Abstract
Gastroparesis, or chronic delayed gastric emptying without mechanical obstruction, affects about 40% of patients with type 1 diabetes and up to 30% of patients with type 2 diabetes. Diabetic gastroparesis (DGP) typically causes nausea, vomiting, early satiety, bloating, and postprandial fullness. These symptoms can be extremely troubling and result in poor quality of life. The diagnosis of DGP is made by documenting the presence of chronic upper gastrointestinal (GI) symptoms, ruling out mechanical obstruction, and demonstrating delayed gastric emptying. The usual treatment for DGP includes dietary modifications, prokinetic agents, and antiemetic agents. Although the majority of patients have mild-to-moderate disease that can be managed using these measures, a substantial percentage of patients have severe DGP that is characterized by inadequate oral intake, malnutrition, weight loss, and frequent hospitalizations. Optimal management of these patients presents a difficult challenge for the clinician, although emerging treatment options, such as gastric neurostimulation, are encouraging. Patients with DGP often present with gastric comorbidities, including gastroesophageal reflux disease, intestinal dysmotility, and fungal and bacterial infections of the GI tract. This monograph will present an overview of the pathophysiology of DGP, review diagnostic testing with a discussion of emerging technology, and present the latest research in treatment options for DGP. In addition, management strategies for refractory DGP and gastric comorbidities will be described.
The Pathophysiology of Diabetic Gastroparesis
Gastroparesis is a chronic, symptomatic disorder of the stomach that is characterized by delayed gastric emptying in the absence of mechanical obstruction. The 3 most common etiologies are diabetes mellitus, idiopathic, and postsurgical.1 Other causes include medication, Parkinson's disease, collagen vascular disorders, thyroid dysfunction, liver disease, chronic renal insufficiency, and intestinal pseudo-obstruction.1 The prevalence of diabetic gastroparesis (DGP) appears to be higher in women than in men, for unknown reasons.2
Diabetic gastroparesis affects about 40% of patients with type 1 diabetes and up to 30% of patients with type 2 diabetes, especially those with long-standing disease.3,4 Both symptomatic and asymptomatic DGP seem to be associated with poor glycemic control by causing a mismatch between the action of insulin (or an oral hypogly-cemic drug) and the absorption of nutrients.5 Diabetic gastroparesis does not appear to be associated with an increased risk of death, however.6
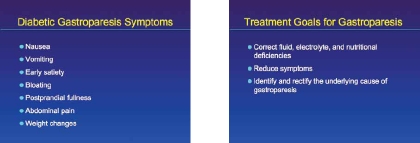
The symptoms associated with DGP often include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight changes.7 In a study by Soykan and colleagues of 146 patients with gastroparesis of various etiologies, nausea was present in 92% of the patients, vomiting in 84%, abdominal bloating in 75%, early satiety in 60%, and abdominal pain in 46%.8 Constipation may also be associated with gastroparesis. It should be noted that the severity of DGP symptoms does not always correlate with the rate of gastric empty-ing.2 Complications of gastroparesis include esophagitis, Mallory-Weiss tear from chronic nausea and vomiting, malnutrition, volume depletion with acute renal failure, electrolyte disturbances, and bezoar formation.9,10
A patient-based instrument called the gastroparesis cardinal symptom index (GCSI) has been developed to assess the severity of gastroparesis (Table 1).11 The GCSI index is based on 3 symptom subscales of a larger upper gastrointestinal disorders–symptom severity index that was previously developed. These 3 scales were selected as part of the GCSI because they assess common symptoms related to gastroparesis—nausea/vomiting, postprandial fullness/early satiety, and bloating. The GCSI is used to rate symptom change by either the physician or the patient over a 2-week recall period. In 2009, the American Neurogastroenterology and Motility Society (ANMS) published a daily diary version of the GCSI that has been validated for use in clinical trials.12 Such a daily diary can aid patients who may have difficulty with remembering symptoms over a 2-week recall period.
Table 1.
Gastroparesis Cardinal Symptom Index (GCSI): 9 Symptoms of Gastroparesis Are Graded by the Patient According to Their Severity Over the Prior 2 Weeks
| None | Very Mild | Mild | Moderate | Severe | Very Severe | |
|---|---|---|---|---|---|---|
| 1. Nausea (feeling sick to your stomach as if you were going to vomit or throw up) | 0 | 1 | 2 | 3 | 4 | 5 |
| 2. Retching (heaving as if to vomit, but nothing comes up) | 0 | 1 | 2 | 3 | 4 | 5 |
| 3. Vomiting | 0 | 1 | 2 | 3 | 4 | 5 |
| 4. Stomach fullness | 0 | 1 | 2 | 3 | 4 | 5 |
| 5. Not able to finish a normal-sized meal | 0 | 1 | 2 | 3 | 4 | 5 |
| 6. Feeling excessively full after meals | 0 | 1 | 2 | 3 | 4 | 5 |
| 7. Loss of appetite | 0 | 1 | 2 | 3 | 4 | 5 |
| 8. Bloating (feeling like you need to loosen your clothes) | 0 | 1 | 2 | 3 | 4 | 5 |
| 9. Stomach or belly visibly larger | 0 | 1 | 2 | 3 | 4 | 5 |
Reprinted with permission from Revicki DA et al.11 ©2002 Johnson & Johnson Pharmaceutical Services, LLC, Beerse, Belgium.
Diagnostic Testing
Diabetic gastroparesis is diagnosed by the presence of upper GI symptoms suggestive of delayed gastric emptying in patients with diabetes, exclusion of mechanical obstruction that could cause upper GI symptoms, and demonstration of delayed gastric emptying. Abdominal radiography, computed tomography, and magnetic resonance imaging can be used to exclude gastric and intestinal obstruction, and an upper endoscopy is needed to exclude a stricture, mass, or ulcer. Laboratory testing can be used to rule out infectious, metabolic, and immunologic causes of upper GI symptoms. Once mechanical obstruction is excluded, DGP is then diagnosed by demonstrating delayed gastric emptying via gastric emptying scintigraphy (GES), a wireless motility capsule, or the stable isotope breath test (Table 2).
