Abstract
The synthesis of new substituted E-3-(3-indolylmethylene)1,3-dihydroindol-2-ones is reported. The antitumor activity was evaluated according to protocols available at the National Cancer Institute (NCI), Bethesda, MD. Structure-activity relationships are discussed. The action of selected compounds was investigated in MCF-7 breast cancer cells. The ability of these derivatives to inhibit cellular proliferation was accompanied by increased level of p53 and its transcriptional targets p21 and Bax, interference in the cell cycle progression with cell accumulation in the G2/M phase, and lastly activation of apoptosis.
Introduction
In the previous papers of this series2–6 we described several substituted E-3-(2-chloro-3- indolylmethylene)1,3-dihydroindol-2-ones prepared from an indolealdehyde and an oxindole. Compounds with potent cytotoxic activity were obtained when the aldehyde was 2-chloro-5- methoxy-6-methylindole-3-carbaldehyde,4 moreover we noticed6 that in some cases N-methylation increased the activity. Even preliminary attempts to introduce substituents at the positions 4, 5 and 6 of the oxindole portion had been performed.4
The rationale for the synthesis of new analogs described in this paper (Scheme 1, Table 1) is the following:
Scheme 1.
Table 1.
Compounds 4–37
| Comp | Formula | MW | Mp, °C | Comp | Formula | MW | Mp, °C |
|---|---|---|---|---|---|---|---|
| 4 | C19H14BrClN2O2 | 417.69 | 325–328 dec. | 21 | C20H18ClN3O2 | 367.83 | 200–205 dec. |
| 5 | C21H20ClN3O2 | 381.86 | 295–298 dec. | 22 | C21H21ClN4O | 380.87 | 340–343 dec. |
| 6 | C23H19ClN2O5 | 438.86 | 338–340 | 23 | C21H12Cl2N2O | 379.24 | 278–281 dec. |
| 7 | C19H14Cl2N2O2 | 373.23 | 285–290 dec. | 24 | C21H12Cl2N2O | 379.24 | 310–312 dec. |
| 8 | C22H21ClN2O3 | 396.87 | 284–286 dec. | 25 | C21H12ClFN2O | 362.79 | 330–332 dec. |
| 9 | C20H16BrClN2O2 | 431.71 | 290–292 dec. | 26 | C22H15ClN2O2 | 374.82 | 185–187 dec. |
| 10 | C22H22ClN3O2 | 395.88 | 230–233 dec. | 27 | C23H17ClN2O2 | 388.85 | 317–320 dec. |
| 11 | C24H21ClN2O5 | 452.89 | 340–345 dec. | 28 | C22H15ClN2O2 | 374.82 | 335–338 dec. |
| 12 | C20H16Cl2N2O2 | 387.26 | 250–253 dec. | 29 | C18H14N2O2 | 290.32 | 255–258 |
| 13 | C21H19ClN2O2 | 366.84 | 277–280 dec. | 30 | C19H16N2O2 | 304.34 | 288–290 |
| 14 | C21H19ClN2O3 | 382.84 | 348–350 dec. | 31 | C21H20N2O3 | 348.40 | 273–275 |
| 15 | C23H23ClN2O3 | 410.90 | 225–228 | 32 | C19H16N2O2 | 304.34 | 272–275 |
| 16 | C20H17ClN2O2 | 352.82 | 220–222 dec. | 33 | C20H18N2O2 | 318.37 | 277–280 |
| 17 | C19H15Cl2N3O | 372.25 | 335–340 dec. | 34 | C20H18N2O3 | 334.37 | 280–282 |
| 18 | C19H15Cl2N3O | 372.25 | 200–204 dec. | 36 | C20H16N2O4 | 348.35 | 175–180 |
| 19 | C19H16ClN3O2 | 353.80 | 345–350 dec. | 37 | C18H14N2O4 | 322.32 | 340–345 |
| 20 | C20H18ClN3O2 | 367.83 | 180–185 dec. |
Evaluation of the effects induced by different substituents in the oxindole portion (bromine, dimethylamino and succinyl group 4, 5 e 6), and by the position of chlorine (7): the compounds bearing chlorine at the position 4 or 5 were described in previous papers.5,6
Compound 8 was prepared to evaluate whether the methylation of the nitrogen in the chloroindole portion could lead to increase of activity as previously noticed.5,6 This kind of methylation was performed also in four compounds mentioned in the above group (4–7) and gave rise to the derivatives 9–12.
The synthesis of one compound bearing a methyl group at both the nitrogens and its activity on HeLa cells was described in the first paper of this series.2 Its cytotoxicity was weak but when it was tested according to the NCI protocols it showed mean GI50 1.26 μM. This prompted us to reconsider the possibility of bis-methylation and compounds 13–15 were prepared accordingly.
Compound 16 was prepared to evaluate the effect of shifting methyl and methoxy group from the chloroindole to the oxindole portion.
In the compounds so far described, the best results were obtained when the aldehyde was bearing a methoxy group at the 5 position and a methyl group at the 6 position. Compounds 17–22 were prepared to investigate if they could be replaced by different substituents and in particular with a group which can be easily protonated.
In the previous paper6 good results were obtained with an additional condensed benzene ring in the oxindole portion. Compounds 23–28 bear an additional condensed benzene ring but in the chloroaldehyde portion.
With the synthesis of compounds 29–34 we were planning to compare the activity of the 2-chloro with that of the unsubstituted derivatives.
The introduction of a hydrophilic and electron attracting group was considered in compounds 35 and 36.
Compound 37 does not belong to the same series considered so far but could be the first term of a new series (devoid of the methine bridge) if it should display significant antitumor activity.
Chemistry
Most of the compounds were prepared by means of the single step Knoevenagel reaction between the indolinones 1 and the aldehydes 2 in methanol/piperidine. For compounds 4, 6, 11, 24–26, 35 and 37 a mixture of acetic acid/hydrochloric acid has been employed.
Most compounds were obtained as almost pure geometrical isomers which, according to the usual NOE experiments described in the previous papers7,8 were assigned to the E configuration. Compounds 18 and 34 were obtained as evident E/Z mixtures (around 70/30) and submitted as such to the biological tests. The E/Z ratio in solution is time dependent and tends to be 50/50. In our experience with similar derivatives, we described the separations of the two isomers by fractional crystallization7 but no significant difference in the pharmacological behavior was noticed.
The oxindoles 1d, 1j, 1o, the aldehyde 2h and the isatin 3 are commercially available whereas the other starting compounds (oxindoles and aldehydes) have been prepared according to the literature,4, 6, 9–22 except 1h, 2d, 2e and 2j reported under the experimental section.
Biology
a. Cell-based assays
In a preliminary test, compounds were assayed at a single high concentration (10−5 M) in the full NCI 60 cell panel. This panel is organized into subpanels representing leukemia, melanoma and cancers of lung, colon, kidney, ovary, breast, prostate and central nervous system. Only compounds which satisfy pre-determined threshold inhibition criteria in a minimum number of cell lines progress to the full 5-concentration assay. The threshold inhibition criteria for progression to the 5-concentration screen was selected to efficiently capture compounds with anti-proliferative activity based on the analysis of historical DTP screening data. The result is expressed as the percent growth of treated cells at the test concentration of 10−5 M following 48 h incubation (unpublished results).
Compounds 5, 6, 11, 17, 22, 31, 35, 36 and 37 were not considered active enough to enter the 5- concentration test whereas all the others were subjected to this screen. The compounds were dissolved in dimethylsulphoxide (DMSO) and evaluated using five concentrations at ten-fold dilutions (the highest being 10−4 M) following 48 h incubation.
Table 2 reports the results obtained (vincristine is reported for comparison purposes), expressed as μM concentration at three assa y endpoints: the 50% growth inhibitory power (GI50), the cytostatic effect (TGI=Total Growth Inhibition) and the cytotoxic effect (LC50).
TABLE 2.
