The goal of vascular regeneration
Broadly speaking, vascular regeneration includes the restoration of normal vascular function and structure; the reversal of vascular senescence; and the growth of new blood vessels. Therapeutic applications of vascular regeneration for coronary or peripheral arterial diseases are directed to relieving symptoms of ischemia; preventing target organ damage due to hypoxia, reperfusion or capillary leak; and avoiding cardiovascular catastrophes due to acute thrombosis, embolism, plaque rupture or dissection.
Clinicians have sought methods to harness the potential of therapeutic vascular regeneration, but studies focused on gene therapy or small molecular approaches have largely failed, thus far. Recently, efforts have shifted to stem cell-based approaches, given their theoretical capacity to replicate, differentiate and form new blood vessels in a directed fashion. Initial pre-clinical studies evaluated the pluripotent embryonic stem cell (ESC) and the more lineage-committed ‘adult’ stem cells, which include the endothelial progenitor cells (EPCs) found within the bone marrow. Early clinical trials indicate some benefit of EPC therapy in patients with ischemic or cardiomyopathic disease. In the meantime, scientific interest has shifted to a newly described class of stem cell: the induced pluripotential stem cell (iPSC). This fascinating cell is derived from terminally differentiated adult somatic cells that are ‘reprogrammed’ to an embryonic-like state with transcription factors that govern cell differentiation. Interest in iPSCs is high as these cells are autologous (do not require immunosuppression when delivered), pluripotential (can differentiate into tissue from each of the three germline lineages), noncontroversial (are derived from adult tissue), and come from a plentiful source (are derived from any adult cell, eg. skin fibroblasts).
The focus of this review is on the use of stem cell therapies for the growth of new blood vessels, i.e. angiogenesis, vasculogenesis and arteriogenesis. In particular, we will focus on the promise of iPSCs, for cell-based vascular regeneration, by comparison to other stem cell approaches.
General properties of stem cells
A stem cell is defined by its capacity for both self renewal and directed differentiation. Historically, investigators have recognized two broad categories of stem cells, the embryonic stem cell and the so-called adult stem cell. The embryonic stem cell (ESC) is derived from the inner cell mass of the fetal blastula and is pluripotent, i.e. having the ability to differentiate into any cell type found in the adult body. ESCs can replicate via mitotic division while retaining their undifferentiated state (self-renewal), or differentiate into lineage-specific cells under the appropriate stimuli.
By contrast to embryonic stem cells, adult stem cells are partially lineage-committed and therefore have the capacity to give rise only to cells of a given germ layer. In other words, they are multipotent, rather than pluripotent. As an example, the adult hematopoietic stem cell can repopulate the bone marrow of the leukemia patient after transplant, generating all blood cell lineages. However, this multipotent adult stem cell cannot produce cells of endodermal or ectodermal lineage. Another form of multipotent stem cell, the endothelial progenitor cell (EPC) is described in detail below. In addition to mutipotent adult stem cells, unipotent stem cells have been described. Such cells have increased replicative capacity, but can only differentiate into one cell lineage. By comparison to adult differentiated cells, adult stem cells have greater capacity for proliferation and ability to repopulate or repair tissue1. Although adult differentiated cells typically give rise only to cells of identical lineage, there is rare evidence for transdifferentiation between lineages. For example, Barrett's metaplasia is due to transdifferentiation of esophageal epithelial cells into cells resembling intestinal mucin-secreting goblet cells.
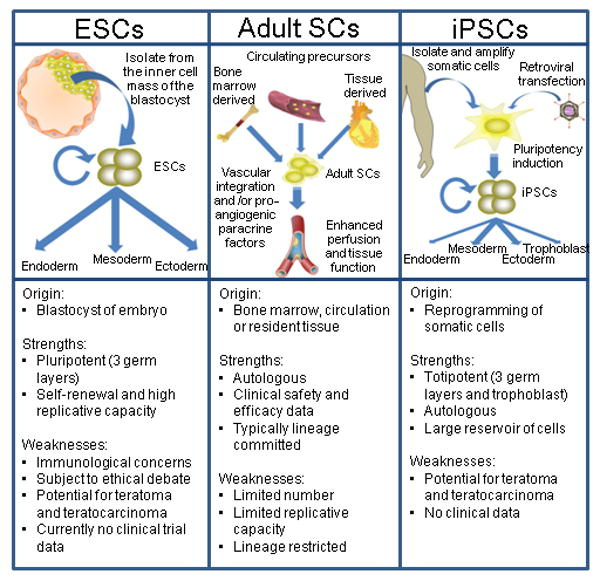
A third form of stem cell that has great potential for regenerative medicine is the iPSC. In 2006, Yamanaka and colleagues reported that mouse fibroblasts could be reprogrammed into iPSCs by viral transduction of four transcription factors2. That a small set of genes can induce “nuclear reprogramming” of adult differentiated cells into cells with many of the same characteristics as pluripotent embryonic stem cells, was soon confirmed by others3-6. In 2007, human fibroblasts were reprogrammed into iPSCs by viral transduction of Oct3/4 and Sox2, in combination with Klf4 and c-Myc, or in combination with Nanog and Lin287, 8. The iPSCs resemble ESCs in that they have the potential to differentiate into any adult cell. Ultimately, iPSCs may represent the most attractive cellular approach for regenerative medicine. In the following sections, each of these three cell types will be discussed in turn with an emphasis on the translation to therapeutic application in patients with vascular disease (Figure 1).