Table 2.
Diagnostic Options for Diabetic Gastroparesis
|
Gastric emptying scintigraphy of a radiolabeled solid meal is used for the diagnosis of gastroparesis because it is noninvasive and it quantifies the emptying of a physiologic caloric meal.13 One drawback of this test is its radiation exposure, which is equivalent to about one-third of the average annual radiation exposure in the United States from natural sources. Historically, GES has been plagued by a lack of standardization, such as the use of different kinds of meals, variations in patient positioning, and the frequency and duration of imaging. This lack of standardization has resulted in difficulties in interpreting study results across institutions, meaning that patients often had to undergo repeat testing using a different protocol when consulting with a different gastroenterologist. This repeat testing can have a logistical and financial impact on both patients and health insurance providers.
More recent guidelines from the Society of Nuclear Medicine and the ANMS have attempted to standardize the protocol used for GES.14 These consensus guidelines recommended a 99mTc-sulfur colloid radiolabeled low-fat, egg-white meal. It is important that a solid meal be administered because liquid emptying may remain normal despite advanced disease. Medications that alter gastric emptying should be discontinued 48 to 72 hours in advance. Furthermore, blood glucose levels in patients with diabetes should be <275 mg/dL on the day of the test because marked hyperglycemia significantly delays gastric emptying.15 Scanning should be performed with the patient in an upright position at 1, 2, and 4 hours after the test meal in order to identify both rapid and slow gastric emptying.
Swallowed wireless motility capsule is a technology that is emerging as an alternative to GES. In this procedure, the patient swallows a wireless motility capsule that measures pH, pressure, and temperature using miniaturized wireless sensor technology. The time it takes for the pill to be expelled from the stomach into the duodenum is measured by monitoring the time point at which the acid readings of the stomach are replaced by the dramatic increase in pH as the capsule enters the duodenum. It has been shown that gastric transit time calculated using a wireless motility capsule correlates well with GES data. In a study by Kuo and colleagues, 87 healthy volunteers and 61 patients with gastroparesis underwent simultaneous use of a wireless motility capsule and GES.16 Images were obtained every 30 minutes for 4 hours. If after 4 hours, 90% of the meal had not emptied, then an additional image was taken at 6 hours. The diagnostic accuracy from the receiver operating characteristic curve between patients with gastroparesis and healthy volunteers was 0.83 for the wireless motility capsule and 0.82 for GES at 4 hours. The advantages to this method are the ambulatory nature of the test, lack of radiation exposure, and ability to measure motility of the entire GI tract. Current drawbacks include the cost of the capsule and a lack of widespread availability.
Another diagnostic option is the stable isotope breath test. In this protocol, 13C-labeled octanoate, a medium chain triglyceride, is bound to a solid meal. After ingestion and stomach emptying, 13C-octanoate is absorbed in the small intestine and metabolized to 13CO2, which is expelled from the lungs. The rate-limiting step for the signal appearing in the breath is the rate of gastric emptying. Viramontes and colleagues compared the stable isotope breath test with GES in 57 healthy volunteers and found good specificity and sensitivity.17 In that study, 33 volunteers received no treatment, 10 received erythromycin, and 14 received atropine. Patients consumed an egg labeled with both 13C-octanoate and 99mTc-sulfur colloid. Breath 13CO2 was measured every 15 minutes for 3 hours, and scintigraphy was performed every 15 to 30 minutes for 5 hours. Researchers found that the breath test detected abnormal gastric emptying with a sensitivity of 86% and specificity of 80%. The main drawbacks of this test are the need for normal small intestinal absorption, liver metabolism, and the need to assess pulmonary excretion to detect radioactivity.
Electrogastrography (EGG) is a useful adjunctive diagnostic test. It measures gastric slow-wave myoelectri-cal activity typically via cutaneous electrodes positioned along the long axis of the stomach. A preprandial recording is captured for approximately 45 to 60 minutes, then the patient is given a meal, followed by a 45-minute to 60-min-ute postprandial recording. Healthy controls produce EGG recordings that exhibit uniform waveforms of 3 cycles per minute, which increase in amplitude after ingestion of a meal. Electrogastrography abnormalities have been found to be present in 75% of patients with gastroparesis.18 One main drawback to ECG is the presence of movement artifacts that make recordings difficult to interpret. Overall, EGG, coupled with GES, wireless motility capsule, or the stable isotope breath test, allows for a more comprehensive evaluation and is particularly useful for patients with refractory symptoms.
Conclusion
Gastroparesis is characterized by delayed gastric emptying in the absence of mechanical obstruction. Diabetes is a common cause of gastroparesis. Diabetic gastroparesis has been associated with symptoms such as nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight changes. The severity of DGP symptoms does not always correlate with the rate of gastric emptying. After mechanical obstruction is excluded, DGP is diagnosed by demonstrating delayed gastric emptying via GES, wireless motility capsule, or the stable isotope breath test.