Nine subpanels at five concentrations: growth inhibition, cytostatic and cytotoxic activity (μM) of the selected compounds (see Supporting Information for the complete list of cell lines employed).
| Comp a | Modes | Leukemia | NSCLC | Colon | CNS | Melanoma | Ovarian | Renal | Prostate | Breast | MG-MIDb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | GI50 | 1.62 | 3.02 | 1.95 | 1.58 | 2.14 | 2.00 | 2.45 | 2.24 | 2.14 | 2.14 |
| TGI | 6.17 | 14.13 | 6.76 | 8.91 | 9.55 | 8.13 | 10.96 | 7.94 | 10.47 | 9.33 | |
| LC50 | 37.15 | 53.70 | 23.44 | 35.48 | 41.69 | 38.90 | 37.15 | 30.90 | 50.12 | 39.81 | |
| 7 c | GI50 | 0.14 | 0.43 | 0.17 | 0.12 | 0.23 | 0.28 | 0.23 | 0.22 | 0.15 | 0.21 |
| TGI | 4.79 | 11.48 | 3.55 | 4.37 | 11.48 | 14.79 | 7.08 | 5.25 | 2.63 | 6.61 | |
| LC50 | 58.88 | 52.48 | 25.70 | 41.69 | 35.48 | 48.98 | 46.77 | 41.69 | 39.81 | 41.69 | |
| 8 c | GI50 | 18.20 | 34.67 | 22.91 | 26.92 | 15.14 | 30.20 | 41.69 | 22.39 | 18.62 | 23.99 |
| TGI | 60.26 | 97.72 | 87.10 | 87.10 | 45.71 | 91.20 | - | 97.72 | 77.62 | 77.62 | |
| LC50 | 97.72 | - | - | - | 57.54 | - | - | - | 95.50 | 91.20 | |
| 9 c | GI50 | 2.51 | 1.91 | 1.55 | 0.93 | 1.55 | 3.47 | 1.70 | 1.55 | 0.95 | 1.66 |
| TGI | 20.89 | 9.33 | 6.03 | 7.08 | 7.41 | 13.49 | 6.76 | 8.91 | 4.90 | 8.32 | |
| LC50 | - | 37.15 | 29.51 | 38.90 | 31.62 | 43.65 | 26.92 | 26.30 | 31.62 | 36.31 | |
| 10 | GI50 | 10.00 | 18.20 | 12.30 | 10.72 | 10.23 | 10.47 | 11.22 | 20.42 | 6.92 | 11.22 |
| TGI | 44.67 | 91.20 | 70.79 | 60.26 | 67.61 | 77.62 | 69.18 | 97.72 | 54.95 | 67.61 | |
| LC50 | 87.10 | - | - | - | 93.33 | 91.20 | - | - | - | 97.72 | |
| 12 c | GI50 | 0.02 | 0.07 | 0.02 | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.05 | 0.03 |
| TGI | 1.74 | 11.48 | 0.95 | 1.20 | 7.08 | 2.34 | 15.85 | 3.02 | 5.62 | 3.80 | |
| LC50 | 39.81 | 93.33 | 25.12 | 23.44 | 50.12 | 52.48 | 70.79 | 21.38 | 72.44 | 48.98 | |
| 13 c | GI50 | 0.25 | 0.58 | 0.20 | 0.20 | 0.34 | 0.38 | 0.33 | 0.39 | 0.28 | 0.32 |
| TGI | 4.07 | 33.11 | 8.51 | 4.47 | 14.13 | 18.20 | 10.23 | 10.00 | 13.18 | 11.48 | |
| LC50 | 46.77 | - | 72.44 | 74.13 | 69.18 | 95.50 | 79.43 | 66.07 | 95.50 | 77.62 | |
| 14 c | GI50 | 0.05 | 0.22 | 0.07 | 0.04 | 0.11 | 0.26 | 0.11 | 0.10 | 0.06 | 0.10 |
| TGI | 0.47 | 6.03 | 3.55 | 1.74 | 7.41 | 11.75 | 4.27 | 3.16 | 1.70 | 3.39 | |
| LC50 | 19.95 | 42.66 | 26.92 | 29.51 | 33.11 | 47.86 | 36.31 | 23.99 | 40.74 | 33.88 | |
| 15 | GI50 | 6.92 | 19.50 | 20.42 | 10.47 | 16.22 | 18.62 | 15.85 | 35.48 | 6.76 | 14.13 |
| TGI | 56.23 | 69.18 | 83.18 | 26.92 | 67.61 | 60.26 | 41.69 | 67.61 | 36.31 | 52.48 | |
| LC50 | - | 93.33 | 97.72 | 61.66 | - | 89.13 | 77.62 | - | 83.18 | 87.10 | |
| 16 c | GI50 | 0.26 | 0.58 | 0.37 | 0.32 | 0.71 | 0.60 | 0.62 | 0.32 | 0.30 | 0.45 |
| TGI | 7.41 | 12.30 | 9.33 | 7.59 | 18.20 | 11.75 | 12.88 | 21.38 | 8.32 | 10.96 | |
| LC50 | 69.18 | 57.54 | 46.77 | 47.86 | 51.29 | 61.66 | 56.23 | 69.18 | 57.54 | 56.23 | |
| 18 c | GI50 | 1.62 | 2.57 | 2.09 | 1.58 | 2.69 | 3.31 | 1.78 | 2.45 | 1.32 | 2.09 |
| TGI | 17.78 | 10.00 | 7.24 | 5.50 | 11.22 | 11.75 | 8.13 | 13.49 | 9.12 | 9.77 | |
| LC50 | 91.20 | 46.77 | 22.91 | 18.20 | 37.15 | 41.69 | 29.51 | 46.77 | 54.95 | 38.90 | |
| 19 | GI50 | 2.24 | 5.13 | 3.89 | 3.80 | 5.25 | 7.24 | 5.13 | 8.91 | 2.88 | 4.37 |
| TGI | 13.49 | 31.62 | 20.42 | 36.31 | 25.70 | 52.48 | 33.88 | 53.70 | 33.88 | 29.51 | |
| LC50 | - | 95.50 | 69.18 | - | 79.43 | - | 91.20 | - | - | 91.20 | |
| 20 c | GI50 | 1.62 | 3.02 | 1.55 | 2.09 | 3.55 | 3.39 | 3.02 | 2.88 | 1.15 | 2.29 |
| TGI | 10.23 | 15.49 | 9.55 | 7.94 | 15.49 | 15.14 | 13.80 | 19.05 | 8.51 | 12.02 | |
| LC50 | 72.44 | 58.88 | 30.20 | 32.36 | 44.67 | 50.12 | 52.48 | 50.12 | 54.95 | 48.98 | |
| 21 | GI50 | 16.60 | 6.61 | 4.90 | 6.03 | 4.57 | 8.32 | 4.37 | 12.88 | 3.80 | 6.03 |
| TGI | - | 46.77 | 20.89 | 33.88 | 26.30 | 54.95 | 31.62 | - | 30.90 | 38.02 | |
| LC50 | - | 85.11 | 61.66 | 85.11 | 66.07 | 95.50 | 87.10 | - | - | 83.18 | |
| 23c | GI50 | 5.50 | 9.55 | 4.90 | 3.63 | 4.57 | 2.51 | 8.91 | 10.23 | 5.01 | 5.50 |
| TGI | 25.70 | 28.18 | 17.38 | 17.78 | 19.05 | 13.18 | 23.44 | 31.62 | 19.95 | 20.89 | |
| LC50 | 75.86 | 72.44 | 47.86 | 53.70 | 46.77 | 57.54 | 56.23 | 63.10 | 67.61 | 58.88 | |
| 24c | GI50 | 0.19 | 0.87 | 0.49 | 0.36 | 0.66 | 0.59 | 0.55 | 0.38 | 0.38 | 0.49 |
| TGI | 7.24 | 12.59 | 6.17 | 5.37 | 11.22 | 11.22 | 8.51 | 8.91 | 5.62 | 8.32 | |
| LC50 | 72.44 | 58.88 | 35.48 | 47.86 | 40.74 | 61.66 | 52.48 | 38.02 | 54.95 | 51.29 | |
| 25 c | GI50 | 0.49 | 0.65 | 0.35 | 0.40 | 0.56 | 0.33 | 0.63 | 0.39 | 0.40 | 0.47 |
| TGI | 8.71 | 12.59 | 6.92 | 6.46 | 10.96 | 7.41 | 10.96 | 9.77 | 6.76 | 8.91 | |
| LC50 | 87.10 | 70.79 | 57.54 | 51.29 | 44.67 | 41.69 | 43.65 | 51.29 | 70.79 | 56.23 | |
| 26 | GI50 | 3.31 | 7.94 | 6.61 | 8.71 | 6.46 | 9.12 | 8.71 | 5.37 | 3.72 | 6.31 |
| TGI | 38.02 | 30.20 | 20.89 | 24.55 | 20.89 | 46.77 | 33.11 | 26.30 | 19.05 | 26.92 | |
| LC50 | - | 81.28 | 61.66 | 67.61 | 57.54 | 87.10 | 75.86 | 74.13 | 69.18 | 72.44 | |
| 27 c | GI50 | 0.19 | 0.63 | 0.31 | 0.31 | 0.56 | 0.40 | 0.49 | 0.25 | 0.23 | 0.37 |
| TGI | 1.00 | 9.33 | 2.88 | 3.39 | 7.94 | 7.24 | 8.13 | 3.02 | 4.37 | 4.79 | |
| LC50 | 19.05 | 47.86 | 18.62 | 30.90 | 32.36 | 33.88 | 35.48 | 25.70 | 47.86 | 30.90 | |
| 28 c | GI50 | 0.01 | 0.06 | 0.02 | 0.02 | 0.03 | 0.04 | 0.04 | 0.01 | 0.02 | 0.03 |
| TGI | 0.08 | 4.07 | 0.71 | 1.95 | 4.68 | 4.68 | 5.62 | 0.68 | 1.32 | 1.78 | |
| LC50 | 5.75 | 32.36 | 9.12 | 22.39 | 27.54 | 27.54 | 51.29 | 16.22 | 66.07 | 23.99 | |