Figure 1.
Embryonic stem cells, adult stem cells and induced pluripotential stem cells. The three progenitor cell types available for vascular regenerative therapy- their promise and peril.
Adult stem cells in vascular regeneration
The therapeutic application of adult stem cells is farther along in clinical development than any of the other stem cell approaches. Adult stem cells exist in the bone marrow, the circulation or as residents within a specific tissue9. Therapies aimed at neovascularization have so far included bone marrow derived and circulating stem cell approaches. The use of adult stem cells for vascular regeneration was presaged by Asahara's discovery of the vasculogenic endothelial progenitor cell (‘EPC’) subpopulation in 1997 and has culminated in recent clinical trials9.
Parenthetically, the widely used term ‘EPC’ is a misnomer. The surface markers that are commonly used for identifying human ‘EPCs’ include markers that are not specific for endothelial lineage, such as CD133 and KDR10. In addition, methods for harvesting, purifying and culturing ‘EPCs’ are not standardized. Thus, semantic confusion is compounded by methodological variation. Casual readers of the literature may not recognize that ‘EPCs’ are a mixed population of progenitor cells of different lineages. Within this population of cells there are true endothelial progenitors that can incorporate into the vascular network, whereas hematopoietic progenitors may contribute by secreting angiogenic cytokines. In cell culture, ‘EPCs’ may form ‘early outgrowth’ and ‘late outgrowth’ colonies. Cells derived from the former colonies are clearly not of endothelial origin, expressing markers of hematopoietic lineage, and are morphologically distinct from the ‘late outgrowth’ cells which grow in a cobblestone pattern reminiscent of endothelial cells. At present, there are no surface markers that clearly distinguish early endothelial progenitors; the best approach currently is to define endothelial lineage morphologically, i.e. with tubulogenesis assays. The formation of a tubular network in matrigel is a defining feature of endothelial progenitors, which can also incorporate into existing tubular networks formed by differentiated endothelial cells.
The ‘EPCs’ are postulated to arise from an earlier progenitor, termed the hemangioblast, which also generates hematopoietic stem cells11. Pre-clinical studies indicate that ‘EPCs’ reside in the marrow (adhering to supporting stromal cells in a ‘stem cell niche’) and circulate in the blood at very low levels (<0.01% of circulating white cells)12. Their prevalence in the blood can change in response to various stimuli. Ischemia increases VEGF expression, which in turn activates matrix metalloproteinases which releases the ‘EPCs’ (CD34+/cKit+ cells) from the vascular niche by cleaving Kit-ligand. The mobilized ‘EPCs’ enter the circulation and home to the site of ischemia11, 13, 14. Bone-marrow derived cells mobilized by ischemia have been shown to incorporate into the vasculature, differentiating into endothelial cells (ECs), pericytes, or smooth muscle cells (SMCs). Alternatively or in addition, they may potentiate local angiogenesis via the elaboration of paracrine factors15-17. Numerous animal studies in both hindlimb- and coronary-ischemia models have indicated that ‘EPCs’ can be harvested, expanded ex vivo, and administered to augment capillary density, perfusion and organ function11, 18-22.
To avoid semantic confusion, it may be best to eschew the term ‘EPC’ and simply describe the isolated cells by their morphology, surface markers and method of isolation. Thus in man, vascular regeneration has been attempted using autologous bone marrow mononuclear cells (BMNCs) or G-CSF-expanded peripheral blood mononuclear cells (PBMNCs), in patients with coronary (CAD) and peripheral arterial disease (PAD). Phase I and II trials have been completed in patients with acute myocardial infarction(MI)9, as well as in subjects with chronic ischemic heart disease23-25. Thus far, the majority of these early trials have suggested modest, but consistent, improvements in cardiac endpoints such as global and regional contractility and little risk for these cell-based therapies9.
Strauer and colleagues26 found that the intracoronary (IC) injection of Ficoll gradient-isolated autologous BMNCs five to nine days after revascularization was associated with a small improvement in ejection fraction (EF) and infarct size (as assessed by left ventriculography) and improved myocardial perfusion by nuclear scintigraphy three months after delivery. This work was quickly followed by several other small non-blinded trials, including TOPCARE-AMI (BMNCs or PBMNCs) and BOOST (sedimentation-protocol isolated BMNCs), both of which confirmed improved pump function globally as well as in the infarct zone at four to six months after IC cell therapy, again with no observed serious adverse events27-29. Enthusiasm was briefly tempered by negative findings reported by Janssens et. al. and in the ASTAMI trial of autologous BMNCs in patients with acute ST segment elevation MI30, 31. In addition, the 18 month BOOST follow-up data failed to show a persisting benefit of cell therapy on contractility over standard care32. It is possible that a single injection of stem cells may not be sufficient to produce long term differences in pump function and that the methods used to isolate and process the BMNCs in the ASTAMI trial may have adversely affected their performance9. Also, while Janssens et. al. did not show global improvements in the EF of the patients in their randomized trial, those that received cell therapy did in fact have smaller infarct sizes and greater recovery of regional cardiac function30.