References
- 1.Waseem S, Moshiree B, Draganov PV. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 3.Lyrenås EB, Olsson EH, Arvidsson UC, et al. Prevalence and determinants of solid and liquid gastric emptying in unstable type I diabetes. Relationship to postprandial blood glucose concentrations. Diabetes Care. 1997;20:413–418. doi: 10.2337/diacare.20.3.413. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (noninsulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–159. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 5.Lyrenas E, Olsson E, Arvidsson U, et al. Prevalence and determinants of solid and liquid gastric emptying in unstable type 1 diabetes: relationship to postprandial blood glucose concentrations. Diabetes Care. 1997;20:413–418. doi: 10.2337/diacare.20.3.413. [DOI] [PubMed] [Google Scholar]
- 6.Kong MF, Horowitz M, Jones KL, et al. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503–507. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 7.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 8.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 9.Parkman HP, Schwartz SS. Esophagitis and gastroduodenal disorders associated with diabetic gastroparesis. Arch Intern Med. 1987;147:1477–1480. [PubMed] [Google Scholar]
- 10.Blam ME, Lichtenstein GR. A new endoscopic technique for the removal of gastric phytobezoars. Gastrointest Endosc. 2000;52:404–408. doi: 10.1067/mge.2000.108407. [DOI] [PubMed] [Google Scholar]
- 11.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833–844. doi: 10.1023/B:QURE.0000021689.86296.e4. [DOI] [PubMed] [Google Scholar]
- 12.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 13.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 14.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterol-ogy and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 15.Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- 16.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 17.Viramontes BE, Kim DY, Camilleri M, et al. Validation of a stable isotope gastric emptying test for normal, accelerated or delayed gastric emptying. Neurogastroenterol Motil. 2001;13:567–574. doi: 10.1046/j.1365-2982.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 18.Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;15:89–102. doi: 10.1046/j.1365-2982.2003.00396.x. [DOI] [PubMed] [Google Scholar]
Treatment Options for Diabetic Gastroparesis
The principal goals for the treatment of gastroparesis are to correct fluid, electrolyte, and nutritional deficiencies, to reduce symptoms, and to identify and rectify the underlying cause of gastroparesis, if possible. This latter goal should be emphasized in clinical practice. Prior to any intervention, the underlying mechanism that may exacerbate an already existing gastroparesis should be managed, and clinicians should be aware that certain medications might exacerbate gastric emptying abnormalities, including H2 receptor antagonists, proton pump inhibitors, antihistamines, opioids, tricyclic antidepressants, benzodiazepines, and calcium channel blockers.1 In the case of diabetic gastroparesis (DGP), optimum glycemic control should be achieved, and repeated fluctuations in glucose levels avoided because gastric emptying is extremely sensitive to changes in the serum glucose levels.2,3
All patients with gastroparesis should be evaluated by a dietitian for proper dietary modifications. An improper diet may exacerbate symptoms and lead to a variety of complications that could be easily avoided.4 In general, patients should shift from a primarily solid food diet to one based mostly on soft and liquid nutrition. Because indigestible fiber and roughage may increase the risk of bezoar formation for patients with DGP, consumption of vegetables or fruits rich in fiber should be minimized. In addition, fatty foods should be avoided because they increase gastric emptying time. Patients with gastroparesis should be encouraged to eat 4 to 6 small meals spread throughout the day instead of 3 large meals. Carbonated beverages should be avoided, except in patients who have developed a bezoar, in which case noncaloric carbonated beverages can be helpful in breaking the fiber and facilitate emptying of the bezoar.5 Alcohol and tobacco smoking, both of which decrease antral contractility, should be avoided as well.
Medical Treatment Options
The first-line medical therapy for patients with DGP is generally a combination of an antiemetic agent and a promotility drug (Table 1). Unfortunately, data from adequately powered clinical trials in patients with gastroparesis are limited, and no study has adequately stratified patients by etiologic subtypes. Thus, these drugs are used empirically.
Table 1.
Gastroparesis Treatment Guidelines
Primary Treatment: Dietary Manipulation and Administration of Antiemetic and Prokinetic Agents
|
For Refractory Gastroparesis
|
Data from Parkman HP et al and the American Gastroenterological Association.23
Antiemetics are helpful in relieving the DGP symptoms of nausea and vomiting, which are often the most disabling for patients and result in a substantial impact on quality of life.6 One class of antiemetics often used for managing these symptoms are the phenothiazines; these are dopamine-receptor antagonists that work at the level of the area postrema of the medulla oblongata. The most commonly prescribed phenothiazine agent for DGP is prochlorperazine, a neuroleptic with a potency 10 to 20 times that of chlorpromazine.7 Adverse effects include drowsiness, dry mouth, constipation, skin rashes, and tardive dyskinesia.
Other, less commonly used antiemetic agents include the antihistamines cyclizine and dimenhydrinate. Cyclizine is available as a tablet and also in injectable form, and dimenhydrinate is available as a tablet, liquid suspension, and suppository. The adverse effects include drowsiness, dry mouth, blurred vision, difficulty urinating, constipation, palpitations, dizziness, insomnia, and tremor. The serotonin receptor antagonists are occasionally used to treat DGP, although they are expensive and there are no data to support their use in this setting. They may be helpful when all other drugs have failed to provide symptom relief.
There are only a few prokinetic agents that are approved in the United States. Their expected effect includes increasing antral contractility, correcting gastric dysrhythmia, and improving antroduodenal coordination.
The only prokinetic agent that is approved in the United States specifically for DGP is metoclopramide, which is available in tablet, intravenous, and orally disintegrating tablet formulations. Metoclopramide is a 5-HT4 receptor agonist that releases acetylcholine from the myen-teric plexus. The net effect is to increase lower esophageal sphincter pressure and fundal tone, as well as increase the amplitude of antral contractions and facilitate antroduodenal coupling. Metoclopramide also has dopamine receptor antagonist properties and is a weak 5-HT3 receptor antagonist. Thus, metoclopramide acts both as a prokinetic agent and an antiemetic agent. Adverse events include drowsiness, fatigue, and lassitude, which occur in approximately 10% of patients receiving the most commonly prescribed dosage of 10 mg 3 times daily.8 Extrapyramidal symptoms, primarily acute dystonic reactions, can also occur. Such symptoms include involuntary movements of the limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of the tongue, bulbar type of speech, trismus, and dystonic reactions resembling tetanus.8 Other adverse events include both physical and mental restlessness, agitation, irritability, and an aggravation of underlying depression.8 In 2009, a black box warning was added for metoclopramide, against long-term (>3 months) or high-dose usage because of the risk of tardive dyskinesia.9 Due to these risks, it is critical that patients who are prescribed metoclopramide receive careful, scheduled follow-up.