| 29 | GI50 | 2.34 | 5.13 | 2.24 | 6.17 | 6.61 | 6.31 | 5.75 | 5.62 | 3.47 | 4.57 |
| TGI | 7.76 | 25.12 | 15.49 | 28.84 | 27.54 | 30.20 | 26.30 | 23.99 | 18.20 | 21.38 | |
| LC50 | 43.65 | 77.62 | 70.79 | 75.86 | 75.86 | 81.28 | 83.18 | 83.18 | 91.20 | 74.13 | |
| 30 c | GI50 | 0.09 | 0.11 | 0.09 | 0.15 | 0.12 | 0.18 | 0.23 | 0.07 | 0.12 | 0.13 |
| TGI | 2.88 | 16.98 | 3.98 | 3.63 | 7.41 | 7.76 | 6.92 | 12.88 | 6.76 | 6.46 | |
| LC50 | 64.57 | 77.62 | 22.91 | 53.70 | 50.12 | 58.88 | 53.70 | 63.10 | 75.86 | 54.95 | |
| 32 | GI50 | 8.71 | 13.49 | 8.13 | 10.00 | 13.18 | 12.59 | 11.48 | 13.80 | 11.75 | 11.22 |
| TGI | 54.95 | 43.65 | 52.48 | 33.88 | 37.15 | 45.71 | 38.02 | 36.31 | 40.74 | 42.66 | |
| LC50 | - | 89.13 | 95.50 | 95.50 | 81.28 | 97.72 | 85.11 | 91.20 | 91.20 | 91.20 | |
| 33 c | GI50 | 2.00 | 6.31 | 0.76 | 1.07 | 3.09 | 7.76 | 4.17 | 4.17 | 1.78 | 2.75 |
| TGI | 35.48 | 66.07 | 60.26 | 42.66 | 74.13 | 60.26 | 60.26 | 81.28 | 54.95 | 57.54 | |
| LC50 | 93.33 | 93.33 | 97.72 | 95.50 | 97.72 | - | - | - | - | 97.72 | |
| 34 | GI50 | 3.47 | 6.03 | 2.95 | 3.72 | 3.24 | 5.62 | 5.89 | 3.55 | 2.75 | 4.07 |
| TGI | 36.31 | 21.88 | 24.55 | 23.99 | 15.14 | 31.62 | 28.18 | 15.85 | 16.22 | 22.91 | |
| LC50 | - | 91.20 | - | 67.61 | 66.07 | 95.50 | 93.33 | - | 63.10 | 83.18 | |
| Vincristine sulfate d | GI50 | 0.10 | 0.25 | 0.10 | 0.13 | 0.16 | 0.32 | 0.32 | 0.13 | 0.32 | 0.20 |
| TGI | 15.85 | 15.85 | 3.98 | 6.31 | 7.94 | 19.95 | 19.95 | 6.31 | 7.94 | 10.00 | |
| LC50 | 630.96 | 251.19 | 79.43 | 199.53 | 251.19 | 316.23 | 251.19 | 316.23 | 316.23 | 251.19 |
Highest conc. = 10−4M unless otherwise reported; only value > 100 μM are reported. The compound exposure time was 48 hours
Mean Graph MIDpoint i.e. the calculated panel mean
Mean of two separate experiments
Highest conc. = 10−3M
For some compounds the 5-concentration test was repeated and no significant differences were found. For these compounds the data reported in Table 2 are the mean values of the two experiments.
The tested compounds showed a mean GI50 range between 23.99 and 0.03 μM and compounds 7, 12, 13, 14, 16, 24, 27, 28 and 30 were submitted to BEC (Biological Evaluation Committee) for possible future development. Further studies continued at the NCI where the maximum tolerated dose (MTD) was determined for compounds 16, 28 and 30. It was found to be 100 mg/Kg for compound 16, whereas it was found to be 400 mg/Kg for compounds 28 and 30 administered IP in DMSO for 16, 28 and 10% DMSO in Saline/Tween 80 for 30. Compound 30 was then chosen for the first in vivo experiment i.e. the “Hollow Fiber Assay”,23 which is a rapid and efficient initial in vivo screening model including multiple cell lines in a single assay. Therefore it was subjected to four experiments against a panel of three tumor cell lines each consisting of the breast, non-small cell lung, colon, ovarian, CNS and melanoma cell lines (administered IP in 10% DMSO and Saline/Tween 80). The cancer cell lines were loaded into biocompatible polyvinylidene fluoride hollow fibers. Duplicate sets of fibers were implanted into mice intraperitoneally (IP) and subcutaneously (SC) such that each mouse carried 3 fibers IP and 3 fibers SC representing 3 distinct cell lines. The mice were treated IP with compound 30 once daily for 4 days, the fibers are collected 24 h after treatment and the viable cell mass in each fiber is determined.
A point system was developed to assess the activity. A value of 2 is assigned for each treatment dose that results in a 50% or greater reduction in viable cell mass in any fiber. Compounds with a combined IP and SC score of 20 or higher, an SC score of 8 or higher, or cell kill of one or more cell lines are considered for further in vivo testing. Compound 30 resulted in a combined IP + SC score of 16, a SC score of 6 and gave cell kill nevertheless at this time BEC decided to suspend further testing.
In the light of the NCI-60 results, the following may be considered: shifting of chlorine from position 4/5 to 6 of the oxindole portion is accompanied by an activity increment which was more evident when the indole portion was N-methylated. In fact compound 12 showed mean GI50 0.03 μM vs. 5.37 of the 4-chloro isomer and 0.45 of the 5-chloro isomer.6
The comparison between the activity of compounds 7 and 12 (mean GI50 0.21 and 0.03 μM respectively) confirms the activity increment observed for some analogs after N-methylation of the chloroalde yde. To a lesser extent an increment was observed even in the comparison of 4 (mean GI50 2.14 μM) vs 9 (1.66 μM) and 5 (inactive in the preliminary test) vs 10 (11.22 μM) whereas the same does not happen in the comparison of 8 (23.99 μM) vs the previously reported NH free analog (2.00 μM)4 and in the comparison of 13–15 vs the monomethyl derivatives.4,5 Shifting of the methyl and methoxy groups from the chloroindole to the oxindole portion (16) did not lead to a significant difference in the biological behavior: mean GI50,TGI, LC50 0.45, 10.96, 56.23 μM vs 0.40, 12.59, 79.43 μM of the previously reported analog.4
The introduction of the dimethylamino group was accompanied by loss of activity with respect to the 5-methoxy-6-methyl analogs;5 compound 22, bearing two dimethylamino groups, was inactive in the preliminary test. Other attempts were unsuccessful such as the introduction of hydrophilic/electron attracting groups or the lack of the methine bridge.
