Abstract
Objective
To determine the prognostic utility of dobutamine cardiovascular magnetic resonance (DCMR) stress test results in women.
Background
To date, the preponderance of studies reporting the utility of DCMR stress results for predicting cardiac prognosis have been performed in men. We sought to determine the utility of DCMR results for predicting cardiac prognosis in women.
Methods
Two hundred sixty-six consecutively referred women underwent DCMR in which left ventricular wall motion (LVWM) was assessed at rest and after intravenous dobutamine and atropine. Inducible LVWM abnormalities were identified during testing. Women were contacted to determine the post DCMR occurrence of a cardiac event. All events were substantiated according to defined criteria, and then verified after a thorough medical record review by individuals blinded to testing data.
Results
Women were contacted an average of 6.2 ± 1.6 (median 6.2, range 0.8 to 10.4) years after DCMR; 27% of the women experienced an inducible LVWM abnormality during testing. In those with and without inducible LVWM abnormalities, the proportion of women with cardiac events were 63% versus 30%, respectively, (hazard ratio [HR] of 2.7 [CI 1.8 – 4.3] for the presence of inducible LVWM abnormalities p<0.0001). The proportion of women with myocardial infarction (MI) and cardiac death were 33.3% and 7.5%, respectively. This resulted in a HR for MI and cardiac death of 4.1 [CI 2.2 – 9.4] for those with versus without inducible LVWM abnormalities; p<0.0001. A subgroup analysis was performed in women without a history of coronary artery disease and in those with LVWM abnormalities, DCMR remained an adverse predictor of cardiac events (HR 4.0, CI 1.8 – 9.0, p=0.003).
Conclusions
Inducible LVWM abnormalities during DCMR predict cardiac death and MI in women. Similar to men, these results indicate DCMR is a valuable noninvasive stress imaging modality for identifying cardiac risk in women with known or suspected ischemic heart disease.
Keywords: Women, Prognosis, Magnetic Resonance
Dobutamine cardiovascular magnetic resonance (DCMR) has been used to identify cardiac risk in individuals with or suspected of having coronary artery disease (1,2). To date, the majority of participants in these prior DCMR studies of prognosis have been men (1,2). Recently, the diagnostic utility of DCMR for identifying flow limiting coronary artery stenoses in women was shown to be high (3). At present, however, it is uncertain whether the results of DCMR can be used to predict cardiac events in women, particularly those that are difficult to risk stratify with other forms of noninvasive imaging. Accordingly, we performed this study to determine the prognostic utility of DCMR results for forecasting future cardiac events in women suspected of having or known to have acquired coronary arteriosclerosis.
Methods
Study population and design
The Institutional Review Board at the Wake Forest University School of Medicine approved this study, and all participants provided witnessed informed consent. Patients with contraindications to DCMR (implanted pacemakers, defibrillators, or intracranial metal) were excluded from enrollment. The study population consisted of 266 consecutive women that had undergone high dose dobutamine and atropine stress testing between 1997, and 2004, secondary to >6 of 17 segments not well seen with second harmonics transthoracic echocardiography with or without microbubble contrast. The study was designed as a prospective cohort analysis in which cardiac outcomes were determined after women received DCMR stress.
DCMR Procedure
As previously described, DCMR stress images were collected on a Horizon 1.5T whole-body imaging system (General Electric Medical Systems) using cine white blood gradient-echo imaging (4). Images were viewed using a software program designed for display of dobutamine stress magnetic resonance images in a multi-window, synchronized format (5). According to previously published techniques (1–5), left ventricular wall motion (LVWM) was assessed at rest and after intravenous dobutamine and atropine administered to achieve 80% of the maximum predicted heart rate response for age. LVWM was identified throughout testing across myocardial segments identified by the American Heart Association as normal, hypokinetic, akinetic, or dyskinetic (6). Akinetic segments that exhibited a dyskinetic response to stress testing, were not included in our analysis. As previously described, the resting left ventricular ejection fraction (LVEF) was measured using a biplane area-length technique (7). Myocardial segments were identified using an 18 segment model previously described (1). Inducible LVWM abnormalities were defined as deterioration of LVWM during infusion (1,2,4,5,8).
Participants’ routine use of medications, including β-receptor antagonists, were not altered before testing. An occurrence of prior Q-wave myocardial infarction (MI), prior coronary artery revascularization, and risk factors for coronary arteriosclerosis, including the treatment or presence of hypertension (blood pressure > 140/90 mm Hg), a total serum cholesterol value ≥ 6.4 mmol/L, a fasting glucose measuring ≥ 7.8 mmol/L, a history of chronic obstructive pulmonary disease (COPD), and a history of smoking, were recorded at the time of DCMR testing.