The prokinetic agent domperidone is a benzimidazole derivative and is a specific dopamine antagonist with similar physiologic effects on the upper gastrointestinal tract as metoclopramide. Patterson and colleagues compared meto-clopramide with domperidone for the alleviation of DGP symptoms in 93 patients with insulin-dependent diabetes mellitus and found both agents to be equally effective.10 Several central nervous system–related adverse events were sig-nifcantly less common with domperidone. However, hyper-prolactinaemia, menstrual disturbance, breast engorgement, and galactorrhea can occur with domperidone because of its antidopaminergic effect. Unlike metoclopramide, domperi-done is not currently approved for use in the United States.
The macrolides, such as erythromycin and azithromycin, are antibiotics that also have motilin receptor agonist activity. Motilin is a hormone present in the endocrine cells of the distal stomach and duodenum. It increases lower esophageal sphincter pressure and is responsible for initiating the migrating motor complex (MMC). Erythromycin has been shown to increase the amplitude of antral peristalsis, trigger premature MMC phase III activity, and stimulate gastric emptying.11 Administration of erythromycin is generally short term because tolerance develops rapidly.12 Erythromycin is available as a tablet and liquid suspension, but it is more potent when administered intravenously. The most common adverse events include nausea, vomiting, and abdominal pain.
Management of Refractory DGP
Unfortunately, many patients with DGP will not experience adequate symptom relief despite education, dietary manipulation, and the use of prokinetic and antiemetic drugs. Thus far, there is no consensus on the proper treatment of patients with refractory disease. The therapeutic options available for these patients include combination prokinetic therapy, psychotropic medications, pyloric botulinum toxin injection, and gastric electric stimulation. Combination prokinetic therapy (eg, metoclopramide and erythromycin) would involve agents that act via different mechanisms to enhance gastric emptying; however, it should be noted that combination therapy has not been specifically studied for gastroparesis of any etiology. In a subset of patients with severe nonmedically responsive gastroparesis, feeding tubes or surgical interventions might be considered.
The use of a low-dose of tricyclic antidepressants is another option for complex patients. It is true that, in general, tricyclic antidepressants impair gastrointestinal motility through anticholinergic activity; however, there are limited data to suggest that low-dose therapy is effective in relieving chronic nausea and vomiting in patients with diabetes. Sawhney and colleagues conducted a retrospective study of 24 patients with diabetes who had been treated with tri-cyclic antidepressants specifically for nausea and vomiting after an unsatisfactory response to prokinetic therapy.13 Symptom patterns and treatment response were determined from chart review and telephone interview. According to the chart review (median antidepressant dosage, 50 mg/d), 88% of the 24 patients had at least moderate improvement in symptoms. In the follow-up interview, 77% of the patients reported at least moderate symptom improvement during therapy, and 68% rated tricyclic antidepressants the most effective treatment received.
The efficacy does not appear to differ between tricyclic preparations. The key to this treatment approach is to go “slow and low.” The starting dose is usually 10 mg, taken 2 hours before bedtime. If the patient tolerates this dose, it can be progressively increased up to 50 to 100 mg. The low-dose tricyclics are usually well-tolerated, although excessive sedation and dry mouth occasionally limit its use. If these events occur, it may be helpful to switch to nortriptyline or desipramine, which are thought to have a lower incidence of adverse events.14
Over the past few years, there has been substantial interest in pyloric injection of botulinum toxin A as a treatment option for gastroparesis. Botulinum toxin A is a bacterial toxin that inhibits acetylcholine release. Consequently, it was assumed that a botulinum toxin injection can relieve DGP-related pylorospasm. A 2005 chart review by Bromer and colleagues identified patients who underwent pyloric botulinum injection (100 or 200 U) for treatment of gas-troparesis and followed them over time to assess therapeutic response.15 The latter was defined as improvement or resolution of the patient's major symptom and/or 2 minor symptoms over a period of 4 weeks. The study subjects consisted of 53 women and 10 men with a mean age of 42 years. About half of the patients had idiopathic gastroparesis. A response was achieved by 43% of the 63 patients (mean duration, 2.0 months). There was no difference in durability of response, however, male sex predicted therapeutic response. A recent double-blind, placebo-controlled, crossover study found that botulinum toxin injection was not superior to saline placebo injection in 23 patients with gastroparesis, 19 of whom had idiopathic gastroparesis.16 Thus, botulinum toxin A injection is currently not commonly used in clinical practice and is considered if all other established treatment options have failed.
One option that has been recently approved in the United States is the placement of a paced gastric neurostim-ulator. This device generates a high-frequency (12 cycles per minute), low-energy, short-duration pulse. Electrical stimulation is delivered by 2 electrodes implanted laparoscopically or during laparotomy onto the serosal surface overlying the pacemaker area of the greater curve of the stomach. Leads from the electrodes connect to a neurostimulator, which resembles a cardiac pacemaker that is implanted in the anterior abdominal wall. A wireless remote control allows the settings to be adjusted from outside the body.17 In a landmark study by Abell and colleagues, the gastric neurostimulator was implanted into 33 patients with chronic gastroparesis (17 diabetic and 16 idiopathic).18 After implantation, patients were randomized in a double-blind, crossover design to stimulation ON or OFF for a 1-month period. After this period of time, all patients were programmed to stimulation ON and evaluated at 6 and 12 months. The outcome measures were vomiting frequency, preference for ON or OFF, upper gastrointestinal tract symptoms, quality of life, gastric emptying, and adverse events. The investigators found that during the double-blind portion of the study, self-reported vomiting frequency was significantly reduced in the ON versus OFF period (P<0.05). There was significant patient preference for the ON period versus the OFF period (P<0.05). In the unblinded portion of the study, vomiting frequency decreased significantly (P<0.05) at 6 and 12 months, and patient scores for symptom severity and quality of life significantly improved (P<0.05) at 6 and 12 months. In 5 patients, the gastric electrical stimulation system was explanted or revised because of infection or other complications. In other studies, gastric electrical stimulation is reported to enhance nutritional status, reduce the requirement for supplemental feeds, and improve glycemic control in patients with diabetes.18,19
For those refractory patients who have difficulty maintaining proper caloric intake, enteral feeding can be considered in those with dysmotility that is limited to the stomach. A first step might be a trial of slow, pump-driven nasogastric infusion, to assess whether a steady liquid drip feed is tolerated. A satisfactory response to a trial of nasogastric feeding may support direct feeding through a percutaneous endoscopically placed gastrostomy tube, which can be used for both liquid feeding and stomach venting.20 If the patient is intolerant of intragastric feeding, a trial of nasojejunal feeding should be carried out.21 If nasojejunal feeding is helpful, an endoscopic or surgically placed jejunostomy tube might be considered.22 In rare instances when enteral nutrition is poorly tolerated because of pain, bloating, or recurrent abdominal wall infection, long-term parenteral nutrition might be necessary.
