far, only the relative configurations of these terpenoids have been established.
Scheme 11.
Top: Terpenoids from cell cultures of Euplotes rariseta. Bottom: MM minimized structures of raristenolide 36 [left, ß H-C(10)] and epirarisetenolide 38 [right, α H-C(10)].
In a series of experiments aimed at elucidating the structures of these terpenoids, the expected mixture of hemiacetal epimers at C(14) was obtained in good yields by DIBAL reduction of the lactone group of 36. At the same time, it was also demonstrated that the α-ß conjugated lactone could be removed by nucleophilic addition at the unsaturated C(3)=C(4) double bond. Indeed, UV irradiation (α 254 nm) of a methanol solution of rarisetenolide (36) in a quartz cuvette at room temperature gave good yields of the corresponding 4-methoxy derivative. Addition of MeOH rather than loss of conjugation under UV irradiation is in accordance with the proposed lactone structures.
As for the C(10) epimeric raikovenals 18/20, the structural similarity between the C(10) epimeric metabolites 36/38 complicates the dereplication process. However, the problem is readily solved using NMR spectroscopy. The 1H resonance of Me-C(10) (δH 2.90, dt) in rarisetenolide is 0.5 ppm downfield with respect to the same proton resonance in epirarisetenolide (δH 2.49, dt), and their vicinal coupling patterns are also different. These signals can easily be detected in 1H-NMR spectra, even in crude cell extracts, and thus it is possible to distinguish which one of the two epimers, 36 or 38, a given strain produces. Until now, a strain of E. rariseta that produces both epimers has not yet been found. This is indicative of a selective and/or different enzymatic pool in populations of this ciliate or the use of enantiomeric biogenetic precursors.
6.2. Biogenetic considerations
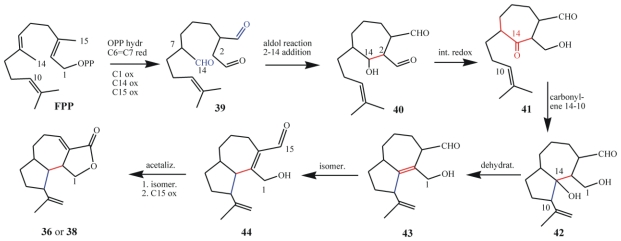
The proposed biosynthesis of rarisetenolide from FPP via the intermediate 44 is shown in Scheme 12. After C1 pyrophosphate hydrolysis of FPP, oxidation of the C(14) and C(15) methyl groups would afford the putative intermediate 39 bearing a saturated aldehyde at what was the C(6)=C(7) double bond. Aldol condensation of this aldehyde with the activated nucleophilic center at C(2), followed by proton tautomerization leads to the intermediate 40. This could then react through an intramolecular C(14)–C(10) carbonyl-ene process affording the bicyclic intermediate 42, which upon dehydration produces 43. This intermediate is proposed to play a pivotal role undergoing acetalization/oxidation processes leading to rarisetenolide-like metabolites through intermediate 44. Herein, C(14) is thus considered as the electrophilic center for the nucleophilic attack of both C(2) and C(10). If it is considered that FPP methyl groups are rarely involved in intramolecular terpenoid cylizations, the enzymatic processes able to build the octahydroazulene ring system of 36 or 38 appears to be a unique feature of ciliate secondary metabolism. It should be noted however, that the putative linear precursor (i.e., “prerarisetenolide”) has not yet been isolated. The C(6)/(7) hydrogenated analog 39, the C(14) oxidized form of preraikovenal (19), should contain all the structural and stereochemical features required to produce the octahydroazulene ring system of 36 and 38. Furthermore, it could also give rise to divergent routes towards 36 or 38, depending on the absolute configuration at its C(7) chiral center. Thus, it is proposed that evolutionary differentiation among E. rariseta strains could rely on C(7) enantiomeric FPP oxidized precursors, such as 39, in a similar way as differentiation among E. raikovi could rely on the enantiomeric FPP oxidized precursors 19 and ent-19.
Scheme 12.
Biogenetic proposal of Euplotes rariseta metabolites.
6.3. Intra-morphospecies phylogenetic analysis
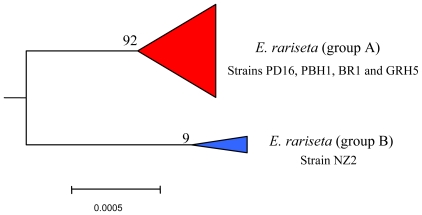
The strains of the morphospecies E. rariseta analyzed for the secondary metabolite production have been collected from various coastal sites, including those of northern and southern Australia (strains PD16 and PBH1, respectively), southern Brazil (strain BR1), Canary Islands (strain GRH5), and New Zealand (NZ2) [65]. All of them are found to be producers of non-aldehydic terpenoids, which is quite unusual in the Euplotes genus. The three sesquiterpenoids identified, rarisetenolide, epoxyrarisetenolide, and epirarisetenolide, share the same octahydroazulene moiety, which seems to be a convincing chemotaxonomic marker of the morphospecies.