Outcomes
Personnel blinded to the study design or stress testing results contacted each subject (or, if deceased, an immediate family member) to determine if cardiac events had occurred since their DCMR test. The date of last contact was recorded. All events were confirmed by review of the participant’s medical records. Hard events were defined as MI (angina of ≥ 20 minutes duration and either ≥ 1 mm ST segment elevation in 2 contiguous electrocardiographic (ECG) leads or a rise in troponin and creatine kinase level and its MB fraction exceeding the 99th percentile) (9), or cardiac death (death during a hospital admission for acute coronary syndrome [ACS], significant cardiac arrhythmia, or refractory congestive heart failure). Other events (termed any events) included hard events, all-cause mortality, coronary arterial revascularization, unstable angina or congestive heart failure warranting hospital admission. When available, ECG, enzymatic, or autopsy data were used to substantiate cardiac mortality. In the case of 2 simultaneous cardiac events, the worst event was selected for use in follow-up (cardiac death>MI>revascularization>unstable angina or congestive heart failure).
Statistical Analysis
Patients were categorized according to the presence, extent, and location of inducible LVWM abnormalities during pharmacologic infusion. All grouped data were expressed as mean ± standard deviation. The association between the risk of future cardiac events with individual patient factors was estimated and tested for significance using Cox proportional hazards model. Univariable patient factors that were tested for their potential association with risk of future cardiac events were selected based upon prior association with cardiovascular events. These potential cardiac risk factors were included in the multivariable Cox proportional hazards regression models if they showed a univariable association with a p<0.20. Collinearity was examined and none of the independent variables had a pairwise correlation >0.50. The increased or decreased risk of future cardiovascular events due to the presence or absence of a given variable was expressed by a hazard ratio (HR) with corresponding 95% confidence interval (CI). Variables were considered significant if the null hypothesis of no contribution could be rejected at the 5% level of significance probability value of > 0.05. The reasonableness of assuming a proportional hazard model for each univariable and multivariable model was assessed using the test for proportional hazards based on Schoenfeld residuals; in no cases did any of the tests give a p-value <0.30 (10,11). The probability of the presence or absence of cardiac events as a function of follow-up duration was estimated by the Kaplan-Meier method and compared between groups by use of the log-rank test.
Results
Contact was made with all participants (100% participant follow-up) at a mean of 6.2 ± 1.6 (median 6.2, range 0.8 to 10.4) years after performance of the DCMR procedure. The mean age of the women at the time of DCMR testing was 63 ± 12 years. Of the 266 participants, 9 underwent coronary arterial revascularization (coronary artery bypass or percutaneous intervention) within 60 days of their DCMR stress test. To exclude the possibility that test results influenced or biased decision making, or that immediate revascularization prevented or promoted subsequent hard events, we excluded these 9 subjects from analysis. Thirty-six (13.5%) women did not achieve maximal heart rate response as a result of the prescribed use of rate limiting calcium antagonists or beta blockers. Since previous studies have identified a reduced sensitivity and specificity of stress test results for identifying inducible LVWM abnormalities at heart rate responses of less than 80% to 85% of the maximum predicted for age (12,13), these individuals’ outcome analyses were assessed separately.
Baseline data on the remaining 221 are displayed in Table 1. As shown, the age and body mass index of the 2 groups of participants were similar. The group with inducible LVWM abnormalities more frequently experienced prior coronary artery revascularization procedures, diabetes, and a mildly reduced LVEF at the time of testing. Hemodynamic data from the participants are shown in Table 2.
TABLE 1.