Conclusion
In the clinical management of patients with gastroparesis, treatment should aim to rectify the underlying cause of gastroparesis. In patients with DGP, optimum glycemic control should be achieved. Necessary dietary modifications should be identified in consultation with a dietician. First-line medical therapy for these patients usually consists of an antiemetic agent and a promotility drug. For patients with refractory disease, options include combination prokinetic therapy, psychotropic medications, pyloric botulinum toxin injection, and gastric electric stimulation.
References
- 1.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterol-ogy and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 2.Fraser R, Horowitz M, Maddox A, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- 3.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 4.Parrish CR. Nutrition concerns for the patient with gastroparesis. Curr Gastroenterol Rep. 2007;9:295–302. doi: 10.1007/s11894-007-0033-0. [DOI] [PubMed] [Google Scholar]
- 5.Lee BJ, Park JJ, Chun HJ, et al. How good is cola for dissolution of gastric phytobezoars? World J Gastroenterol. 2009;15:2265–2269. doi: 10.3748/wjg.15.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielefeldt K, Raza N, Zickmund SL. Different faces of gastroparesis. World J Gastroenterol. 2009;15:6052–6060. doi: 10.3748/wjg.15.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray WD. An appraisal of a new antiemetic drug: prochlorperazine. Int Rec Med Gen Pract Clin. 1957;170:469–472. [PubMed] [Google Scholar]
- 8.Metoclopramide [package insert] Sellersville, PA: Teva Pharmaceuticals USA; 2005. [Google Scholar]
- 9.United States Food and Drug Administration. FDA requires boxed warning andrisk mitigation strategy for metoclopramide-containing drugs. [May 6, 2010]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149533.htm.
- 10.Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–1234. doi: 10.1111/j.1572-0241.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 11.Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol. 1993;88:203–207. [PubMed] [Google Scholar]
- 12.Frazee LA, Mauro LS. Erythromycin in the treatment of diabetic gastroparesis. Am J Ther. 1994;1:287–295. doi: 10.1097/00045391-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Sawhney MS, Prakash C, Lustman PJ, Clouse RE. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–424. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 14.Mertz H, Fass R, Kodner A, et al. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol. 1998;93:160–165. doi: 10.1111/j.1572-0241.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 15.Bromer MQ, Friedenberg F, Miller LS, Fisher RS, Swartz K, Parkman HP. Endoscopic pyloric injection of botulinum toxin A for the treatment of refractory gastroparesis. Gastrointest Endosc. 2005;61:833–839. doi: 10.1016/s0016-5107(05)00328-7. [DOI] [PubMed] [Google Scholar]
- 16.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251–1258. doi: 10.1111/j.1365-2036.2007.03467.x. [DOI] [PubMed] [Google Scholar]
- 17.de Csepel J, Goldfarb B, Shapsis A, Goff S, Gabriel N, Eng HM. Electrical stimulation for gastroparesis. Gastric motility restored. Surg Endosc. 2006;20:302–306. doi: 10.1007/s00464-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 18.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071–1076. doi: 10.2337/diacare.27.5.1071. [DOI] [PubMed] [Google Scholar]
- 20.Jones MP, Maganti K. A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol. 2003;98:2122–2129. doi: 10.1111/j.1572-0241.2003.07721.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacober SJ, Narayan A, Strodel WE, Vinik AI. Jejunostomy feeding in the management of gastroparesis diabeticorum. Diabetes Care. 1986;9:217–219. doi: 10.2337/diacare.9.2.217. [DOI] [PubMed] [Google Scholar]
- 22.Fontana RJ, Barnett JL. Jejunostomy tube placement in refractory diabetic gastroparesis: a retrospective review. Am J Gastroenterol. 1996;91:2174–2178. [PubMed] [Google Scholar]
- 23.Parkman HP, Hasler WL, Fisher RS, the American Gastroenterological Association American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589–1591. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
Challenges Associated With the Treatment of Diabetic Gastroparesis
Treatment of diabetic gastroparesis (DGP) is based on disease severity, which is assessed both by the patient's ability to preserve adequate nutrition and by his or her symptoms as reflected in the gastroparesis cardinal symptom index (GCSI) score, as discussed in Dr. Parkman's article. Mild DGP is managed by controlling weight and symptoms primarily through dietary adjustments to maintain glycemic control. Moderate DGP is characterized by moderate symptoms with more variable glycemic stability. It is managed through diet and lifestyle modifca-tions to maintain nutrition and stabilize glucose levels, as well as with pharmacologic agents. Hospitalization for the treatment of moderate DGP is rarely necessary. In severe decompensated DGP, symptoms respond poorly to medical therapy. Adequate oral intake can be hard to achieve, nutrition and weight difficult to maintain, and hospital visits may occur more frequently. Management of these patients can present a difficult challenge for the clinician.