By contrast, the largest and most rigorously designed trial to date; the multicenter, randomized, double-blind, placebo controlled REPAIR-AMI trial, revealed a modest improvement in global left ventricular EF at 4 months after the delivery of BMNCs three to seven days post infarct33. Though not powered to do so, this study also revealed a significant reduction in death, recurrent MI, and revascularization at one year. The majority of trials completed thus far have not attempted to uncover the mechanism of observed benefit with cell transplantation. Furthermore, most studies do not control for the possible effects of paracrine factors secreted by the administered cells (which could be addressed in part by using a conditioned medium control). Nevertheless, a subset of patients in the REPAIR-AMI trial who underwent coronary flow reserve (CFR) testing did manifest a marked improvement in microvascular function and vasomotor responsiveness at four months suggesting that the administered BMNCs may, in fact, enhance vascular repair34. Overall, the studies of adult stem cell therapy for myocardial ischemia suggest that cell therapy is feasible, safe and associated with modest improvements in contractility. However, the mechanism(s) of this modest benefit remain unknown.
There have also been a number of small pilot trials of adult stem cells in PAD. Clinical benefit has been observed in small uncontrolled studies of BMNC therapy for subjects with claudication14, 35-37. Pathologic specimens of amputation specimens have confirmed the presence of therapeutic angiogenesis in patients who had undergone bone marrow transplant relative to matched controls22. The only controlled trial (TACT) revealed improvements in transcutaneous oxygen pressure, rest pain, and pain free walking time after intramuscular injections of BMNCs compared to vehicle control38. Several international Phase II trials are now underway to confirm these promising early safety and efficacy findings in peripheral vascular disease (clinicaltrials.gov).
Mechanisms of benefit of adult stem cells in vascular regeneration
It appears that only a small subset of ‘EPCs’ are of true endothelial lineage in man. These endothelial colony forming cells (ECFCs) can form vascular structures in vivo, but are rare, only 1-2 per 100 million mononuclear cells39. Another subset of ‘EPCs’, that are more common, are of hematopoietic lineage. These ‘EPCs’ share the same surface markers (CD31, CD105, CD144, CD146, VWF, KDR, and UEA-1) and incorporate acetylated LDL, but express the myeloid surface markers CD45 and CD14, and have other features of the monocyte/macrophage phenotype. Some of these cells may contribute to angiogenesis not by incorporating into the vasculature, but by secreting angiogenic cytokines and matrix metalloproteinases40, 41. Still other bone marrow derived stem cells can form pericytes which associate with and stabilize endothelial networks42. To conclude, circulating adult stem cells mobilized from the bone marrow, termed ‘EPCs’, are a heterogenous population of cells that may promote EC survival and proliferation, contribute to network formation and/or stabilize newly formed vessels.
The horizon for adult stem cell therapies is rapidly broadening. Treatments comprised of two or more cellular components, such as those with both mononuclear and mesenchymal populations, are currently under investigation in patients with coronary and peripheral vascular disease (the Mesendo trials). Approaches that move beyond the injection of a single progenitor cell type are intuitively appealing in that they may enhance paracrine cross-talk between intimal and medial constituents or reinforce the scaffolding of the developing vascular tree43. Investigations are also evolving past the bone marrow-derived and circulating cells studied thus far to include resident tissue stem cells such as the adipocyte-derived and cardiac progenitor cell populations (e.g. the Precise, Apollo, and Caduceus trials)44-48. Cardiospheres expanded after endomyocardial biopsy may have a particular role in repopulating areas of infarcted myocardium49, while mesenchymal adipose-derived stem cells have demonstrated the capacity to differentiate into endothelial, pericytic and smooth muscle cells, indicating less lineage restriction and more plasticity than a comparable EPC50-52. Finally, the future of adult stem cell therapy may lie in even more innovative areas, as highlighted by examples such as the so-called “EPC-capture stent” which is coated with immobilized anti-CD34 antibodies that aim to guide circulating stem cells to the site of vessel injury (e.g., the JACK-EPC trial)53. Novel approaches that enhance progenitor homing, cell-cell interaction and cell integration, or that incorporate progenitor cells with vascular matrices and synthetic conduits are likely to define the future of adult stem cell therapies for vascular disease54, 55.
Strengths and weaknesses of adult stem cells
All adult stem cells - whether harvested from the marrow, blood, or tissue - share a number of traits which make them appealing as candidates to lead the field of cell-based regeneration. The first is that these cells are harvested from the patient in which they are ultimately to be used, and do not need to overcome an immunological barrier. Also, this approach is not burdened by the ethical concerns surrounding the use of human embryos. Adult stem cells, and EPCs in particular, have undergone the most translational and human studies of all stem cell approaches. The trials done to date show little increased risk associated with their therapeutic use in humans.
On the other hand, there are deficiencies of adult stem cell therapy. Autologous delivery of cells inevitably means that there is a delay in treatment, due to the time needed to collect the cells, isolate and then propagate progenitors ex vivo so as to obtain adequate numbers before injection. Adverse effects of their delivery could include microvascular embolism, as well as unintended acceleration of pathological neovascularization, as in the case of an occult malignancy. Furthermore, most investigators use a small set of surface markers to define “EPCs” which is problematic56. As discussed above, the typical surface markers used to isolate ‘EPCs’ generate a mixed population of cells that have not been completely characterized11, 14, 57, 58. It is likely that a greater effect size and/or more persistent benefit of cell therapy could be realized with a more precisely defined cell population. It is not known what combination of progenitor cells might be most therapeutic in humans, e.g. purified endothelial progenitors or some combination with smooth muscle precursors and/or subsets of hematopoietic progenitors,59-61. Of importance, the mechanisms by which these adult stem cells interact to confer benefit must be further defined, so that they can be therapeutically manipulated62. Reproducible and efficient methods to isolate, expand and deliver angiogenic cells are needed. Finally, in the patients which most need EPC therapy, these cells are rare, have limited replicative capacity and are often dysfunctional. For example, ‘EPCs’ derived from older individuals demonstrate reduced ability to proliferate, incorporate into existing capillary networks and enhance limb perfusion in preclinical models. Conditions typically associated with vascular disease, such as age and diabetes, impair angiogenic functionality11, 63. The limitations of adult stem cell therapy for vascular disease have increased the interest in other sources for stem cell therapy, such as embryonic stem cells.