The evaluation of the antitumor activity of the compounds lacking chlorine at the 2-position of the indole portion (29–34) did not indicate that this class of compounds is superior to the 2-chloro derivatives: only compound 29 was more active than the corresponding 2-chloro analog (mean GI50 4.57 vs. 77.62 μM3) whereas 30–34 were about as active as the chloro analogs: compound 30 was selected by NCI for in vivo studies.
The replacement of the indole by a benzoindole portion led to the most interesting compounds: in particular 28 was the most active of the whole series described in this paper thus confirming that the bulky benzoindole system is a suitable pharmacophoric group in this class of compounds.
b. Effect on growth of MCF-7 breast cancer cells
A subset of compounds was used for further assays in a biological model. Firstly, we examined the influence on the growth and death of MCF-7 breast cancer cells of compounds 12, 28 (which in the 60 cell panel were very active) and 16, a less-active compound.
Figure 1A shows that, even at a low concentration (200 nM), 12 and 28 blocked MCF-7 cell growth within 48 h. At the same concentration, the less effective compound 16 initially decreased cell proliferation, however after 24 h, the cells recovered and started to grow. In some preliminary experiments, apoptotic cell death was observed after a longer treatment time (72–96 h). At shorter times, a significant toxic effect became evident only at concentrations in the micromolar range, and cell death was detectable after a 48 h incubation (data not shown). To understand whether the toxicity of these new derivatives was associated with activation of apoptosis, the cells were treated with a 5 μM concentration of the compounds, a concentration that caused about 30–40% of cell death, and the activation of caspases acting on the peptide substrate sequence Asp-Glu-Val-Asp (DEVD), was determined. This sequence is the optimal substrate for effector caspases 3, and 7, activated as the result of all caspase cascades.24 However, since MCF-7 cells lack caspase 3, the assay measures mainly the activation of caspase 7, which is highly expressed in these cells.25 The activation of the caspase proteases, considered the molecular effectors of apoptosis,26 was significant only after 48 h of treatment (Figure 1B).
Figure 1.
Effects on growth and death of MCF-7 cells. (A) Rate of cell growth determined as total cell number. The cells were incubated in the presence of the indicated compounds (200 nM) and viable cells were counted daily. The differences of all treated cells vs control cells were significant (P<0.05) at either 24 and 48 h. (B) Caspase activity acting on the peptide sequence DEVD (mainly caspase 7) was measured in extracts obtained from cells treated for 24 or 48 h with the indicated compounds (5 μM). The reported data are means ± s.e.m. obtained in five determinations. The differences of all treated cells vs. control cells were significant (P<0.05) only at 48 h.
We next examined the effect of the derivatives on some biochemical pathways correlated with cell proliferation and cell death (Figure 2). In these studies, the cells were treated with the different agents at 5 μM concentration for 20 hours. Firstly, we determined whether the antiproliferative effect was accompanied by changes in the expression of the tumor suppressor p53. Figure 2A shows that compounds 12, 16 and 28 caused a p53 increase. Next, we investigated the effect on ERK1/2 mitogen-activated protein kinases, which are generally associated with tumor cell growth.27 In this case, the three compounds had different effects. In fact, 12 and especially 16 decreased ERK phosphorylation which, on the contrary, was increased by compound 28. The three derivatives had minimal effects on the kinase Akt, an oncoprotein upregulated in several cancers and responsible for resistance to cell death,28 Akt-phosphorylation was only slightly decreased by compound 12.
Figure 2.
. Effect on biochemical pathways correlated with cell growth in MCF-7 cells. (A) The cells were incubated for 20 h in the presence of 5 μM of the indicated compound. Then the content and phosphorylation status of the indicated proteins were determined in cell extracts by Western blotting (80 μg of protein/lane). Immunoblots reported are from one experiment representative of at least three that gave similar results. (B) Quantitative determination of p53 and p21 proteins by densitometry of immunoblotting in cells incubated 24 h in the presence of 5 μM compound 12. Results are means ± s.e.m. obtained in four separate determinations. Differences between treated cells and control cells were significant (P<0.05) for both p53 and p21. (C) Effect of compound 12 (5 μM for 24 h) on cellular Bax detected by immunofluorescence confocal microscopy. Nuclei were evidenced by incubation with propidium iodide (red fluorescence). Bax was stained with FITC-antibody and is evidenced as green fluorescence. The image is representative of three experiments.
These data indicate that interference in the function of growth-related kinases ERKs and Akt do not represent a common mechanism of the antiproliferative effect of the tested compounds, whereas p53 appears correlated to the action of these derivatives. To confirm the involvement of p53, the effects of the potent compound 12 on the levels of the p53 transcriptional target p2129 were determined. In cells incubated 24 h with 12, together with a threefold induction of p53, also p21 was increased by about 90% (Figure 2B). Another important protein associated to the tumor suppressive role of p53 is Bax, that is an effector of p53-induced apoptosis and is either a transcriptional target of 53 and a partner of p53 in its non-transcriptional actions.30 The cellular content of the Bax protein was assayed by immunofluorescence microscopy. Figure 2C shows that treatment with compound 12 caused a considerable Bax accumulation, evidenced by a large increase in green fluorescence. Densitometric analysis of bands obtained by Western blotting (n=3) indicated an increase of 2.8 times (data not shown).
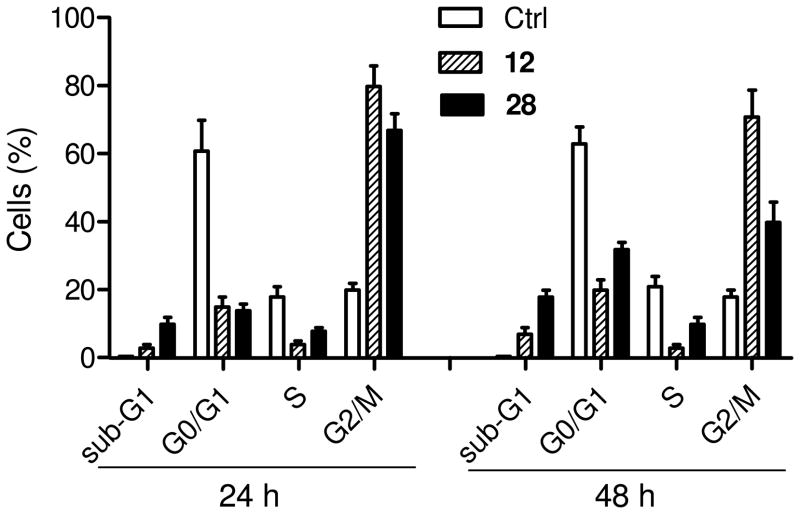
The p53 tumor suppressor can either cause apoptosis and influence the cell cycle.31 The increase of p21 caused by 12 suggested that the anticancer action of the novel compounds could be also associated with interference in cell cycle progression. Therefore, we examined the effect of some derivatives on the cell c ycle profile of MC F-7 cells. The cells were incubated for 24 and 48 h in the presence of 200 nM of compounds 12 and 28, and the analysis of DNA profiles was performed by flow cytometry. As previously detailed, at this concentration both compounds strongly inhibit cell growth, without causing massive cell death. Figure 3 shows that they caused a marked accumulation of MCF-7 cells in the G2/M phase, whereas the majority of control cells were in the G0/G1. Moreover, after treatment with the analogues, a notable cell population was observed in a sub-G1-peak caused by fragmented DNA in apoptotic cells. This population increased with incubation time, confirming activation of apoptosis.
Figure 3.
Effects on cell cycle in MCF-7 cells. Cells were treated with 200 nM of the indicated compound for 24 h, afterward cell cycle distribution was determined by flow cytometry. Results are means ± s.e.m. of three determinations.
In conclusion, the most active compounds tested on MCF-7 breast cancer cells gave rise to a block in cell cycle progression, with cell arrest in the G2/M phase. The arrest of the cell cycle probably causes the late induction of apoptosis, as indicated by activation of caspase proteases and appearance of a sub-G1 cell population. From the biochemical point of view, these events can be explained by the induction of the tumor suppressor p53, which is known to exert cell cycle blocking effect and eventual activation of apoptosis directly and by means of several effectors such as p21 or Bax,31 that were also increased.
Experimental Section
Chemistry
All the compounds prepared have a purity of at least 95% as determined by combustion analysis.