Although a major part of the assessment of DGP dis-ease severity is based on the patient's gastrointestinal (GI) symptoms, in actuality, symptoms do not correlate well with the severity of delayed gastric emptying as seen by gastric emptying scintigraphy (GES).1 Because any part of the GI tract can be affected by diabetes, symptoms will depend on the amalgam of dysfunctional elements involved. While it is often assumed that the major cause of symptoms in DGP is autonomic dysfunction, symptoms can precede or may not correlate well with the presence of autonomic neuropathy.2 Hypoglycemia and hyperglycemia have a reversible effect on the metabolic and signaling pathways of enteric neurons and as a result can alter intestinal func-tion.3 The functional delay in gastric emptying that often occurs in the setting of significant hyperglycemia can be challenging to differentiate from autonomic neuropathy or motor dysfunction. Infection, noncompliance with diet, ischemia, hypoadrenal state, and noncompliance with treatments are other known contributing factors to exacerbations of gastroparesis requiring hospitalization.4
Upper GI symptoms are common in the general community. In a 2010 study, Kim and colleagues showed that, among 190 patients with diabetes and 190 healthy volunteers, upper GI symptoms were reported by 72% and 62% of patients, respectively.5 In fact, many diabetic patients with documented delayed gastric emptying are asymptomatic. In a clinical setting, the presence or absence of symptoms in a diabetic patient may be difficult to sort out and can impact decisions on how and when to treat.6 Failure to diferentiate DPG from alterations in gastric emptying that are not associated with autonomic disturbance could potentially lead to inappropriate treatment and ineffective diabetes management.
GI Comorbidities
One of the challenges of managing the patient with severe DGP is the frequent occurrence of GI comorbidities (Table 1). The effects of diabetes on a number of cellular targets is associated with the development of dysphagia and gastroesophageal reflux, intestinal pseudo-obstruction, slow transit constipation, diarrhea, and fecal incontinence.7 Esophageal sensorimotor dysfunction is common, with more than half of all patients with diabetes reporting symptoms of swallowing disorders or acid reflux.8 Dysphagia symptoms are typically difficult to treat, particularly in late-stage diabetes characterized by advanced motor neuropathy. Therefore, diagnosing dysphagia early when improved glycemic control can be initiated and when the condition is reversible may improve long-term clinical outcome.
Table 1.
Comorbidities of Diabetic Gastroparesis
|
Data from Folwaczny C et al.7
The use of oral hypoglycemic agents, increased body mass index, duration of disease, and variability of glucose control in DGP patients can influence the incidence of gastroesophageal reflux disease (GERD). GERD affects 14% of all Americans, yet the overall prevalence of GERD symptoms in diabetic patients is twice that (30%) of people without diabetes.9 Neuropathy appears to play a key role. Heartburn has been identified in 42% of patients with neuropathy compared to 24% of patients without neuropathy. Heartburn is a sensation likely to be blunted by neuropathy, so the actual incidence of GERD may be higher still. Esophageal dysfunction correlates with the presence of diabetic motor neuropathy as measured by motor nerve conduction velocity better than with autonomic neuropathy as measured by the R-R intervals on an electro-cardiogram.10 In patients with diabetic motor neuropathy and GERD, reduced amplitude of peristaltic waves, reduced rate of smooth muscle contractions, diminished peristaltic efficacy, and decreased lower esophageal sphincter tone and function are more common. Symptoms of GERD in patients with mild-to-moderate DGP can often be managed effectively with conventional agents and conservative anti-reflux measures (Table 2). Surgical measures are typically reserved for patients with refractory GERD with significantly compromised quality of life. Although the role of gastric emptying has not been clearly established, in the case of profound diabetic enteropathy, fundoplication may not effectively improve symptoms, in spite of the fact that this procedure has been shown to speed gastric emptying.11 Use of pyloroplasty or a gastric pacemaker may be necessary in severe gastroparesis.
Table 2.
Conservative Anti-reflux Measures in Diabetic Gastroparesis
|
Data from Folwaczny C et al.7
Candida esophagitis is also more common in patients with diabetes, particularly in those patients with poor glycemic control and in those with DGP.12 Impaired esophageal clearance because of poor motility and secretions high in glucose are factors that may contribute to the increased prevalence. Patients with both DGP and Candida esophagitis often present with persistent upper GI symptoms that do not improve with antiemetic and prokinetic therapy. Treatment involves improving glycemic control and using antifungal agents like fluconazole and promotil-ity agents to aid esophageal clearance. Agents that stimulate motilin receptors, like erythromycin, may be effective in the short term.13
Although upper GI symptoms of nausea, bloating, fullness, distension, and reflux are common in patients with DGP, chronic abdominal pain is not a hallmark symptom of the disease. Because acute pain is not typical of DGP, upper endoscopy is warranted in the evaluation of patients with acute pain or change in usual symptoms. Indeed, pain may signify a complication, such as gastric or duodenal ulcers, or an unrelated condition. Small intestinal bacterial overgrowth occurring in patients with DGP can present with a predominance of abdominal pain and bloating, especially in those patients with a longer duration of disease.14 Clinicians should keep this possibility in mind when a patient with longstanding DGP presents with new abdominal pain and bloating symptoms.
Treatments Under Investigation for DGP Symptoms
When abdominal pain is present, it can be disabling in patients with gastroparesis. Agents commonly used in clinical practice to treat abdominal pain include tricyclic antidepressants, gabapentin, or pregabalin, but these agents provide limited benefit in effectively managing the pain.15 Nerve blockade and alternative therapies have been used with some success. A case report of celiac plexus block with local anesthesia and steroid injections in which adequate analgesia was achieved and maintained for 10 weeks has recently been published.16 This approach allowed for elimination of opiates, avoidance of the narcotic-associated constipation, continuation of percutaneous endoscopic jejunostomy tube feedings, and avoidance of long-term enteral nutrition. In an open-label study using a gastric electrical stimulation device—electroacupuncture—provided significant symptom relief with decreased nausea and vomiting that persisted for the duration of the trial.17 In another study, 19 patients with type 2 diabetes who had symptoms of gastroparesis for more than 3 months were randomized to receive electroacu-puncture at the Zusanli (ST 36) and Hegu (LI 4) points or sham electroacupuncture repeated over 4 sessions in 2 weeks.18 GCSI scores and solid gastric half-emptying time on scintigraphy were measured at baseline, at the end of treatment, and 2 weeks post-treatment. The authors found that the average gastric half-emptying time was significantly shortened by 45 minutes with electroacupuncture treatment as compared with baseline (143.8 ± 55.9 minutes vs 98.8 ± 28.6 minutes; P<.03). Half-emptying time did not change in the sham group. The GCSI total score improved significantly over baseline both at the end of treatment and 2 weeks after the end of the trial in the electroacupuncture group, but it did not change from baseline with sham EA treatment. Larger trials of both celiac plexus blockade and electroacupuncture will be needed to draw definitive conclusions about their efficacy in treating DGP-related abdominal pain and symptoms.