As with E. crassus and E. vannus, this morphospecies also shows intra-morphospecific variability in its secondary metabolite production that is reflected in the phylogenetic partition. The four strains of E. rariseta (PD16, PBH1, BR1 and GRH5) produce both rarisetenolide and epoxyrarisetenolide. These strains, although collected from geographically distinct locations, are all phylogenetically grouped together in the same clade (Figure 8). It is difficult to believe that this is a coincidence. The production of secondary metabolites is related to enzymes coded by the organism’s genome, their presence reflecting the expression of functional genes, and the inference of a close phylogenetic relationship among the above strains is warranted. In contrast to the other strains, the strain NZ2 gives epirarisetenolide, revealing a subtle variability in secondary metabolism for this ciliated morphospecies. The epimeric relationship of this metabolite with rarisetenolide implies the presence of different metabolic pathways in strain NZ2 with respect to the other strains. This is indicative of genetic differentiation of strain NZ2 as revealed by the phylogenetic tree (Figure 8).
Figure 8.
Schematic representation of the phylogenetic tree, based on SSU-rRNA gene sequence comparisons, of different strains of Euplotes rariseta. The numbers at the nodes are bootstrap percentages from 1000 replicates. The scale bars correspond to a distance of 5 substitutions per 10000 nucleotide positions.
6.4. Biological activities
Cytotoxicity of rarisetenolide (36) and its congeners (37 and 38) against representatives of the marine interstitial ciliate community, comprising E. rariseta itself, was detected only at relatively high doses, i.e., >20 μg/mL. The only exception observed was towards the ciliate Litonotus lamella, which proved sensitive to 36 at lower doses. It is worth noting that L. lamella differs from all other ciliates involved in the bioassays due to its behavior as a predator. It can be proposed that the effectiveness of 36 against L. lamella is part of a defensive strategy of E. rariseta to avoid predation. Since lower cytotoxicity of the chemically more reactive epoxide 37 with respect to 36 or 38 was found in the ecological tests, interactions with membrane receptors of the Euplotes morphospecies might be non-covalent in nature.
7. Terpenoids from Euplotes focardii
7.1. Structures, chemical reactivity and synthesis
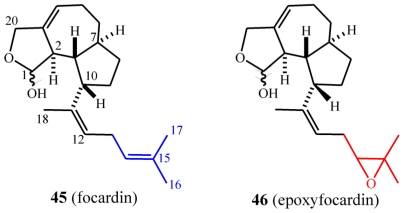
Strains assigned to the species E. focardii have been found to produce the focardins (Scheme 13) [66], which show much of the structural characters of the rarisetenolide/epirarisetenolide family. From the morphospecies collected from Antarctica (strain TN1) and inter-tropical coastal waters (strains GBS1 and GBS5), a new skeletal class of diterpenoid, epoxyfocardin (46), was isolated. Focardin (42), which is a possible biogenetic precursor of 46, was also isolated as a minor component. Focardins constitute the first example of ecologically relevant metabolites from ciliate species that inhabit polar ecosystems.
Scheme 13.
Diterpenoids from cell cultures of Euplotes focardii.
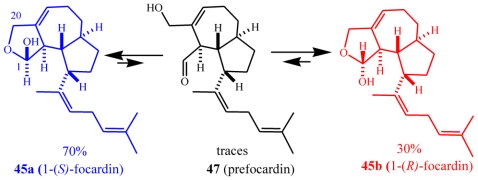
Focardin (45) was isolated as a 70:30 mixture of hemiacetals 45a and 45b. These equilibrated through the unstable ring-opened, aldehyde intermediate 47 (Scheme 14), to ultimately give epoxyfocardin (46) as an 85:15 mixture of its corresponding hemiacetals 46a and 46b. Evidence of the equilibrium of the diasteroisomeric hemiacetals came from the analysis 1H-NMR spectra of pure 46a obtained by reversed-phase chromatography of raw extract containing 46 which was superimposable to that obtained before the separation. An unaltered ratio of products clearly suggests equilibrium conditions among the epimers. The absolute configuration of 46a/46b was determined from Mosher’s method [30] through esterification of the hemiacetal hydroxyl group.
Scheme 14.
Hemi-acetal equilibration among C(20) epimers of focardin (45).
7.2. Biogenetic considerations
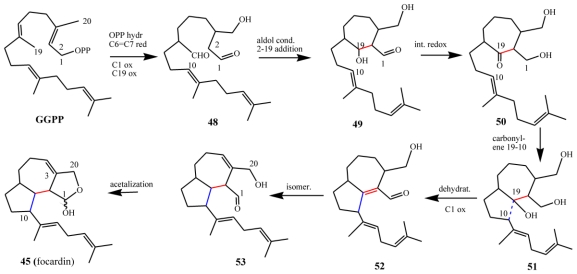
The proposed biosynthesis of the E. focardii metabolites described here commences from geranylgeraniol pyrophosphate (GGPP) and involves (in the first steps) similar intermediates to those involved in rarisetenolide biosynthesis, albeit with one prenyl unit more (Schemes 12 and 15). Herein, C(19) is playing the same role as C(14) in the raristenolide pathway, but a striking difference between rarisetenolide and focardin biosynthesis is represented by the different oxidation states of C(1) and C(15)/C(20). Whereas in the formation of 36 (or 38), C(1) is in a lower oxidation state than C(15), in 45 (or 46) it is in a higher oxidation state than C(20).
Scheme 15.
Biogenetic proposal for Euplotes focardii metabolites.