The melting points are uncorrected. TLC was performed on Bakerflex plates (Silica gel IB2-F) and column chromatography on Kieselgel 60 (Merck): the eluent was a mixture of petroleum ether/acetone in various proportions. The IR spectra were recorded in nujol on a Nicolet Avatar 320 E.S.P.; νmax is expressed in cm−1. The 1H-NMR spectra were recorded on a Varian Gemini (300 MHz); the chemical shift (referenced to solvent signal) is expressed in δ (ppm) and J in Hz (abbreviations: ar=aromatic, bzind=benzoindole, ind=indole, ox=oxindole). For the spectra that are not reported here, see Supporting Information. The oxindoles 1d, 1j, 1o, the aldehyde 2h and the isatin 3 are commercially available. The following compounds were prepared according to the literature: 1a,9 1b,11 1c,12 1e,10 1f,13 1g14 1i,16 1k,17 1l,18 1m,19 1n,4 2a,10 2b,6 2c,15 2f,20 2g21 and 3522.
Synthesis of 5-methoxy-1,6-dimethyl-1,3-dihydro-2H-indol-2-one (1h)
5-Methoxy-6-methyl-1,3-dihydro-2H-indol-2-one10 (4 mmol) in acetone (40 mL) was treated with dimethyl sulfate (9.5 mmol) in the presence of anhydrous potassium carbonate (6.5 mmol). The reaction mixture was refluxed for 15 h, cooled and filtered. The solution was evaporated and the expected compound was purified by column chromatography (petroleum ether/acetone, 70/30), with a yield of 52%. It was crystallized from acetone/petroleum ether: mp 93–95°C.
IR: 1697, 1240, 1151, 1069. 1NMR: 2.17 (3H, s, CH3), 3.06 (3H, s, N-CH3), 3.47 (2H, s, CH2), 3.73 (3H, s, OCH3), 6.79 (1H, s, ar), 6.94 (1H, s, ar). Anal. Calcd for C11H13NO2 (MW 191.23): C, 69.09; H, 6.85; N, 7.32. Found: C, 69.12; H, 6.78; N, 7.36.
Synthesis of 5-(dimethylamino)-3-[(dimethylamino)methylene]-2-oxoindoline-1-carbaldehyde (1p)
The Vilsmeier reagent was prepared at 0–5°C by dropping POCl3 (32 mmol) into a stirred solution of DMF (43 mmol) in CHCl3 (5 mL). 5-(Dimethylamino)-1,3-dihydroindol-2-one11 (1b) (6 mmol) was dissolved in CHCl3 and treated with the Vislmeier reagent. After 3h at room temperature, CHCl3 was evaporated under reduced pressure and the residue was poured into ice water. After neutralization with NaHCO3 the expected compound was collected by filtration and crystallized from EtOH with a yield of 82%: mp 154–157°C.
I.R.: 1671, 1703, 1177, 1108. 1H-NMR: 2.89 (6H, s, 2CH3), 3.37 (3H, s, CH3), 3.56 (3H, s, CH3), 6.40 (1H, dd, ind-6, J=8.6, J=2.6), 6.85 (1H, d, ind-4, J=2.6), 7.69 (1H, d, ind-7, J=8.6), 7.85 (1H, s, CH), 9.14 (1H, s, CH). Anal. Calcd for C14H17N3O2 (MW 259.31): C, 64.85; H, 6.61; N, 16.20. Found: C, 64.87; H, 6.58; N, 16.23.
Synthesis of 2-chloro-5-(dimethylamino)-indole-3-carbaldehyde (2d)
Compound 1p (2mmol) was refluxed for 15 min with POCl3 (22 mmol). The mixture was cooled, poured into ice water and basified with 30% NH4OH.32 The aldehyde 2d was collected by filtration with a yield of 30%: mp 180–190°C dec.
I.R.: 3125, 1634, 830, 787. 1H-NMR: 2.92 (6H, s, 2CH3), 6.87 (1H, dd, ind-6, J=8.7, J=2.4), 7.28 (1H, d, ind-7, J=8.7), 7.39 (1H, d, ind-4, J=2.4), 9.95 (1H, s, CHO), 12.72 (1H, broad, NH). Anal. Calcd for C11H11ClN2O (MW 222.67): C, 59.33; H, 4.98; N, 12.58. Found: C, 59.29; H, 5.01; N, 12.60.
Synthesis of 2-chloro-1H-benzo[g]indole-3-carbaldehyde (2e) and 3-formyl-5-methoxy-6-methyl-1H-indole-2-carboxylic acid (2j)
The Vilsmeier reagent was prepared at 0–5°C by dropping POCl3 (54 mmol) into a stirred solution of DMF (65 mmol) in CHCl3 (5 mL). The 1,3-dihydro-2H-benzo[g]indol-2-one, prepared as reported in the literature33 or 5-methox y-6-meth ylindole-2-carbox ylic acid34 (5mmol) was suspended in CHCl3 (20 mL) and treated with the Vislmeier reagent. The reaction mixture was kept under reflux for 10–15 h, according to a TLC test. Chloroform was removed under reduced pressure and the resulting oil was poured into ice. The crude aldehyde thus obtained was collected by filtration with a yield of 70%.
Data for 2e. Crystallized from ethanol had mp 220–225°C.
I.R.: 3160, 1649, 856, 805. 1H-NMR: 7.52 (1H, t, ar, J=7.9), 7.64 (1H, t, ar, J=7.9), 7.73 (1H, d, ar, J=7.9), 8.00 (1H, d, ar, J=7.9), 8.16 (1H, d, ar, J=7.9), 8.38 (1H, d, ar, J=7.9), 10.08 (1H, s, CHO), 13.77 (1H, s, NH). Anal. Calcd for C13H8ClNO (MW 229.66): C, 67.99; H, 3.51; N, 6.10. Found: C, 68.02; H, 3.49; N, 6.13.
Data for 2j. Crystallized from methanol had mp 260°C dec.
I.R.: 3247, 1672, 1643, 1102, 1025. 1H-NMR: 2.26 (3H, s, CH3), 3.83 (3H, s, OCH3), 7.30 (1H, s, ind-4/7), 7.64 (1H, s, ind-4/7), 10.60 (1H, s, CHO), 12.52 (1H, s, NH), 13.20 (1H, broad, COOH). Anal. Calcd for C12H11NO4 (MW 233.22): C, 61.80; H, 4.75; N, 6.01. Found: C, 61.82; H, 4.73; N, 5.98.
General procedure for the synthesis of compounds 5, 7–10, 12–23, 27–34 and 36
The appropriate 2-indolinone 1 (10 mmol) was dissolved in methanol (100 mL) and treated with the equivalent of the appropriate indole-3-carbaldehyde 2 and piperidine (1 mL). The reaction mixture was refluxed for 3–5 h (according to a TLC test) and the precipitate formed on cooling was collected by filtration with a yield of 20% for compound 5, 7, 28 and 33, 45–50% for compounds 13, 15, 16, 23, 27 and 31 and 75–85% for compounds 8–10, 12, 14, 29, 30, 32, 34 and 36.
Compounds 17–22 were purified by column chromatography with a yield of 20%.
Data for 7. I.R.: 3355, 1667, 1593, 1207, 917. 1H-NMR: 2.25 (3H, s, CH3), 3.56 (3H, s, OCH3), 6.47 (1H, s, ind-4), 6.76 (1H, d, ox-4, J=8.2), 6.89 (1H, d, ox-7, J=1.8), 6.95 (1H, dd, ox-5, J=8.2, J=1.8), 7.23 (1H, s, ind-7), 7.69 (1H, s, CH), 10.71 (1H, s, NH-ox), 12.60 (1H, broad, NH-ind). Anal. C19H14Cl2N2O2 (C, H, N).
Data for 9. I.R.: 3150, 1692, 1594, 1298, 922. 1H-NMR: 2.32 (3H, s, CH3), 3.62 (3H, s, N-CH3), 3.86 (3H, s, OCH3), 6.48 (1H, s, ind-4), 6.86 (2H, m, ind+ox), 7.35 (1H, d, ox, J=8.4), 7.52 (1H, s, ox), 7.74 (1H, s, CH), 10.73 (1H, s, NH-ox). Anal. C20H16BrClN2O2 (C, H, N).