Conclusion
Treatment options for DGP will vary according to the severity of the disease. For patients with DGP, the etiology of symptoms is often multifactorial, involving both reversible and irreversible processes. Mild-to-moderate cases often respond to diet and lifestyle adjustments and pharmaco-therapy, while patients with severe decompensated DGP respond poorly to treatment. Strict glycemic control early on may reduce progression of disease. Hospitalization may be required when gastroparesis is exacerbated by illness such as infection, systemic disorders, ischemia, and non-compliance with diet or treatments. Severe DGP may be associated with comorbidities such as development of esophageal motor dysfunction, GERD, pseudo-obstruction, slow transit constipation, diarrhea, and incontinence. New treatments such as celiac nerve block and electroacupunc-ture are being investigated, with larger well-designed trials needed to confirm effects.
References
- 1.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 2.Punkkinen J, Färkkilä M, Mätzke S, et al. Upper abdominal symptoms in patients with type 1 diabetes: unrelated to impairment in gastric emptying caused by autonomic neuropathy. Diabet Med. 2008;25:570–577. doi: 10.1111/j.1464-5491.2008.02428.x. [DOI] [PubMed] [Google Scholar]
- 3.Koch CA, Uwaifo GI. Are gastrointestinal symptoms related to diabetes mellitus and glycemic control? Eur J Gastroenterol Hepatol. 2008;20:822–825. doi: 10.1097/MEG.0b013e3282f5f75e. [DOI] [PubMed] [Google Scholar]
- 4.Uppalapati SS, Ramzan Z, Fisher RS, et al. Factors contributing to hospitalization for gastroparesis exacerbations. Dig Dis Sci. 2009;54:2404–2409. doi: 10.1007/s10620-009-0975-1. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Park HS, Ko SY, et al. Diabetic factors associated with gastrointestinal symptoms in patients with type 2 diabetes. World J Gastroenterol. 2010;16:1782–1787. doi: 10.3748/wjg.v16.i14.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samsom M, Bharucha A, Gerich JE, et al. Diabetes mellitus and gastric emptying: questions and issues in clinical practice. Diabetes Metab Res Rev. 2009;25:502–514. doi: 10.1002/dmrr.974. [DOI] [PubMed] [Google Scholar]
- 7.Folwaczny C, Riepl R, Tschöp M, Landgraf R. Gastrointestinal involvement in patients with diabetes mellitus: part I (first of two parts). Epidemiology, pathophysiology, clinical findings. Z Gastroenterol. 1999;37:803–815. [PubMed] [Google Scholar]
- 8.Hüppe D, Tegenthoff M, Faig J, et al. Esophageal dysfunction in diabetes mellitus: is there a relation to clinical manifestation of neuropathy? Clin Investig. 1992;70:740–747. doi: 10.1007/BF00180740. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Pitchumoni CS, Chandrarana K, Shah N. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol. 2008;14:709–712. doi: 10.3748/wjg.14.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinekawa F, Kubo F, Matsuda K, et al. Relationship between esophageal dysfunction and neuropathy in diabetic patients. Am J Gastroenterol. 2001;96:2026–2032. doi: 10.1111/j.1572-0241.2001.03862.x. [DOI] [PubMed] [Google Scholar]
- 11.Hinder RA, Stein HJ, Bremner CG, DeMeester TR. Relationship of a satisfactory outcome to normalization of delayed gastric emptying after Nissen fundoplication. Ann Surg. 1989;210:458–464. doi: 10.1097/00000658-198910000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkman HP, Schwartz SS. Esophagitis and gastroduodenal disorders associated with diabetic gastroparesis. Arch Intern Med. 1987;147:1477–1480. [PubMed] [Google Scholar]
- 13.Sellin JH, Chang EB. Therapy insight: gastrointestinal complications of dia-betes—pathophysiology and management. Nat Clin Pract Gastroenterol Hepatol. 2008;5:162–171. doi: 10.1038/ncpgasthep1054. [DOI] [PubMed] [Google Scholar]
- 14.Reddymasu SC, McCallum RW. Small intestinal bacterial overgrowth in gastroparesis: are there any predictors? J Clin Gastroenterol. 2010;44:e8–e13. doi: 10.1097/MCG.0b013e3181aec746. [DOI] [PubMed] [Google Scholar]
- 15.Reddymasu SC, McCallum RW. Pharmacotherapy of gastroparesis. Expert Opin Pharmacother. 2009;10:469–484. doi: 10.1517/14656560902722505. [DOI] [PubMed] [Google Scholar]
- 16.Wu DJY, Dib C, Hoelzer B, McMahon M, Mueller P. Coeliac plexus block in the management of chronic abdominal pain due to severe diabetic gastroparesis. BMJ Case Reports. 2009. doi:10.1136/bcr.06.2009.1986. [DOI] [PMC free article] [PubMed]
- 17.McCallum R, Brody FJ, Parkman HP, et al. 376 Enterra® gastric electrical stimulation for diabetic gastroparesis: results from a multicenter randomized study. Gastroenterology. 2009;136(suppl 1):A61–A62. [Google Scholar]
- 18.Wang CP, Kao CH, Chen WK, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Altern Complement Med. 2008;14:833–839. doi: 10.1089/acm.2008.0107. [DOI] [PubMed] [Google Scholar]
Question and Answer Forum
Q: It was mentioned that dietary changes are one of the primary means of treating DGP, particularly mild-to-moderate disease. How can patient adherence to dietary changes be improved?