7.3. Intra-morphospecies phylogenetic analysis
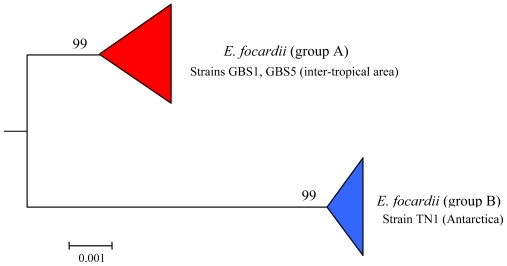
By morphological and morphometrical analysis, all Antarctic and inter-tropical populations of E. focardii constituted a single cluster of indistinguishable units, thus allowing their attribution to the same morphotype. Nevertheless, within the morphospecies two distinct groups, which corresponded to the two geographical areas of sampling (Antarctic and inter-tropical regions), were detected by phylogenetic analysis (Figure 9). Interestingly, this genetic polymorphism is also reflected by secondary metabolite production. In fact 45 and 46 can be isolated in higher yields from the inter-tropical strains GBS1 and GBS5, whereas the Antarctic TN1 strain produces them only in low amounts (Figure 9). This observation suggests that competition for exploitable niches and food varies with latitudes, thus the survivor strategies are also expected to vary. In particular in the inter-tropical area the metabolite expression would be expected to be higher, since E. focardii inter-tropical populations must cope with higher species competition than the Antarctic strains of the same morphospecies.
Figure 9.
Schematic representation of the phylogenetic tree, based on SSU-rRNA gene sequence comparisons, of different strains of Euplotes focardii. The numbers at the nodes are bootstrap percentages from 1000 replicates. The scale bars correspond to a distance of 1 substitution per 1000 nucleotide positions.
7.4. Biological activities
The paucity of suitable test organisms prevented the investigation of the ecological role of focardin and epoxyfocardin, but the high bioactivity of the latter against predacious ciliates such as L. lamella and L. cygnus suggests a defensive role for these substances. This would indicate that ciliates are in strong competition even in Antarctica. Of the two diterpenoids, focardin (45) showed the least cytotoxic activity. E. focardii showed a significant cytotoxic activity vs. various ciliate morphospecies, including congeneric species of the ciliate Litonotus, a notorious predator of Euplotes specimens. Due to difficulties in accomplishing long lasting experiments using psychrophilic ciliate strains (growing at temperatures close to water-freezing), after 24 h of Litonotus predaction activity, the disappearance of Euplotes representatives of different morphospecies in the sample community was significantly larger than that observed when the sample ciliate community included specimens of E. focardi. Toxic activity counteracting predation by Litonotus may be guessed for the substances produced by E. focardii. Focardin’s low bioactivity and low abundance in E. focardii infers a minor role for this metabolite in nature.
8. Functional Role and Phylogenetic Significance
The modern construction of phylogenetic trees and evolutionary events concerning species is based on molecules that are found in all organisms, such as ribosomal RNA (rRNA) and highly conserved proteins, or polymorphic enzymes for population structure and dynamics. Some species, however, compete for space and resources, or defend themselves from predators, largely due to their secondary metabolites, which are potentially their best taxonomic and phylogenetic markers [67].
It is well known that terpenes act as defensive substances for a variety of marine organisms including algae, sponges, corals, mollusks and fish [71]. For example, caulerpenyne is an acetylenic sequiterpene found in certain species of green algae, which is rapidly converted into highly reactive 1,4 dialdehydes by esterase enzymes that are activated upon wounding [69]. Chemical defense is also typical in the poorly defended, shell-less opisthobranch mollusks, which accumulate terpenes both from their diet and from de novo biosynthesis [70].
As described recently [71], biosynthetic pathways leading to secondary metabolites may have evolved to favor molecular diversity, although the explanation on how the evolution has followed its course is a matter of debate. From the point of view of the “target-based” model of natural product evolution [72,74], certain secondary metabolites could confer a survival benefit to an organism through their ability to specifically bind to cellular targets in competing organisms. This belief, however, relies on the assumption that natural product/receptor interactions are comparable to enzyme/substrate interactions [72]. From the point of view of the “diversity-based” model (originally named the “screening” model [73,75]), evolution would favor organisms that “could generate and retain chemical diversity at low cost”. Here the assumption is that organisms producing a wider chemical diversity should have greater chances of producing metabolites with potent biological activity [75]. Herein, almost two decades of comparative analysis of the secondary metabolism and phylogenesis of marine ciliates of the family Euplotidae from temperate and tropical marine environments is described. The synergistic contribution of expertise coming from biological (microbiology, molecular biology and ecology) and chemical (analytical and synthetic organic chemistry) disciplines has created the opportunity to propose a global picture of the ciliate protistan world to-date.
Marine interstitial ciliates belonging to the genus Euplotes have emerged as a rich source of new terpenoids bearing various functionalities. Furthermore, these terpenoids display activities against other ciliates, competing for space and resources, and also possess interesting biological properties such as apoptotic activity towards mouse and rat tumor cell lines [29,45]. Generally, the lipophilic nature of these compounds indicates that their principal targets are likely to be cell membranes, wherein they could play a role in chemioosmotic control [46]. Moreover, there is evidence from bioassays that terpenoids are non-covalently bound to the ciliate’s external membrane.