Embryonic stem cells as a source of vascular regeneration
Embryonic stem cells (ESCs) are derived from the inner cell mass of the blastocyst and, unlike adult stem cells, can differentiate into any cell type or any organ of endodermal, mesodermal, or ectodermal lineage64. The ESC can be directed to differentiate into vascular endothelial and smooth muscle cells as well as cardiomyocytes65-67. The ESC-derived endothelial cells (ESC-ECs) manifest endothelial surface markers (such as CD31), express endothelial proteins (such as von Willebrand factor and PECAM), and manifest endothelial functions (such as uptake of acetylated-LDL and formation of capillary tubes in Matrigel). Animal studies have shown that ESC-derived endothelial and mural cells incorporate into the vasculature of the ischemic limb or myocardium68-70. We tracked the fate and function of transplanted ESC-ECs in the ischemic murine myocardium. Murine ESCs were first transduced with a construct encoding luciferase (for bioluminescence imaging) and red fluorescent protein (for histological tracking). After differentiating the ESCs into endothelial cells, the ESC-ECs or vehicle were injected into the ischemic zone of the left ventricle after ligation of the left anterior descending coronary artery. Bioluminescence imaging showed persistence of the ESC-ECs out to 8 weeks, and echocardiography revealed improved systolic function in hearts injected with ESC-ECs by comparison to vehicle. Histological studies revealed increased myocardial capillary density in the hearts treated with cell therapy. Pre-clinical studies of ESC-ECs for vascular regeneration are at an early stage. Although the ESC cell has not yet transitioned from bench to bedside for patients with cardiovascular diseases, the world's first trial of human ESC therapy was recently granted clearance by the FDA. This trial of ESC-derived neuronal cells for use in acute spinal cord injury is an important milestone in ESC therapy, and the results are likely to have a dramatic impact on the future of ESCs in regenerative medicine, including vascular regeneration.
Strengths and weaknesses of embryonic stem cells
By comparison to adult stem cells, ESCs have the advantage of pluripotentiality and greater proliferative capacity. On the other hand, their clinical use remains conflicted by the ethical debate surrounding the use of human embryos and is challenged by an immunological barrier. Cells derived from ESCs will be allogeneic and their use is likely to require the co-administration of immunosuppressive agents, which carry their own substantial risks. Additionally, the great regenerative potential of these cells has raised fears about the inadvertent administration of even a single pluripotent ESC. The undirected growth and pathological differentiation post transplant of an ESC creates a risk of teratoma - a complication that can occur late after administration71. Much more robust differentiation and purification protocols are required, and studies proving the long term safety profile of embryonic progenitors are needed before widespread use in humans will be possible.
Induced pluripotent stem cells for vascular regeneration
iPSC background
An exciting new milestone in the field of regenerative medicine is the development of the iPSC, which has opened a new avenue for cardiac and vascular regeneration72. Previous work had revealed that the differentiation state of adult somatic cells can be fluid. It was known that the nuclear DNA of differentiated cells could be “reprogrammed” to express stem cell genes by fusion with ESCs or after nuclear transfer into oocytes73, 74. More recently it has been shown that a handful of reprogramming factors are sufficient to reprogram adult differentiated cells into pluripotential stem cells2. These iPSCs are capable of giving rise to all three germ layers (endoderm, ectoderm and mesoderm) and subsequently differentiating into adult cells3-6.
The set of reprogramming factors used by Yamanaka and colleagues were octamer-binding transcription factor-3/4 (Oct 3/4), SRY-related high-mobility-group (HMG)-box protein-2 (Sox2), Kruppel-like factor 4 (Klf4), and c-Myc2. In parallel studies, Thomson and colleagues focused on Oct 3/4 and Sox2, together with Nanog and Lin288. Both groups initially used viral vectors to overexpress the genes encoding the reprogramming factors in human fibroblasts, so as to generate iPSCs. While the exact mechanism by which these genes induce de-differentiation remains incompletely understood75, putative pathways have been theorized2, 74. The proto-oncogene c-Myc likely promotes histone acetylation and chromatin remodeling to facilitate access of Oct 3/4 and Sox 2 to their binding sites, and to accelerate cellular proliferation. Tumor suppressor-induced apoptosis, which should naturally follow, is avoided by a second oncogene, Klf4 which inhibits cell death by its suppression of p53 and upregulation of the renewal gene Nanog. The pluripotency-related factors Oct3/4 and Sox2 activate other critical embryonic genes and/or recruit chromatin remodeling complexes to promote reprogramming. Lin28 is an RNA-binding, microRNA-regulated protein that is involved in regulation of developmental timing. The creation of iPSCs does not require antibiotic selection5, 76, nor the insertion of the retroviruses in specific genomic locations77. Furthermore, these transcription factors are required only for the induction of, and not the maintenance of, pluripotency78. Indeed, the persistent expression of these exogenously introduced genes (a particular problem with the lentiviral constructs), seems to impair the ability to differentiate the iPSCs into the desired lineage.