Data for 12. I.R.: 3140, 1714, 1611, 1040, 799. 1H-NMR: 2.29 (3H, s, CH3), 3.56 (3H, s, N-CH3), 3.82 (3H, s, OCH3), 6.50 (1H, s, ind-4), 6.74 (1H, d, ox-4, J=7.5), 6.89 (1H, d, ox-7, J=1.2), 6.92 (1H, dd, ox-5, J=7.5, J=1.2), 7.48 (1H, s, ind-7), 7.68 (1H, s, CH), 10.74 (1H, s, NH-ox). Anal. C20H16Cl2N2O2 (C, H, N).
Data for 13. I.R.: 1709, 1605, 1040, 912, 851. 1H-NMR: 2.30 (3H, s, CH3), 3.34 (3H, s, N-CH3), 3.53 (3H, s, N-CH3), 3.84 (3H, s, OCH3), 6.52 (1H, s, ind-4), 6.83 (1H, d, ox-4/7, J=7.2), 6.94 (1H, t, ox-5/6, J=7.2), 7.05 (1H, d, ox-4/7, J=7.2), 7.29 (1H, t, ox-5/6, J=7.2), 7.49 (1H, s, ind-7), 7.73 (1H, s, CH). Anal. C21H19ClN2O2 (C, H, N).
Data for 14. I.R.: 3155, 1667, 1592, 1119, 912. 1H-NMR: 2.31 (3H, s, CH3), 3.19 (3H, s, N-CH3), 3.60 (3H, s, N-CH3), 3.84 (3H, s, OCH3), 6.40 (1H, d, ox-4, J=2.3), 6.57 (1H, s, ind-4), 6.68 (1H, dd, ox-6, J=8.2, J=2.3), 6.83 (1H, d, ox-7, J=8.2), 7.48 (1H, s, ind-7), 7.68 (1H, s, CH), 8.94 (1H, s, OH). Anal. C21H19ClN2O3 (C, H, N).
Data for 16. I.R.: 1686, 1602, 1052, 897, 739. 1H-NMR: 2.17 (3H, s, CH3), 3.20 (3H, s, N-CH3), 3.25 (3H, s, OCH3), 6.43 (1H, s, ox), 6.88 (1H, s, ox), 7.22 (3H, m, ind), 7.46 (1H, d, ind, J=7.8), 7.64 (1H, s, CH), 12.78 (1H, broad, NH-ind). Anal. C20H17ClN2O2 (C, H, N).
Data for 18. I.R.: 3165, 1692, 1600, 1215, 804. 1H-NMR: 2.74 (6H, s, N(CH3)2), 6.20 (1H, d, ox/ind-4, J=2.2), 6.70 (1H, d, ox/ind-4, J=2.2), 6.89 (1H, d, ox/ind-7, J=8.4), 6.92 (1H, dd, ox/ind-6, J=8.4, J=2.2), 7.21 (1H, dd, ox/ind-6, J=8.4, J=2.2), 7.34 (1H, d, ox/ind-7, J=8.4), 7.74 (1H, s, CH), 10.69 (1H, s, NH-ox), 12.60 (1H, broad, NH-ind). Anal. C19H15Cl2N3O (C, H, N).
Data for 20. I.R.: 3135, 1683, 1608, 1199, 810. 1H-NMR: 2.72 (6H, s, N(CH3)2), 3.43 (3H, s, OCH3), 6.25 (1H, d, ind/ox-4, J=2.2), 6.39 (1H, s, ind/ox), 6.77 (2H, s, ind/ox), 6.89 (1H, dd, ind/ox-6, J=9, J=2.2), 7.31 (1H, d, ind/ox-7, J=9), 7.65 (1H, s, CH), 10.36 (1H, s, NH-ox), 12.70 (1H, broad, NH-ind). Anal. C20H18ClN3O2 (C, H, N).
Data for 27. I.R.: 3408, 3172, 1721, 1674, 1086. 1H-NMR: 2.11 (3H, s, CH3), 3.17 (3H, s, OCH3), 6.49 (1H, s, ox), 6.68 (1H, s, ox), 7.31 (1H, d, bzind, J=8.1), 7.48 (2H, t, bzind, J=8.1), 7.61 (2H, m, bzind+CH), 7.96 (1H, d, bzind, J=8.1), 8.42 (1H, d, bzind, J=8.1), 10.35 (1H, s, NH-ox), 13.46 (1H, broad, NH-bzind). Anal. C23H17ClN2O2 (C, H, N).
Data for 28. I.R.: 3200, 1666, 1583, 805, 671. 1H-NMR: 3.19 (3H, s, CH3), 6.39 (1H, d, ox-4, J=2.3), 6.68 (1H, dd, ox-6, J=8.4, J=2.3), 6.84 (1H, d, ox-7, J=8.4), 7.28 (1H, d, bzind, J=8.1), 7.50 (1H, t, bzind, J=8.1), 7.60 (1H, d, bzind, J=8.1), 7.62 (1H, t, bzind, J=8.1), 7.70 (1H, s, CH), 7.99 (1H, d, bzind, J=8.1), 8.39 (1H, d, bzind, J=8.1), 8.82 (1H, s, OH), 13.45 (1H, s, NH-bzind). Anal. C22H15ClN2O2 (C, H, N).
Data for 30. I.R.: 3364, 3131, 1684, 1603, 1100. 1H-NMR: 2.27 (3H, s, CH3), 3.93 (3H, s, OCH3), 6.83 (1H, d, ox-4/7, J=7.5), 6.98 (1H, t, ox-5/6, J=7.5), 7.12 (1H, t, ox-5/6, J=7.5), 7.27 (1H, s, ind), 7.67 (1H, s, ind), 7.92 (1H, d, ox-4/7), 8.14 (1H, s, CH), 9.33 (1H, s, ind-2), 10.47 (1H, s, NH-ox), 11.78 (1H, broad, NH-ind). Anal. C19H16N2O2 (C, H, N).
General procedure for the synthesis of compounds 4, 6, 11, 24–26 and 37
The appropriate oxindole 1 (5 mmol) was dissolved in acetic acid (25 mL) and treated with an equivalent of indole-3-carbaldehyde 2 or 5-methoxyindole-2,3-dione 3 (for compound 37) and 37% hydrochloric acid (1 mL). The reaction mixture was refluxed for 24 h and, after cooling, the precipitate was collected by filtration with a yield of 35–40% for compounds 4, 24–26 and 70–80% for compounds 6, 11 and 37.
Data for 4. I.R.: 3124, 1672, 1604, 1108, 1021. 1H-NMR: 2.28 (3H, s, CH3), 3.62 (3H, s, OCH3), 6.48 (1H, s, ind-4), 6.86 (1H, d, ox-7, J=8.2), 6.90 (1H, d, ox-4, J=1.5), 7.27 (1H, s, ind-7), 7.36 (1H, dd, ox-6, J=8.2, J=1.5), 7.73 (1H, s, CH), 10.74 (1H, s, NH-ox), 12.77 (1H, broad, NH-ind). Anal. C19H14BrClN2O2 (C, H, N).
Data for 24. I.R.: 3426, 1698, 1623, 1198, 809. 1H-NMR: 6.79 (1H, d, ox-4, J=2.3), 6.89 (1H, d, ox-7, J=8.3), 7.22 (1H, dd, ox-6, J=8.3, J=2.3), 7.28 (1H, d, bzind, J=8.2), 7.51 (2H, t, bzind, J=8.2), 7.63 (1H, d, bzind, J=8.2), 7.77 (1H, s, CH), 8.00 (1H, d, bzind, J=8.2), 8.43 (1H, d, bzind, J=8.2), 10.76 (1H, s, NH-ox), 13.55 (1H, broad, NH-bzind). Anal. C21H12Cl2N2O (C, H, N).
Data for 25. I.R.: 3200, 1607, 1170, 815, 682. 1H-NMR: 6.57 (1H, dd, ox, J=9, J=2.7), 6.88 (1H, dd, ox, J=8, J=4.5), 7.04 (1H, td, ox, J=9, J=2.7), 7.30 (1H, d, bzind, J=8.3), 7.53 (2H, m, bzind), 7.63 (1H, d, bzind, J=8.3), 7.77 (1H, s, CH), 8.00 (1H, d, bzind, J=8.3), 8.44 (1H, d, bzind, J=8.3), 10.65 (1H, s, NH-ox), 13.56 (1H, broad, NH-bzind). Anal. C21H12ClFN2O (C, H, N).