From an evolutionary point of view, the structural chemo-diversity among ciliate metabolites is not unexpected because it reflects the biological imperative to maximize biodiversity starting with both a minimal substrate pool and minimal genetic alterations [76]. It also provides evidence that the biodiversity and adaptive ability of marine ciliates is higher than what was previously thought. From a structural point of view, many of the ciliate secondary metabolites isolated so far are regular, often polycyclic terpenoids, which are proposed to originate from the biogenetic precursors FPP or GGP through the involvement of species-specific enzymes. In particular, four different sesquiterpenoid skeletons have been isolated and demonstrated to be species-specific: i.e., euplotane (E. crassus), raikovane (E raikovi), rarisetane (E. rariseta) and hemivannusane (E. vannus) (Scheme 16). The latter is thought to be the biogenetic precursor of the unusual C30 vannusane metabolites. The putative linear precursors to three of the above (preuplotin, preraikovenal and prevannusadial A), deriving from methyl oxidations of FPP along with C(6)=C(7) double bond isomerizations, have been isolated from strains of the corresponding morphospecies (E. crassus, E. raikovi and E. vannus, respectively). On the other hand, the proposed linear precursor of rarisetenolide (“preraristenolide”) has never been found in any strain of E. rariseta. The unifying picture of the secondary metabolites in marine ciliates depicted in Scheme 16 assumes that epimeric products 18/20 observed in E. raikovi, and 36/38 in E. rariseta, should derive from enantiomeric forms of the corresponding linear precursors. It has also been demonstrated that another diterpenoid structure, focardane, is a chemotaxonomic marker of the morphospecies E. focardii.
Scheme 16.
Metabolic routes towards terpenoids produced by marine ciliates of the genus Euplotes.
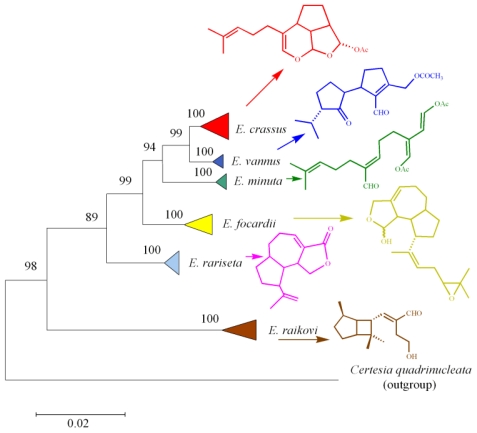
To gain insight into the phylogenetic relationships among Euplotes species, SSU-rRNA gene sequences of representatives belonging to the different marine species were determined and aligned in order to compare molecular phylogenesis with secondary metabolite production. The results obtained clearly define not only the relationships among the different populations of the same morphospecies (intraspecific phylogenetic trees) as already discussed in previous sections (see Figures 4, 5, 7–10), but also the relationships among different Euplotes morphospecies (interspecific phylogenetic tree). The latter indicates the monophyly of the taxon Euplotes, characterized by the following particular features: (1) E. raikovi branches basal to the other species; (2) E. crassus, E. vannus, and E. minuta form a stable group positioned at the apex of the phylogenetic tree; (3) E. focardii is the sister species of the previous group; (4) E. rariseta occupies an intermediate position and is more phylogenetically related to the clade E. crassus - E. vannus - E. minuta - E. focardii than to E. raikovi species (Figure 10).
Figure 10.
Schematic representation of the phylogenetic tree, based on SSU-rRNA gene sequence comparisons, of different species of Euplotes and their main terpenoid secondary metabolites isolated to-date. The numbers at the nodes are the bootstrap percentage from 1000 replicates. The scale bars correspond to a distance of 2 substitutions per 100 nucleotide positions.
The production of secondary metabolites at the morphospecies level is consistent with the results described above. Three different compounds, the euplotins A–C and their precursor preuplotin, have been isolated from E. crassus. The other two members of the E. vannus-crassus-minuta complex produce only small amounts of preuplotin (E. minuta) or are characterized by the complete absence of euplotins (E. vannus). This latter species is instead characterized by the production of a variety of compounds, with different populations of E. vannus giving regular sesquiterpenes such as hemivannusal, prevannusadial A, and prevannusadial B, while others give dimeric sesquiterpenes such as vannusal A and vannusal B, and others appear not to produce any terpenoid secondary metabolites. These results suggest (Scheme 16 and Figure 10) that E. crassus is a species that has developed a homogeneous set of secondary molecular characters, and evolution of this species is destined either to maintain all these characters (euplotins A–C) or to lose all of them. In contrast, the evolution of E. vannus utilizes different sets of secondary molecular characters to distinguish different populations.
From an evolutionary perspective, it could be said that genes implied in the secondary metabolism of the TB6 strain (E. vannus) have evolved from divergence of ancestral homologous genes of the CM1 strain, since the latter are (and were) not able to encode enzymes for the biosynthesis (Figures 5 and 6) of the bicyclic terpenoid hemivannusal from prevannusadial A. On the other hand, strains Sil21 and BUN3 should have the most diverse genes since they are able to carry out the reaction even further than hemivannusal, affording “dimeric” C30 terpenoids such as vannusal A. Of course, it also possible that it is not new genes, which have arisen by gene duplication followed by divergence, but an increased expression of genes encoding new enzymes and, hence, new chemical processes which are not expressed in the ancestors.
Some morphospecies appear to be much more homogeneous than others (e.g., E. raikovi), suggesting different evolutionary strategies. This result must be interpreted according to the recent reported evidence [39], which shows that within ciliate morphospecies, there is sufficient isolation to allow separation of genomes without morphological differentiation. Moreover, the high number of strains genetically analyzed for each morphospecies indicates the existence of an internal genetic variability that undermines the general assumption that morphospecies equate to an evolutionary unit. Interestingly, these genetic intra-morphospecific groups have their exact counterpart in the outcome of the chemotaxonomic approach. This analysis shows that different strains belonging to the same morphospecies but grouped in different genetic clades, are characterized, both qualitatively and quantitatively, by a different profile of secondary metabolites.