Although fibroblasts were the first target of reprogramming efforts, other cell types have been induced to form pluripotential cells, with differences in the ease of reprogramming noted77. For example, murine neural progenitor cells can be reprogrammed with overexpression of a single factor, Oct3/4. By comparison to human fibroblasts, induction efficiencies of human scalp keratinocytes are 100 fold higher79. The difference in the ease of reprogramming probably relates to differences in the basal epigenetic state and transcriptional activation of cells. Other advances have been made to streamline the reprogramming process6, 7. Exogenous introduction of c-Myc and Klf4 is dispensable, though the differentiation efficiency is reduced without ectopic Myc73, 80. The addition of small molecules that enhance chromatin remodeling can enhance the reprogramming progress. For example, with the use of valproic acid (which is a histone deacetylase inhibitor at high concentrations), only overexpression of Sox2 and Oct3/4 are required for reprogramming73.
The promise and perils of iPSCs
The therapeutic potential of iPSCs is considerable as they are patient-specific stem cells that do not face the immunological barrier which confront cells derived from ESCs. Furthermore, these cells can be derived from plentiful and easily accessible sources of tissue-such as the donor's skin, fat or hair. Thus, immediate advantages over other progenitors include their immune-privileged status as autologous tissue (compared to allogeneic ES cells) and their potential abundance (relative to the rare EPC). Furthermore, these cells are “almost indistinguishable from ES cells”66 and have “almost completely identical” differentiation properties81, epigenetic states, surface markers and gene expression profiles as the pluripotential embryonic stem cell73 (Figure 2). iPSCs are germline competent and form trophoblastic tissue, actually making them totipotent cells82. The generation and use of iPSCs are not encumbered by the ethical concerns and political barriers faced by those using ESC83. Additionally, because they can be derived from subjects with genetic diseases, and easily propagated in vitro, they represent an ideal vehicle for the investigation of heritable disorders, and for screening of novel therapeutics84, 85.
Figure 2.
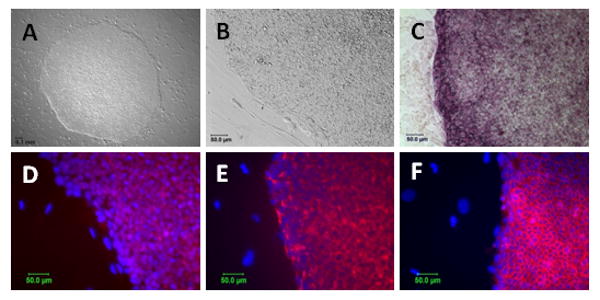
Characterization of iPSCs. iPSCs derived from human fibroblast reprogrammed with lentiviral vectors expressing Oct-3/4, Sox2 and Klf4 maintain colony morphology characteristic of ESC (A: 4X, B: 20X), demonstrate alkaline phosphatase activity (C) and express pluripotency markers as detected by immunocytochemistry including Nanog (D), TRA-1-81 (E) and SSEA-4 (F). For panels D-F pluripotency markers were detected using antibodies tagged by Alexa-594 (red) and nuclei are marked by dapi staining (blue). Notably, control plates of differentiated fibroblasts that were mock transfected failed to give rise to any hESC-like colonies.
A number of hurdles remain for the clinical development of these cells. The combination of gene manipulation with cell therapy increases safety concerns and regulatory barriers. For example, the use of retroviruses or lentiviruses leads to integration of viral DNA into the chromosome, which raises the risk of silencing indispensable genes and/or inducing oncogenesis78. These concerns are partially overcome with the use of adenoviruses or plasmid constructs86-88, but even these episomal vectors carry a risk of DNA integration. Accordingly, any iPSCs created with DNA-based strategies will still need to be carefully screened to exclude any DNA integration. Most recently, Cre/LoxP and piggyBAC transposable elements have been used to generate iPSCs89, 90. These strategies offer certain advantages in that the elements can be silenced or excised; decreasing the possibility of reactivation. However, in the case of piggyBAC transposons the efficiency of excision is extremely low and silencing of genes via the Cre/LoxP system leaves behind vector elements that can still disrupt the genomic insertion site. Thus, this approach requires intensive screening and would be challenging to apply to high-throughput production of autologous lines for regenerative medicine.
Non-viral methodologies that may overcome these concerns include the use of microRNA, cell permeant reprogramming proteins and/or small molecules. We are employing fusion peptides composed of the individual reprogramming factors together with a polyarginine functionality to promote protein transduction. These short polyarginine peptides typically are comprised of a chain of 7-15 arginine molecules, and are expressed as a fusion peptide with a short linker between them and the reprogramming factor. These fusion peptides readily enter the target cells with unbiased uptake, and without cytotoxicity. The polyarginine functionality does not affect DNA binding of the reprogramming proteins, and early results using these peptides to induce reprogramming are promising. Studies are underway to refine the dose, duration and timing of exposure to induce iPSC formation, in combination with small molecules that affect chromatin remodeling (valproic acid) or otherwise accelerate reprogramming (TGFβ inhibitors).