Biology
a. Cell-based Screening Assay
The NCI screening is a two stage process,35 beginning with the evaluation of all compounds against the 60 cell lines at a single concentration of 10−5 M. Compounds exhibiting significant growth inhibition were evaluated against the 60 cell panel at five concentration levels by the NCI according to standard procedures (http://dtp.nci.nih.gov/branches/btb/ivclsp.html). In both cases the exposure time was 48h.
b. Acute Toxicity
To a single female athymic nude mouse (APA) was given a single injection of 400 mg/Kg; a second mouse received a dose of 200 mg/Kg and a third mouse received a dose of 100 mg/kg of compounds 16, 28 and 30. The mice were allowed ad libitum feed and water and they were observed for 2 weeks. The doses of compound 16 were administered IP in DMSO. After 14 days the mice treated with 400 and 200 mg/kg were sacrificed since they had lost more than 20% of their body weight. Therefore MTD was 100 mg/Kg. The doses of compound 28 and 30 were administered IP in DMSO and DMSO in Saline/Tween 80 respectively. After 14 days no mice showed weight loss or other signs of significant toxicity, therefore MTD was 400 mg/Kg.
c. Hollow Fiber Assay
Twelve tumor cell lines (NCI-H23, NCI-H522, MDA-MB-231, MDA-MB- 435, SW-620, COLO 205, LOX, UACC-62, OVCAR-3, OVCAR-5, U251 and SF-295) were cultivated in RPMI-1640 containing 10% FBS and 2 mM glutamine. On the day preceding hollow fiber preparation, the cells were given a supplementation of fresh medium to maintain log phase growth. For fiber preparation, the cells were harvested by standard trypsinization technique and resuspended at the desired cell density (2–10 × 106 cells/ml). The cell suspension was flushed into 1 mm (internal diameter) polyvinylidene fluoride hollow fibers with a molecular weight exclusion of 500,000 Da. The hollow fibers were heat-sealed at 2 cm intervals and the samples generated from these seals were placed into tissue culture medium and incubated at 37°C in 5% CO2 for 24 to 48 hours prior to implantation. A total of three different tumor lines were prepared for each experiment so that each mouse received three intraperitoneal implants (one of each tumor line) and three subcutaneous implants (one of each tumor line). On the day of implantation, samples of each tumor cell line preparation were quantitated for viable cell mass by a stable endpoint MTT assay so that the time zero cell mass is known. Mice were treated with compound 30 starting on day 4 after fiber implantation and continuing daily for 4 days. The compound was administered by IP injection at two dose levels: 150 mg/Kg and 100.5 mg/Kg. The vehicle was 10% DMSO in saline/0.05% Tween 80. The fibers were collected from the mice on the day following the fourth treatment and subjected to the stable endpoint MTT assay. The optical density of each sample was determined spectrophotometrically at 540 nm and the mean of each treatment group was calculated. The percent net growth for each cell line in each treatment group was calculated and compared to the percent net growth in the vehicle treated controls. A 50% or greater reduction in percent growth of the treated samples compared to the vehicle control samples was considered a positive result. Each positive result was given a score of 2 and all of the scores were totaled. The maximum possible score for the described experiment is 96 (12 cell lines x 2 sites x 2 dose levels x 2 [score]).
d. Cell culture and treatment
The human breast adenocarcinoma cell line MCF-7 was maintained in RPMI 1640 medium (Euroclone, Italy), supplemented with 10% FBS (Euroclone, Italy) and 2 mM L-glutamine (Sigma, USA) at 37°C and 5% CO2. Working solutions of compounds under test were prepared in DMSO at 10 mmol/L immediately before use. Cells were plated at 2×104 cells/cm2 in plastic culture wells and after 24 hours the derivatives were added to the medium to obtain the indicated concentrations. In control cells, DMSO was added to the culture medium. At the end of the treatment, cells were harvested using 0.11 % trypsin (Sigma, USA) 0.02% EDTA (Sigma, USA) and counted. Cell viability was determined by trypan blue exclusion.
e. Cell cycle analysis
MCF-7 control and treated cells were detached with 0.11% trypsin (Sigma-Aldrich, St Louis MO), 0.02% EDTA, washed twice in PBS and centrifuged. The pellet was suspended in 0.01% nonidet P-40 (Sigma, USA) 10 μg/mL RNase, and 0.1% sodium citrate (Sigma USA) and 50μg/mL propidium iodide (PI) (Sigma, USA), for 30 min at room temperature in the dark. Propidium iodide fluorescence was analyzed using a Bryte flow cytometer (Biorad) and cell cycle analysis was performed by means of a Coulter Epics XL MCL Cytometer. Cell cycle analysis was performed using Modfit 5.0 software.
f. Western blotting
The cells were collected in 5 mM dithiothreitol, 2 mM EDTA, 0.1% CHAPS, 0.1% Triton X-100, and protease inhibitors in 20 mM HEPES pH 7.5 and subjected to two cycles of freeze-thawing. The cell proteins in the homogenate were extracted, separated by SDS-PAGE, and detected as described.36 Primary antibodies against total and phosphorylated ERK 1/2 and Akt were from Cell Signaling Technology, Danvers, MA; anti-p53, anti-p21 and anti β-actin antibodies were from Santa Cruz Biotechnology, Santa Cruz, CA. Quantitative assay of immunoblotting was obtained by densitometry with a Fluor-S Max Multilmager instrument (Bio-Rad).
g. Immunofluorescence confocal microscopy
MCF-7 cells seeded at 1×104cells/cm2 on glass coverslips. At the end of the incubation, cells were washed twice and fixed with 3% p-formaldehyde, washed with 0.1 M glycine in PBS and permeabilized in 70% ice-cold ethanol. Bax staining was obtained by incubation for 1 h at room temperature with a mouse monoclonal anti-Bax antibody (BioS ource). Cells were then washed, incubated with FITC-coniugated anti-mouse IgG antibody (Sigma) for 1 h and finally stained with propidium iodide. All preparations were embedded in Mowiol and analyzed using a Nikon C1s confocal laser-scanning microscope, equipped with Nikon eclipse TE300. Multiple images were acquired by using sequential laser excitations at 488 nm and 568 nm to reduce spectral bleed-through artifacts.
h. Caspase activity
The activity of caspase enzymes hydrolyzing the peptide sequence DEVD, indicated as DEVDase activity, was measured in cell extracts by a fluorometric assay.36
i. Data analysis
All the shown experiments about the effects of studied compounds on MCF-7 cells were performed independently three or four times with comparable results. The results are expressed as means ± s.e.m. of the data obtained in the indicated numbers of independent experiments. When statistical analysis was applicable, data were compared by Student t-test. Differences were considered significant for P<0.05.
Supplementary Material
Acknowledgments
This work has been supported by a grant from the University of Bologna (RFO). We are grateful to the National Cancer Institute (Bethesda, MD) for the anticancer tests.
Abbreviations
- NCI
National Cancer Institute
- DTP
Developmental Therapeutics Program
- GI
growth inhibition
- TGI
total growth inhibition
- LC
lethal concentration
- BEC
Biological Evaluation Committee
- MTD
maximum tolerated dose
- DMSO
dimethyl sulfoxide
- FITC
fluorescein isothiocyanate
- EDTA
ethylenediaminetetraacetic
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- PI
propidium iodide
Footnotes
This material is available free of charge at http://pubs.acs.org.
Supporting Information Available
Additional IR and 1H-NMR spectra. Elemental analyses (Table S1), NSC numbers (Table S2), Antitumor activity expressed as the negative log of the molar concentration at three assay endpoints (Table S3). Mean graph of compound 30 where all the cell lines employed are reported (Figure S1).
References
- 1.Potential Antitumor Agents 46. For part 45, see the following: Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Landi L, Prata C, Vieceli Dalla Sega F, Caliceti C, Shoemaker RH. Antitumor Activity and COMPARE Analysis of Bis-indole Derivatives. Bioorg Med Chem. doi: 10.1016/j.bmc.2010.03.063. In press.