9. Conclusions
Unicellular ciliated protists represent an important component of the grazing marine food web, thus playing a key, although scarcely investigated, role in the marine ecosystem. From the structural chemist’s point of view the most fascinating aspect of this trip into the world of ciliates is represented by the high skeletal chemical diversity found in this phylum. Using sesquiterpenoids as an example, the isolation of four new C15 skeleta was unexpected at the beginning of the study, since more than a century of research had been dedicated not only to the identification of new metabolites but also to explain the mechanism of their biosynthesis. Although experimental biogenetic investigations on the origin of ciliate secondary metabolites have not yet been carried out, it is quite clear from the isolated metabolites that marine ciliates have a unique enzymatic system able to deviate from the usual course of the linear terpenoid precursors. Typically, sesquiterpene synthases (cyclases) catalyze the cyclization of FPP into any one of the 300 known hydrocarbon skeletons. This involves ionization of the FPP pyrophosphate leaving group, followed by the generation of a reactive allylic carbocation and subsequent C-C bond formation (along with alkyl shifts), leading to a terminal carbocation that is finally quenched by a base [77]. The analysis of the sesquiterpenoid skeleta elucidated from marine ciliates so far strongly suggests that the methyl groups of FPP are oxidized in the early steps of the biosynthetic route affording the novel linear precursors. Thereafter, the corresponding aldehyde carbonyl groups are extensively used as electrophilic centers in subsequent reactions. Thus, in the biosynthetic proposals herein, the oxidations which occur in the early steps may predispose the linear precursors to follow a particular path towards the novel end-products observed. The challenges for the future will be to substantiate this view with experimental findings, to isolate and characterize the terpene cyclases involved and to exploit the great advancements of molecular biologists towards mapping of the marine ciliate genome. The powerful tools of molecular biology coupled with recent advances in analytical chemistry will enable future investigation into terpene production from marine ciliates “manipulated” by genetic knockouts or by the use of low-molecular-weight elicitors.
Finally, it is often reported that secondary metabolite production could be mediated by prokaryotic (bacteria) endosymbionts harboured by eukaryotic microorganisms. Actually, ciliate cells represent a suitable niche for endosymbiotic colonizers, mainly of prokaryotic nature. Furthermore, specific bacterial endosymbionts characterize several Euplotes taxa and are known to infect all the members of the comprised populations. Treatment with antibiotic drugs (e.g., penicillin) has demonstrated that specific features of the eukaryotic guests are bestowed on the prokaryotic hosts. Euplotes vannus intra-population variability allowed us to isolate both the significantly infected strain TB6 and the endosymbiont-free strain Male5 [54]. According to the phylogenetic analysis based on 18S rRNA, ITS1, ITS2 (nuclear), and 16S rRNA (mitochondrial) gene sequences, Male5 has been demonstrated to be genetically identical to the TB6 strain. The HPLC-MS analysis of an organic extract obtained from a small-scale cell culture of this strain (E. vannus, Male5) clearly indicated the presence of the same major secondary metabolite (hemivannusal (22)) in almost the same amount as that produced by the E. vannus TB6 infected strain. This result strongly suggests that 22, but reasonably also all the metabolites so far isolated from marine ciliates belonging to the genus Euplotes, are “true ciliate metabolites” rather than products of the harbored prokaryotic endosymbionts.
References
- 1.Finlay BJM, Fenchel T. Divergent perspective on Protist species richness. Protist. 1999;150:229–233. doi: 10.1016/S1434-4610(99)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Finlay BJM, Corliss JO, Esteban GF, Fenchel T. Biodiversity at the microbial level: the number of free-living ciliates in the biosphere. Quart. Rev. Biol. 1996;72:221–237. [Google Scholar]
- 3.Lee JJ, Leedale GF, Bradbury P. The Illustrated Guide to the Protozoa. 2nd ed. Wiley-Blackwell; Lawrence, KS, USA: 2000. pp. 1–1475. [Google Scholar]
- 4.Nanney DL. Experimental Ciliatology: An Introduction to Genetic and Developmental Analysis in Ciliates. John Wiley & Sons; New York, NY, USA: 1980. pp. 1–230. [Google Scholar]
- 5.Dini F, Nyberg D. Sex in ciliates. In: Gwynfryn JJ, editor. Advances in Microbial Ecology. Vol. 13. Plenum Press; New York, NY, USA: 1993. pp. 85–153. [Google Scholar]