Amy E. Foxx-Orenstein, DO (AF): We know that gly-cemic control is key to managing DGP. In addition, when patients experience flares in DGP symptoms, small meals of easily absorbed soft, pureed foods or liquids can help ease those symptoms and prevent exacerbations that may require hospitalization. Yet these kinds of dietary changes are difficult for patients to manage. They require lifestyle modifications. Patients may no longer be able to share mealtimes normally with their families, they may not be able to participate in social gatherings in the same way, and they may face awkward situations if their work requires them to attend functions that revolve around food or drink consumption. What patients need is intensive education so that they can understand why glycemic control and dietary changes are so important, and they should be given useful strategies for making these modifications in the real world. Patients with DGP will see their doctors on a regular basis to manage their diabetes; thus, there is always an opportunity for clinicians to better educate patients on how to manage their oral intake. I also recommend that patients visit a dietician on a regular basis so that they can address their oral intake and evaluate how it is affecting their symptoms.
Q: What are some common pitfalls to avoid when diagnosing and treating DGP?
Henry P. Parkman, MD (HP): Interpreting the results of a gastric emptying study can be a challenge in a patient with diabetes. One must always keep in mind that delayed gastric emptying might actually be related to transient hypergly-cemia and not to long-lasting underlying chronic illness. A good strategy is to test the patient glucose levels before the test to ensure that levels are within the normal range.
Ronnie Fass, MD (RF): Sometimes, the medications that we prescribe for motility disorders have a tendency to overtreat the problem. For example, erythromycin can cause gastric emptying to occur too rapidly, which can result in high glucose levels. High glucose levels can then cause delayed gastric emptying. There is a potential for a vicious cycle here.
AF: Sometimes in the course of diabetes, a patient will experience overly rapid stomach emptying. Interestingly, these patients often present with symptoms similar to those seen with DGP. Typically, the patient complains of bloating and abdominal discomfort, which are usually caused by rapid filling, nutrient shifts, and small bowel distension rather than gastric distention. These symptoms will likely become worse when the patient is given a prokinetic agent, and this reaction can be a clue to the underlying etiology.
Q: What treatments are on the horizon for diabetic gastroparesis?
HP: There are several areas that are currently being investigated. The increase in use of a swallowed wireless motility capsule has revealed that DGP is characterized not only by a delay in stomach emptying, but that there can also be a delay in small bowel transit and colon transit. Pinpointing which areas of the gastrointestinal tract have delays will be helpful in the future in order to better customize treatment for each patient.
AF: There is an older agent called pyridostigmine, which is a reversible cholinesterase inhibitor that can stimulate intestinal contraction. This agent may be helpful in patients who have intestinal dysmotility,2 and I would like to see some studies utilizing that therapy in DGP, in particular. As we better define the predisposing genetic and environmental causes of neural and motor dysfunction in diabetes, we should see more treatments coming forward.
Another very interesting area of research is the use of stem cells to improve contractility of the stomach. Studies performed by the National Institutes of Health Gastro-paresis Consortium have shown that there is a paucity of interstitial cells of Cajal in the stomach of patients with DGP.1 These cells are called the pacemaker cells of the stomach. There is substantial interest in trying to increase the number of interstitial cells and thereby increase contractility. However, this research is still very preliminary.
References
- 1.Harberson J, Thomas RM, Harbison SP, Parkman HP. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci. 2010;55:359–370. doi: 10.1007/s10620-009-1071-2. [DOI] [PubMed] [Google Scholar]
- 2.Pasha SF, Lunsford TN, Lennon VA. Autoimmune gastrointestinal dysmotility treated successfully with pyridostigmine. Gastroenterology. 2006;131:1592–1596. doi: 10.1053/j.gastro.2006.06.018. [DOI] [PubMed] [Google Scholar]
Biographies



Slide Library
For a free electronic download of these slides, please direct your browser to the following web address: http://www.clinicaladvances.com/index.php/our_publications/gastro_hep-issue/gh_june_2010/
Footnotes
Supported through an educational grant from Salix Pharmaceuticals, Inc.
Sponsored by Postgraduate Institute for Medicine.
Disclosure of Conflicts of Interest:Postgraduate Institute for Medicine (PIM) assesses conflict of interest with its instructors, planners, managers, and other individuals who are in a position to control the content of CME activities. All relevant conflicts of interest that are identified are thoroughly vetted by PIM for fair balance, scientific objectivity of studies utilized in this activity, and patient care recommendations. PIM is committed to providing its learners with high-quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
The faculty reported the following financial relationships or relationships to products or devices they or their spouse/life partner have with commercial interests related to the content of this CME activity:
Henry P. Parkman, MD—Consultant: SmartPill, Tranzyme; Contracted research: SmartPill, Medtronic
Ronnie Fass, MD—No real or apparent conflicts of interest to report.
Amy E. Foxx-Orenstein, DO—No real or apparent conflicts of interest to report.
The following PIM planners and managers, Jan Hixon, RN, BSN, MA, Trace Hutchison, PharmD, Julia Kimball, RN, BSN, Samantha Mattiucci, PharmD, Jan Schultz, RN, MSN, CCMEP, and Patricia Staples, MSN, NP-C, CCRN, hereby state that they or their spouse/life partner do not have any financial relationships or relationships to products or devices with any commercial interest related to the content of this activity of any amount during the past 12 months. Jacquelyn Matos: No real or apparent conflicts of interest.
Disclosure of Unlabeled Use:This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. Postgraduate Institute for Medicine (PIM), Gastroenterology & Hepatology, and Salix Pharmaceuticals do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of PIM, Gastro-Hep Communications, or Salix Pharmaceuticals. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer:Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient's conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
Funding for this Clinical Roundtable Monograph has been provided through an educational grant from Salix Pharmaceuticals, Inc. Support of this monograph does not imply the supporter's agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporter, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.
Contributor Information
Henry P. Parkman, Internal-General Medicine & Gastroenterology Temple Clinical Research Philadelphia, Pennsylvania.
Ronnie Fass, Professor of Medicine University of Arizona Tucson, Arizona.
Amy E. Foxx-Orenstein, Division of Gastroenterology & Hepatology Mayo Clinic Rochester, Minnesota.