In addition to safety concerns, there are manufacturing hurdles to overcome for therapeutic application. Animal products or non-human iPSC feeder cells used to generate and passage cells, will need to be replaced by defined media and matrices to avoid immune responses to animal protein and to obviate the risk of xenotransmission of zoonotic infections73, 91. A major problem with current reprogramming methods is their inefficiency. The induction of, iPSCs from human fibroblasts takes weeks (generally 3-4 weeks)92 and the yield is low (∼0.01 to 0.1%)78. The inefficiency of nuclear reprogramming is likely related to our incomplete knowledge regarding the determinants of cell fate. As this knowledge increases, other reprogramming factors, or small molecules that regulate chromatin remodeling, for example, may accelerate the process.
Application of iPSCs for vascular regeneration
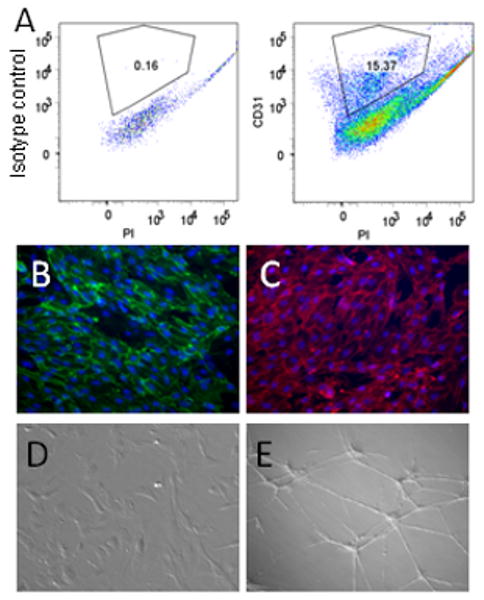
Experimentally, iPSCs have been shown to differentiate into each of the major cardiovascular components including smooth muscle cells93, endothelial cells, vascular mural cells, and cardiomyocytes66, 81. Figure 3 demonstrates the derivation of iPSC-derived endothelial cells (iPSC-ECs) and their vascular potential. Previous work with ESC-derived endothelial cells has documented that these cells are capable of incorporating into the microvasculature of ischemic tissue, enhancing perfusion and improving function94, 95.
Figure 3.
Characterization of human iPSC-derived ECs. iPSCs were differentiated and selected for expression of CD34 by magnetic sorting. (A) CD34+ cells contain a population that demonstrates expression of PECAM-1/CD31 by FACS analysis. EC phenotype of these iPSC-derived CD31+/CD34+ cells is confirmed by expression of EC markers (B) PECAM-1/CD31 and (C) VE-Cadherin, (D) morphological appearance under phase contrast microscopy and by manifestations of EC function including (E) tube formation in matrigel.
There remain substantial hurdles to overcome before iPSC-derived ECs are ready for clinical trials. Currently, the differentiation of iPSCs into therapeutic cells takes the form of directed empiricism, with combinations of growth factors, media and matrices which have been found to favor the desired lineage. For vascular regeneration, more robust selection markers and refined experimental protocols are required to reproducibly guide iPSCs to a vascular lineage66, 91. Furthermore, effective negative selection against pluripotential cells is necessary, to avoid teratoma formation. In this regard, a recent report provided evidence that allogeneic iPSCs, injected directly into the ischemic region in a murine model of myocardial ischemia, did not induce teratoma, and were actually capable of differentiating into cardiac, smooth muscle and endothelial tissue, restoring cardiac architecture and function96. If this surprising observation is confirmed, it is possible that allogeneic iPSCs may be of some utility, or that immune mechanisms might be modulated to reduce the concerns of teratoma formation during therapy with autologous iPSC-derived therapeutic cells. With autologous iPSC-derived cells, there is also the concern that genetic or acquired abnormalities that predisposed a patient to a particular disease will be recapitulated in their iPSCs. In such case, the patient-derived iPSCs may be dysfunctional, e.g. with reduced regenerative capacity, impaired ability to differentiate, or even a tendency to contribute to the disease itself when transplanted back into the host from whence it came. For example, iPSC-derived endothelial cells, when returned to the patient with peripheral arterial disease, might enhance angiogenesis and/or resurface the damaged intima. However, a dysfunctional iPSC-derived EC could potentially contribute to vascular inflammation by manifesting endothelial adhesion molecules, chemokines and pro-thrombotic factors. Finally, as with any potential angiogenic therapy, one must be aware of possible “off-target” effects, such as the potential effect of the iPSC-EC to promote tumor angiogenesis, pathological retinopathy, or neovascularization and progression of atheromatous plaque.
Conclusions
Stem cell therapy arguably has the potential to provide for effective vascular regeneration, though numerous obstacles must still be overcome. Of the available stem cell approaches, iPSCs seem to have the greatest promise. Although ESCs are appealing because of their pluripotency and replicative capacity, they will continue to be limited by ethical and immunological concerns. The use of adult stem cells, in particular EPCs for cardiovascular disease, is supported by early human safety data and an efficacy signal. However, adult stem cells are already lineage committed and grow slowly. In the very patients that they are needed, they are rare and often dysfunctional. By contrast, iPSCs are pluripotent and have high replicative capacity. Furthermore, iPSCs are autologous cells without the ethical or immunological concerns incurred by the use of ESCs. However, much remains to be done to harness these cells for therapeutic purposes. A better understanding of epigenetic alterations, transcriptional activity, and microRNA patterns associated with induction of pluripotency, and with directed differentiation, is required for efficient generation of therapeutic cells. Non-viral strategies for induction of pluripotency will avoid the hazards of DNA integration. Effective differentiation protocols using defined media and matrices (to avoid xenotransmission and immune reactions), together with robust selection and purification strategies (to avoid teratoma or malignancy) are needed. And while genetically-identical patient-derived cells may become easier to produce in the coming years, it is also possible that a tissue bank of renewable, GMP-grade fully HLA-matched vascular cells could be a more practical clinical development. The optimal approach for delivery of iPSC-derived vascular cells will depend upon the indication and patient population, but bioengineered vessels derived from iPSCs, and/or the incorporation of iPSCs into bypass grafts or stents, are a likely future development11, 75, 97. As with all cell-based approaches, long term safety data must be obtained. With diligent efforts to understand the molecular mechanisms of pluripotentiality and cell fate and a commitment to rigorous placebo controlled clinical trials, stem cell regenerative therapy may ultimately become a reality and shift the paradigm of cardiovascular care.