- 6.Petroni G, Rosati G, Vannini C, Modeo L, Dini F, Verni F. In situ identification by fluorescently labeled oligonucleotide probes of morphologically similar, closely related ciliate species. Microb. Ecol. 2003;45:156–162. doi: 10.1007/s00248-002-2016-x. [DOI] [PubMed] [Google Scholar]
- 7.Guella G. Terpenoids from marine ciliates: structure, biological activity, ecological role and phylogenetic relationship. Invited Lecture to 3rd European Conference on Marine Natural Products; Munich, Germany. September 2002. [Google Scholar]
- 8.Luporini P, Alimenti C, Ortenzi C, Vallesi A. Ciliate mating types and their specific protein pheromones. Acta Protozool. 2005;44:89–101. and references therein. [Google Scholar]
- 9.Tao N, Orlando M, Hyon J, Gross M, Song P. A new photoreceptor molecule from Stentor coeruleus. J. Am. Chem. Soc. 1993;115:2526–2528. [Google Scholar]
- 10.Checcucci G, Shoemaker RS, Bini E, Cerny R, Tao N, Hyon J, Gioffre D, Ghetti F, Lenci F, Song P. Chemical structure of blepharismin, the photosensor pigment for Blepharisma japonicum. J. Am. Chem. Soc. 1997;119:5762–5763. [Google Scholar]
- 11.Maeda M, Naoki H, Matsuoka T, Kato Y, Kotsuki H, Utsumi K, Tanaka T. Blepharismin 1–5, novel photoreceptor from the unicellular organism Blepharisma japonicum. Tetrahedron Lett. 1997;38:7411–7414. [Google Scholar]
- 12.Höfle G, Pohlan S, Uhlig G, Kabbe K, Schumacher D. Keronopsins A and B, chemical defense substances of the marine ciliate Pseudokeronopsis rubra (Protozoa): identification by Ex vivo HPLC. Angew. Chem. Int. Ed. Engl. 1994;33:1495–1497. [Google Scholar]
- 13.Guella G, Frassanito R, Mancini I, Sandron T, Modeo L, Verni F, Dini F, Petroni G. Keronopsamides, a new class of pigments from marine ciliates. Eur. J. Org. Chem. 2010;3:427–434. [Google Scholar]
- 14.Masaki ME, Harumoto T, Terazima MN, Miyake A, Usuki Y, Iio H. Climacostol, a defense toxin of the heterotrich ciliate Climacostomum virens against predators. Tetrahedron Lett. 1999;40:8227–8229. [Google Scholar]
- 15.Abe I, Rohmer M. Enyzmatic cyclization of 2,3-dihydrosqualene into euph-7-ene by a cell-free system from the protozoon Tetrahymena pyriformis. J. Chem. Soc., Chem. Commun. 1991;13:902–903. [Google Scholar]
- 16.Foissner W, Berger H, Schaumburg J. Identification and Ecology of limnetic plankton ciliates. Bavarian State Office for Water Management; Munich, Germany: 1999. pp. 1–793. [Google Scholar]
- 17.Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 18.Petroni G, Dini F, Verni F, Rosati G. A molecular approach to the tangled intrageneric relationships underlying phylogeny in Euplotes (Ciliophora, Spirotrichea) Mol. Phylogenet. Evol. 2002;22:118–130. doi: 10.1006/mpev.2001.1030. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;4:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. 1999;41:95–98. [Google Scholar]
- 21.Van de Peer Y, Baldauf SL, Doodlittle WF, Meyer A. An updated and comprehensive rRNA phylogeny of (Crown) eukaryotes based on rate-calibrated evolutionary distances. J. Mol. Evol. 2000;51:565–576. doi: 10.1007/s002390010120. [DOI] [PubMed] [Google Scholar]
- 22.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sinauer Associates; Sunderland, MA, USA: 1998. pp. 1–140. [Google Scholar]
- 23.Posada D, Crandall KA. MODELTEST: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 25.Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Ann. Rev. Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- 26.Dini F, Guella G, Giubbilini P, Mancini I, Pietra F. Control of interspecific relationships in marine ciliate protists by most evolved natural-products. Naturwissenschaften. 1993;80:84–86. [Google Scholar]
- 27.Guella G, Dini F, Tomei A, Pietra F. Preuplotin, a putative biogenetic precursor of the euplotins, bioactive sesquiterpenoids of the marine ciliated protist Euplotes crassus. J. Chem. Soc., Perkin Trans. I. 1994;2:161–166. [Google Scholar]
- 28.Nakatsu T, Ravi BN, Faulkner DJ. Antimicrobial constituents of Udotea flabellum. J. Org. Chem. 1981;46:2435–2438. [Google Scholar]
- 29.Guella G, Dini F, Pietra F. Hydrolytic breakdown of the euplotins, highly strained, adaptive, hemiacetal esters of the marine ciliate Euplotes crassus: A mimic of degradative pathways in nature and a trick for the assignment of the absolute configuration. Helv Chim. Acta. 1996;79:710–717. [Google Scholar]
- 30.Dale JA, Mosher HS. Nuclear magnetic resonance enantiomer reagents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973;95:512–519. [Google Scholar]
- 31.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]
- 32.Tascioglu S. Micellar solutions as reaction media. Tetrahedron. 1996;52:11113–11152. [Google Scholar]
- 33.Aungst RA, Jr, Funk RL. Synthesis of (Z)-2-acyl-2-enals via retrocycloadditions of 5-acyl-4-alkyl-4H-1,3-dioxins: Application in the total synthesis of the cytotoxin (±)-euplotin A. J. Am. Chem. Soc. 2001;123:9455–9456. doi: 10.1021/ja011470b. [DOI] [PubMed] [Google Scholar]