- 2.Andreani A, Locatelli A, Rambaldi M, Leoni A, Bossa R, Fraccari A, Galatulas I. Potential Antitumor Agents. 25. Synthesis and Cytotoxic Activity of 3-(2-Chloro-3- indolylmethylene)1,3-dihydroindol-2-ones. Anticancer Res. 1996;16:3585–3588. [PubMed] [Google Scholar]
- 3.Andreani A, Locatelli A, Leoni A, Morigi R, Chiericozzi M, Fraccari A, Galatulas I, Salvatore G. Synthesis and Potential Coanthracyclinic Activity of Pyridylmethylene and Indolylmethylene Lactams. Eur J Med Chem. 1998;33:905–909. [Google Scholar]
- 4.Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Garaliene V. Synthesis and Antitumor Activity of 1,5,6-Substituted 3-(2-Chloro-3-indolylmethylene)1,3- dihydroindol-2-ones. J Med Chem. 2002;45:2666–2669. doi: 10.1021/jm011123c. [DOI] [PubMed] [Google Scholar]
- 5.Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Garaliene V, Farruggia G, Masotti L. Substituted E-3-(2-Chloro-3-indolylmethylene)1,3-dihydroindol-2- ones With Antitumor Activity. Bioorg Med Chem. 2004;12:1121–1128. doi: 10.1016/j.bmc.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Calonghi N, Cappadone C, Farruggia G, Zini M, Stefanelli C, Masotti L. Substituted E-3-(2-Chloro-3-indolylmethylene)1,3-dihydroindol-2-ones with Antitumor Activity: Effect on the Cell Cycle and Apoptosis. J Med Chem. 2007;50:3167–3172. doi: 10.1021/jm070235m. [DOI] [PubMed] [Google Scholar]
- 7.Andreani A, Locatelli A, Leoni A, Rambaldi M, Morigi R, Bossa R, Chiericozzi M, Fraccari A, Galatulas I. Synthesis and Potential Coanthracyclinic Activity of Substituted 3-(5- Imidazo[2,1-b]thiazolylmethylene)-2-indolinones. Eur J Med Chem. 1997;32:919–924. [Google Scholar]
- 8.Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Giorgi G, Salvini L, Garaliene V. Synthesis and Antitumor Activity of Substituted 3-(5-Imidazo[2,1- b]thiazolylmethylene)-2-indolinones. Anticancer Drug Design. 2001;16:167–174. [PubMed] [Google Scholar]
- 9.Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, Tang C. Synthesis and Biological Evaluations of 3-Substituted Indolin-2-ones: A Novel Class of Tyrosine Kinase Inhibitors that Exhibit Selectivity toward Particular Receptor Tyrosine Kinases. J Med Chem. 1998;41:2588–2603. doi: 10.1021/jm980123i. [DOI] [PubMed] [Google Scholar]
- 10.Andreani A, Rambaldi M, Bonazzi D, Greci L, Andreani F. Potential Antitumor Agents. III. Hydrazone Derivatives of 5-Substituted 2-Chloro-3-formyl-6-methylindole. Farmaco. 1979;34:132–138. doi: 10.1002/chin.197924211. [DOI] [PubMed] [Google Scholar]
- 11.Minisci F, Galli R, Cecere M. Amminazione Radicalica di Composti Aromatici Attivati: Acetammidi. Nuovo Processo per la Sintesi di para-Ammino-N,N-dialchilaniline. La Chimica e l’Industria. 1966;48:1324–1326. [Google Scholar]
- 12.Nakagawa K, Sato T, Nishi T, Oshiro Y, Yamamoto K Carbostyril and Oxindole Derivatives. Jpn. Kokai Tokkyo Koho. 52073866 19770621 Showa. CODEN: JKXXAF JP. 1977:5. Patent written in Japanese. Application: JP 75–150935 19751216. CAN 87:167899 AN 1977:567899 CAPLUS.
- 13.Hodges R, Shannon JS, Jamieson WD, Taylor A. Chemical and Biological Properties of Some Oxindol-3-ylidene Methines. Can J Chem. 1968;46:2189–2194. [Google Scholar]
- 14.Porter JC, Robinson R, Wyler M. Monothiophthalimide and Some Derivatives of Oxindole. J Chem Soc. 1941:620–624. [Google Scholar]
- 15.Schulte KE, Reisch J, Stoess U. Chloroformylation of α-Pyrrolones. Angew Chemie, Int Ed. 1965;4:1081–1082. [Google Scholar]
- 16.Romeo A, Corrodi H, Hardegger E. Umsetzungen des o-Nitrophenylessigesters und des 2-Chlor-6-nitro-phenyl-brenztraubensäureesters. Helv Chim Acta. 1955;38:463–467. [Google Scholar]
- 17.Beer RJS, Davenport HF, Robertson A. Extensions of the Synthesis of Hydroxyindoles from p-Benzoquinones. J Chem Soc. 1953:1262–1264. [Google Scholar]
- 18.Koelsch CF. A Synthesis of Ethyl Quininate from m-Cresol. J Amer Chem Soc. 1944;66:2019–2020. [Google Scholar]
- 19.Zakrzewska A, Kolehmainen E, Osmialowski B, Gawinecki R. 4-Fluoroanilines: Synthesis and Decomposition. J Fluorine Chem. 2001;111:1–10. [Google Scholar]
- 20.Steven E, Klohr SE, Cassady JM. An Intramolecular Photocyclization to from the Azepino [3,4,5-cd] Indole System. Synth Commun. 1988;18:671–674. [Google Scholar]
- 21.Allen GR, Binovi LJ, Weiss MJ. The Mitomycin Antibiotics. Synthetic Studies. XVI. The Utilization of 5-Methoxy-4-nitro-3-indolecarboxaldehydes for the Synthesis of Related 4,7- Indoloquinones. J Med Chem. 1967;10:7–13. doi: 10.1021/jm00313a002. [DOI] [PubMed] [Google Scholar]
- 22.Buzzetti F, Pinciroli V, Brasca MG, Crugnola A, Fustinoni S, Longo A. Synthesis and Configuration of Some New Bicyclic 3-Arylidene- and 3-Heteroarylidene-2-oxindoles. Gazz Chim Ital. 1995;125:69–75. [Google Scholar]
- 23.Hollingshead M, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In Vivo Cultivation of Tumor Cells in Hollow Fibers. Life Sciences. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson DW, Thornberry NA. Caspases: Killer Proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 25.Jänicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for α-fodrin Cleavage but Dispensable for Cleavage of other Death Substrates in Apoptosis. J Biol Chem. 1998;25:15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 26.Kurokawa M, Kornbluth S. Caspases and Kinases in a Death Grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balmanno K, Cook SJ. Tumour Cell Survival Signalling by the ERK1/2 Pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 28.Engelman JA. Targeting PI3K Signalling in Cancer: Opportunities, Challenges and Limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 29.Abbas T, Dutta A. p21 in Cancer: Intricate Networks and Multiple Activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent Pro-apoptotic Functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Kuribayashi K, El-Deiry WS. Regulation of Programmed Cell Death by the p53 Pathway. Adv Exp Med Biol. 2008;615:201–221. doi: 10.1007/978-1-4020-6554-5_10. [DOI] [PubMed] [Google Scholar]
- 32.Engqvist R, Javaid A, Bergman J. Synthesis of Thienodolin. Eur J Org Chem. 2004;12:2589–2592. [Google Scholar]
- 33.Mayer F, Oppenheimer T. Über Naphthyl-essigsäuren. 3. Abhandlung: 1-Nitronaphthyl-2-brenztraubensäure und 1-Nitronaphthyl-2-essigsäure. Chem Ber. 1918;51:1239–1245. [Google Scholar]
- 34.Allen GR, Poletto JF, Weiss MJ. The Mitomycin Antibiotics. Synthetic Studies. V. Preparation of 7-Methoxymitosene. J Org Chem. 1965;30:2897–2904. doi: 10.1021/jo01020a006. [DOI] [PubMed] [Google Scholar]
- 35.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 36.Zini M, Passariello CL, Gottardi D, Cetrullo S, Flamigni F, Pignatti C, Minarini A, Tumiatti V, Milelli A, Melchiorre C, Stefanelli C. Cytotoxicity of Methoctramine and Methoctramine-related Polyamines. Chem Biol Interact. 2009;181:409–416. doi: 10.1016/j.cbi.2009.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.