Acknowledgments
Funding sources: This review and related research in our laboratory is supported by grants from the National Institutes of Health (RO1 HL-75774, RC2HL103400, 1U01HL100397, K12 HL087746), the California Tobacco Related Disease Research Program of the University of California (18XT-0098), the American Heart Association (#0970036N), The Wallace H. Coulter Translational Research Grant Program, and the California Institute for Regenerative Medicine (RS1-00183), and the Stanford Cardiovascular Institute.
Footnotes
Disclosures: None
References
- 1.Young HE, Duplaa C, Katz R, Thompson T, Hawkins KC, Boev AN, Henson NL, Heaton M, Sood R, Ashley D, Stout C, Morgan JH, 3rd, Uchakin PN, Rimando M, Long GF, Thomas C, Yoon JI, Park JE, Hunt DJ, Walsh NM, Davis JC, Lightner JE, Hutchings AM, Murphy ML, Boswell E, McAbee JA, Gray BM, Piskurich J, Blake L, Collins JA, Moreau C, Hixson D, Bowyer FP, 3rd, Black AC., Jr Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J Cell Mol Med. 2005;9:753–769. doi: 10.1111/j.1582-4934.2005.tb00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 6.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 10.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 12.Khoo CP, Pozzilli P, Alison MR. Endothelial progenitor cells and their potential therapeutic applications. Regen Med. 2008;3:863–876. doi: 10.2217/17460751.3.6.863. [DOI] [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Kalka C, Baumgartner I. Gene and stem cell therapy in peripheral arterial occlusive disease. Vasc Med. 2008;13:157–172. doi: 10.1177/1358863x08088616. [DOI] [PubMed] [Google Scholar]
- 15.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamagna C, Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80:677–681. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 17.Ruger BM, Breuss J, Hollemann D, Yanagida G, Fischer MB, Mosberger I, Chott A, Lang I, Davis PF, Hocker P, Dettke M. Vascular morphogenesis by adult bone marrow progenitor cells in three-dimensional fibrin matrices. Differentiation. 2008;76:772–783. doi: 10.1111/j.1432-0436.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 18.Ikenaga S, Hamano K, Nishida M, Kobayashi T, Li TS, Kobayashi S, Matsuzaki M, Zempo N, Esato K. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J Surg Res. 2001;96:277–283. doi: 10.1006/jsre.2000.6080. [DOI] [PubMed] [Google Scholar]
- 19.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 22.Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal-Cortivo L, Julia P, Fiessinger JN, Cavazzana-Calvo M, Aiach M, Emmerich J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol. 2008;21:837–846. doi: 10.1038/modpathol.2008.48. [DOI] [PubMed] [Google Scholar]
- 23.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 24.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 25.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, Thomas P, Bastian B, Chan JK, Lo G, Ho CL, Chan WS, Kwong RY, Parker A, Hauser TH, Chan J, Fong DY, Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 26.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 27.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, Gomez-Bueno M, Cantalapiedra A, Fernandez J, Gutierrez O, Sanchez PL, Hernandez C, Sanz R, Garcia-Sancho J, Sanchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 29.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 30.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 31.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 32.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 33.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 34.Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 35.Arai M, Misao Y, Nagai H, Kawasaki M, Nagashima K, Suzuki K, Tsuchiya K, Otsuka S, Uno Y, Takemura G, Nishigaki K, Minatoguchi S, Fujiwara H. Granulocyte colony-stimulating factor: a noninvasive regeneration therapy for treating atherosclerotic peripheral artery disease. Circ J. 2006;70:1093–1098. doi: 10.1253/circj.70.1093. [DOI] [PubMed] [Google Scholar]
- 36.Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Sueda T, Goto C, Matsubara H, Murohara T, Yoshizumi M. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–1218. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- 37.Napoli C, Williams-Ignarro S, de Nigris F, de Rosa G, Lerman LO, Farzati B, Matarazzo A, Sica G, Botti C, Fiore A, Byrns RE, Sumi D, Sica V, Ignarro LJ. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci U S A. 2005;102:17202–17206. doi: 10.1073/pnas.0508534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 39.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004 Nov 1;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 40.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 42.Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL, Tse VC. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- 43.Schafer R, Northoff H. Cardioprotection and cardiac regeneration by mesenchymal stem cells. Panminerva Med. 2008;50:31–39. [PubMed] [Google Scholar]
- 44.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Kajstura J, Urbanek K, Rota M, Bearzi C, Hosoda T, Bolli R, Anversa P, Leri A. Cardiac stem cells and myocardial disease. J Mol Cell Cardiol. 2008;45:505–513. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 46.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 47.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 48.Zimmet H, Krum H. Using adult stem cells to treat heart failure--fact or fiction? Heart Lung Circ. 2008;17 4:S48–54. doi: 10.1016/j.hlc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 50.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 51.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 52.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 53.Co M, Tay E, Lee CH, Poh KK, Low A, Lim J, Lim IH, Lim YT, Tan HC. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J. 2008;155:128–132. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 54.Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv. 2007;70:477–484. doi: 10.1002/ccd.21292. [DOI] [PubMed] [Google Scholar]