- 34.Guella G, Callone E, Mancini I, Uccello-Barretta G, Balzano F, Dini F. Chemical and structural properties of the inclusion complex of euplotin C with heptakis(2,6-di-O-methyl)-beta-cyclodextrin through NMR spectroscopy, electrospray mass spectrometry and molecular mechanics investigations. Eur. J. Org. Chem. 2004;6:1308–1317. [Google Scholar]
- 35.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA. 2000;97:13172–13177. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapp SC, Croteau RB. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics. 2001;158:811–832. doi: 10.1093/genetics/158.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter-Franklin JN, Butler A. Vanadium bromoperoxidase-catalyzed biosynthesis of halogenated marine natural products. J. Am. Chem. Soc. 2004;126:15060–15066. doi: 10.1021/ja047925p. [DOI] [PubMed] [Google Scholar]
- 39.Dini F. On the evolutionary significance of autogamy in the marine Euplotes (Ciliophora: Hypotrichida) Am. Nat. 1984;123:151–162. [Google Scholar]
- 40.Deitmer JW, Machemer H. Osmotic tolerance of Ca-dependent excitability in the marine ciliate Paramecium calkinsi. J. Exp. Biol. 1982;97:311–324. [Google Scholar]
- 41.Savoia D, Avanzini C, Allice T, Callone E, Guella G, Dini F. Antimicrobial activity of euplotin C, the sesquiterpene taxonomic marker from the marine ciliate Euplotes crassus. Antimicrob. Agents Chemoth. 2004;48:3828–3833. doi: 10.1128/AAC.48.10.3828-3833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramoino P, Usai C, Maccione S, Beltrame F, Diaspro A, Fato M, Guella G, Dini F. Effect of the bioactive metabolite euplotin C on phagocytosis and fluid-phase endocytosis in the single-celled eukaryote Paramecium. Aquat. Toxicol. 2007;85:67–75. doi: 10.1016/j.aquatox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Ramoino P, Dini F, Bianchini P, Diaspro A, Guella G, Usai C. Biophysical effects of the natural product euplotin C on the Paramecium membrane. J. Comp. Physiol. A. 2009;195:1061–1069. doi: 10.1007/s00359-009-0479-7. [DOI] [PubMed] [Google Scholar]
- 44.Cervia D, Martini D, Garcia-Gil M, Di Giuseppe G, Guella G, Dini F, Bagnoli P. Cytotoxic effects and apoptotic signalling mechanisms of the sesquiterpenoid euplotin C, a secondary metabolite of the marine ciliate Euplotes crassus, in tumor cells. Apoptosis. 2006;11:829–843. doi: 10.1007/s10495-006-5700-3. [DOI] [PubMed] [Google Scholar]
- 45.Cervia D, Garcia-Gil M, Simonetti E, Di Giuseppe G, Guella G, Bagnoli P, Dini F. Molecular mechanisms of euplotin C-induced apoptosis: Involvement of mitochondrial dysfunction, oxidative stress and proteases. Apoptosis. 2007;12:1349–1363. doi: 10.1007/s10495-007-0075-7. [DOI] [PubMed] [Google Scholar]
- 46.Trielli F, Cervia D, Di Giuseppe G, Ristori C, Kruppel T, Burlando B, Guella G, Viarengo A, Bagnoli P, Corrado MU, Dini F. Action mechanisms of the secondary metabolite euplotin C: signaling and functional role in Euplotes. J. Eukaryot. Microbiol. 2008;55:365–373. doi: 10.1111/j.1550-7408.2008.00335.x. [DOI] [PubMed] [Google Scholar]
- 47.Guella G, Dini F, Erra F, Pietra F. Raikovenal, a new sesquiterpenoid favoring adaptive radiation of the marine ciliate Euplotes raikovi, and its putative biogenetic precursor, preraikovenal. J. Chem. Soc., Chem. Comm. 1994;22:2585–2586. [Google Scholar]
- 48.Guella G, Dini F, Pietra F. From epiraikovenal, an instrumental niche-exploitation sesquiterpenoid of some strains of the marine ciliated protist Euplotes raikovi, to an unusual intramolecular tele-dienone-olefin [2+2] photocycloaddition. Helv. Chim. Acta. 1995;78:1747–1754. [Google Scholar]
- 49.Guella G, Pietra F. Photochemical conversion of the xenicane into crenulatane skeleton with diterpenoids of the brown seaweed Dictyota sp. of the coasts of Senegal. J. Chem. Soc., Chem. Commun. 1993;20:1539–1539. [Google Scholar]
- 50.Guella G, Chiasera G, Ndiaye I, Pietra F. Xenicane diterpenes revisited - Thermal (E)→(Z) isomerization and conformational motions - A unifying picture. Helv. Chim. Acta. 1994;77:1203–1221. [Google Scholar]
- 51.Snider BB, Lu Q. Syntheses of (±)-raikovenal, (±)-preraikovenal, and (±)-epiraikovenal. Synth. Commun. 1997;27:1583–1600. [Google Scholar]
- 52.Rosini G, Gonfalonieri G, Marotta E, Rama F, Righi P. Preparation of bicyclo[3.2.0]hept-3-en-6-ones: 1,4-dimethylbicyclo[3.2.0]hept-3-en-6-one (Bicyclo[3.2.0]hept-3-en-6-one, 1,4-dimethyl-, cis-(±)-) Org. Synth. 1997;74:158–166. [Google Scholar]
- 53.Guella G, Dini F, Pietra F. Metabolites with a novel C30-backbone from marine ciliates. Angew. Chem. Int. Ed. 1999;38:1134–1136. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1134::AID-ANIE1134>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 54.Guella G, Callone E, Di Giuseppe G, Frassanito R, Frontini F, Mancini I, Dini F. Hemivannusal and prevannusadials, new sesquiterpenoids from the marine ciliate protist, Euplotes vannus, the putative biogenetic precursors of vannusals dimeric terpenoids. Eur. J. Org. Chem. 2007;31:5226–5234. [Google Scholar]