- 55.Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Review: application of stem cells for vascular tissue engineering. Tissue Eng. 2005;11:1535–1552. doi: 10.1089/ten.2005.11.1535. [DOI] [PubMed] [Google Scholar]
- 56.Yoder MC. Judging a proangiogenic cell by its cover. Blood. 2009;114:756–757. doi: 10.1182/blood-2009-05-219378. [DOI] [PubMed] [Google Scholar]
- 57.Barber CL, Iruela-Arispe ML. The ever-elusive endothelial progenitor cell: identities, functions and clinical implications. Pediatr Res. 2006;59:26R–32R. doi: 10.1203/01.pdr.0000203553.46471.18. [DOI] [PubMed] [Google Scholar]
- 58.Brixius K, Funcke F, Graf C, Bloch W. Endothelial progenitor cells: a new target for the prevention of cardiovascular diseases. Eur J Cardiovasc Prev Rehabil. 2006;13:705–710. doi: 10.1097/01.hjr.0000221862.34662.31. [DOI] [PubMed] [Google Scholar]
- 59.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loffredo F, Lee RT. Therapeutic vasculogenesis: it takes two. Circ Res. 2008;103:128–130. doi: 10.1161/CIRCRESAHA.108.180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roche R, Hoareau L, Mounet F, Festy F. Adult stem cells for cardiovascular diseases: the adipose tissue potential. Expert Opin Biol Ther. 2007;7:791–798. doi: 10.1517/14712598.7.6.791. [DOI] [PubMed] [Google Scholar]
- 62.Pearson JD. Endothelial progenitor cells - hype or hope? J Thromb Haemost. 2009;7:255–262. doi: 10.1111/j.1538-7836.2008.03214.x. [DOI] [PubMed] [Google Scholar]
- 63.Spinetti G, Kraenkel N, Emanueli C, Madeddu P. Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res. 2008;78:265–273. doi: 10.1093/cvr/cvn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu SJ, Ivanova Y, Feng Q, Luo C, Lanza R. Hemangioblasts from human embryonic stem cells generate multilayered blood vessels with functional smooth muscle cells. Regen Med. 2009;4:37–47. doi: 10.2217/17460751.4.1.37. [DOI] [PubMed] [Google Scholar]
- 65.Adams B, Xiao Q, Xu Q. Stem cell therapy for vascular disease. Trends Cardiovasc Med. 2007;17:246–251. doi: 10.1016/j.tcm.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 67.Tateishi K, Takehara N, Matsubara H, Oh H. Stemming heart failure with cardiac- or reprogrammed-stem cells. J Cell Mol Med. 2008;12:2217–2232. doi: 10.1111/j.1582-4934.2008.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 69.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 70.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 72.Kennedy D. Breakthrough of the year. Science. 2007;318:1833. doi: 10.1126/science.1154158. [DOI] [PubMed] [Google Scholar]
- 73.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 74.Yamanaka S. Pluripotency and nuclear reprogramming. Philos Trans R Soc Lond B Biol Sci. 2008;363:2079–2087. doi: 10.1098/rstb.2008.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao R, Daley GQ. From fibroblasts to iPS cells: induced pluripotency by defined factors. J Cell Biochem. 2008;105:949–955. doi: 10.1002/jcb.21871. [DOI] [PubMed] [Google Scholar]
- 76.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 77.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 78.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 79.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 80.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 82.Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, Zou J, Cheng L. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998–2005. doi: 10.1634/stemcells.2008-0346. [DOI] [PubMed] [Google Scholar]
- 83.Liu SV. iPS cells: a more critical review. Stem Cells Dev. 2008;17:391–397. doi: 10.1089/scd.2008.0062. [DOI] [PubMed] [Google Scholar]
- 84.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 85.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 87.Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. iPS Cell Generation Using a Single Lentiviral Stem Cell Cassette. Stem Cells. 2008 doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009 doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009 doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 92.Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3:1180–1186. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- 93.Xie C, Huang H, Wei S, Song LS, Zhang J, Ritchie RP, Chen L, Zhang M, Chen YE. A Comparison of Murine Smooth Muscle Cells Generated from Embryonic versus Induced Pluripotent Stem Cells. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116(11 Suppl):I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamahara K, Sone M, Itoh H, Yamashita JK, Yurugi-Kobayashi T, Homma K, Chao TH, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Fukunaga Y, Tamura N, Nakao K. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLoS ONE. 2008;3:e1666. doi: 10.1371/journal.pone.0001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L, Zhou J, Lu Q, Wei Y, Hu S. A novel small-diameter vascular graft: in vivo behavior of biodegradable three-layered tubular scaffolds. Biotechnol Bioeng. 2008;99:1007–1015. doi: 10.1002/bit.21629. [DOI] [PubMed] [Google Scholar]