- 55.Nicolaou KC, Ortiz A, Zhang H. The true structures of the vannusals, part 2: Total synthesis and revised structure of vannusal b. Angew. Chem. Int. Ed. 2009;48:5648–5652. doi: 10.1002/anie.200902029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicolaou KC, Zhang H, Ortiz A. The true structures of the vannusals, part 1: Initial forays into suspected structures and intelligence gathering. Angew. Chem. Int. Ed. 2009;48:5642–5647. doi: 10.1002/anie.200902028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicolaou KC, Zhang H, Ortiz A, Dagneau P. Total synthesis of the originally assigned structure of vannusal B. Angew. Chem. Int. Ed. 2008;47:8605–8610. doi: 10.1002/anie.200804228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicolaou KC, Tang W, Dagneau P, Faraoni R. A catalytic asymmetric three-component 1,4-addition/aldol reaction: Enantioselective synthesis of the spirocyclic system of vannusal A. Angew. Chem. Int. Ed. 2005;44:3874–3879. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]
- 59.Nicolaou KC, Jennings MP, Dagneau P. An expedient entry into the fused polycyclic skeleton of vannusal A. Chem. Commun. 2002;8:2480–2481. [Google Scholar]
- 60.Nicolaou KC, Ortiz A, Zhang H, Dagneau P, Lanver A, Jennings MP, Arseniyadis S, Faraoni R, Lizos DE. Total synthesis and structural revision of vannusal A and B: synthesis of the originally assigned structure of vannusal B. J. Am. Chem. Soc. 2010;132:7138–7152. doi: 10.1021/ja100740t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicolaou KC, Ortiz A, Zhang H, Guella G. Total synthesis and structural revision of vannusal A and B: synthesis of the true structures of vannusal B. J. Am. Chem. Soc. 2010;132:7153–7176. doi: 10.1021/ja100742b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paul VJ, Fenical W. Bioactive terpenoids from caribbean marine-algae of the genera Penicillus and Udotea (Chlorophyta) Tetrahedron. 1984;40:2913–2918. [Google Scholar]
- 63.Fattorusso E, Magno S, Mayol L, Novellino F. Petiodial, a new monocyclic diterpenoid from the mediterranean green-alga Udotea-petiolata. Experientia. 1983;39:1275–1276. [Google Scholar]
- 64.Snider BB. In: Comprehensive Organic Synthesis. Fleming I, editor. Vol. 2. Pergamon; Oxford, UK: 1991. p. 527. [Google Scholar]
- 65.Guella G, Dini F, Pietra F. Rarisetenolide, epoxyrarisetenolide, and epirarisetenolide, new-skeleton sesquiterpene lactones as taxonomic markers and defensive agents of the marine ciliated morphospecies Euplotes rariseta. Helv. Chim. Acta. 1996;79:2180–2189. [Google Scholar]
- 66.Guella G, Dini F, Pietra F. Epoxyfocardin and its putative biogenetic precursor, focardin, bioactive, new-skeleton diterpenoids of the marine ciliate Euplotes focardii from Antarctica. Helv. Chim. Acta. 1996;79:439–448. [Google Scholar]
- 67.Pietra F. Evolution of the secondary metabolite versus evolution of the species. Pure Appl. Chem. 2002;74:2207–2211. [Google Scholar]
- 68.Paul VJ, Puglisi MP, Ritson-Williams R. Marine chemical ecology. Nat. Prod. Rep. 2006;23:153–180. doi: 10.1039/b404735b. [DOI] [PubMed] [Google Scholar]
- 69.Jung V, Pohnert W. Rapid wound-activated transformation of the green algal defensive metabolites caulerpenyne. Tetrahedron. 2001;57:7169–7172. [Google Scholar]
- 70.Gavagnin M, Fontana A. Diterpenes from maribe opisthobranch mollusks. Curr. Org. Chem. 2000;4:1201–1248. [Google Scholar]
- 71.Fischbach MA, Clardy J. One pathway, many products. Nature Chem. Biol. 2007;3:353–355. doi: 10.1038/nchembio0707-353. [DOI] [PubMed] [Google Scholar]
- 72.Stone MJ, Williams DH. On the evolution of functional secondary metabolites (natural products) Mol. Microbiol. 1992;6:29–34. doi: 10.1111/j.1365-2958.1992.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 73.Williams DH, Stone MJ, Hauck PR, Rahman SK. Why are secondary metabolites (natural products) biosynthesized? J. Nat. Prod. 1989;52:1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]
- 74.Firn RD, Jones CG. The evolution of secondary metabolism-a unifying model. Mol. Microbiol. 2000;37:989–994. doi: 10.1046/j.1365-2958.2000.02098.x. [DOI] [PubMed] [Google Scholar]
- 75.Firn RD, Jones CG. Natural products–a simple model to explain chemical diversity. Nat. Prod. Rep. 2003;20:382–391. doi: 10.1039/b208815k. [DOI] [PubMed] [Google Scholar]
- 76.Lesburg CA, Caruthers JM, Paschall CM, Christianson DW. Managing and manipulating carbocations in biology: Terpenoid cyclase structure and mechanism. Curr. Opin. Struct. Biol. 1998;8:695–703. doi: 10.1016/s0959-440x(98)80088-2. [DOI] [PubMed] [Google Scholar]
- 77.Davis EM, Croteau R. Biosynthesis Aromatic Polyketides Isoprenoids Alkaloids. Vol. 209. Springer; Berlin, Germany: 2000. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes; pp. 53–95. [Google Scholar]