Demographic Data and Summary of Events
| All | Inducible LVWMA | No Inducible LVWMA | P-value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Patients, % | n=221 | n=60 (27) | n=161 (73) | |
| Age, years | 63 ±12 | 62 ±12 | 63 ±12 | 0.56 |
| Weight, kg | 189 ±48 | 87±20 | 85 ±23 | 0.7 |
| BMI | 33 ±8 | 34 ±8 | 32 ±9 | 0.18 |
| LVEF, % | 59 ±11 | 51 ±12 | 62 ±10 | <0.0001 |
| Historical information (%) | ||||
| Prior Q-wave MI | 62 (28) | 22 (37) | 40 (25) | 0.08 |
| Prior Re-vascularization | 81 (37) | 32 (53) | 49 (30) | 0.001 |
| Prior PCI | 55 (25) | 24 (40) | 30 (19) | 0.001 |
| Prior CABG | 42 (19) | 16 (27) | 26 (16) | 0.08 |
| Hypertension | 162 (73) | 49 (82) | 113 (70) | 0.087 |
| Diabetes | 84 (38) | 30 (50) | 54 (34) | 0.025 |
| Hyperlipidemia | 127 (57) | 38 (63) | 89 (55) | 0.28 |
| Family History | 115 (52) | 31 (52) | 84 (52) | 0.95 |
| Smoking | 83 (38) | 26 (43) | 57 (35) | 0.28 |
| COPD/Asthma | 47 (21) | 9 (15) | 38 (24) | 0.17 |
| Congestive Heart Failure | 25 (11) | 11 (18) | 14 (9) | 0.044 |
| Medications (%) | ||||
| Digoxin | 13 (6) | 3 (5) | 10 (6) | 0.74 |
| Diuretic | 103 (47) | 33 (55) | 70 (43) | 0.13 |
| Beta Blocker | 77 (35) | 23 (38) | 54 (34) | 0.51 |
| Calcium Antagonist | 48 (22) | 18 (30) | 30 (19) | 0.069 |
| Aspirin | 110 (50) | 34 (57) | 76 (47) | 0.21 |
| Nitrate | 66 (30) | 27 (45) | 39 (24) | 0.0026 |
| ACE inhibitor/ARB | 62 (28) | 26 (43) | 36 (22) | 0.002 |
| Anti-coagulation | 18 (8) | 4 (7) | 14 (9) | 0.63 |
| Anti-Lipid | 86 (39) | 24 (40) | 62 (39) | 0.84 |
| Events (%) | ||||
| Cardiac Death | 21 (10) | 15 (25) | 6 (4) | <0.0001 |
| Myocardial infarction | 19 (11) | 10 (17) | 9 (6) | <0.01 |
| Revascularization | 46 (21) | 19 (32) | 27 (16) | 0.011 |
| Unstable angina | 46 (21) | 22 (37) | 24 (14) | <0.001 |
| Congestive Heart Failure | 17 (8) | 6 (10) | 11 (6) | 0.34 |
Values are expressed as n (%) unless otherwise indicated.
Abbreviations: ACE, angiotension converting enzyme; ARB, angiotension receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; LVWMA, left ventricular wall motion abnormalities; PCI, percutaneous coronary intervention.
TABLE 2.
Hemodynamic Data
| All | Inducible LVWMA | No Inducible LVWMA | P-value | |
|---|---|---|---|---|
| Resting HR (bpm) | 76 ±12 | 72 ±12 | 77 ±14 | 0.35 |
| Peak HR (bpm) | 131 ±14 | 121 ±18 | 148 ±24 | <0.0001 |
| % MPHRR** | 83 ±8% | 77 ±11% | 85 ±4% | <0.0001 |
| Resting SBP (mmHg) | 142 ±23 | 137 ±23 | 145 ±22 | 0.058 |
| Peak SBP (mmHg) | 146 ±26 | 140 ±30 | 148 ±24 | 0.052 |
| Resting DBP (mmHg) | 79 ±14 | 75 ±16 | 81 ±13 | 0.002 |
| Peak DBP (mmHg) | 74 ±15 | 72 ±17 | 75 ±14 | 0.15 |
| Rest Rate/Pressure Product | 10838 ±2652 | 9978 ±2250 | 11159 ±2732 | 0.0035 |
| Peak Rate/Pressure Product | 19121 ±3882 | 16947 ±4547 | 19865 ±3275 | <0.0001 |
Abbreviations: BPM, beats per minute; LVWMA indicates left ventricular wall motion abnormalities; MPHRR, Maximum predicted heart rate response for age
There were 149 total cardiac events among 89 participants over the course of the study; 36 of these were hard events. Of the hard events, there were 15 cardiac deaths among 60 participants with inducible LVWM abnormalities, and 6 among the 161 participants without inducible LVWM abnormalities. The mean follow-up for event free patients was 6.1 (25th percentile 4.8, 75th percentile 7.4) years, nearly the same for women experiencing a cardiac event. The occurrences of all cardiac events are displayed in Table 1. Four patients experienced a non-fatal MI in a separate hospitalization from their cardiac death. As shown, deterioration in LVWM predicted a future increase in the occurrence of cardiac death, MI, coronary artery revascularization, and unstable angina warranting hospitalization.
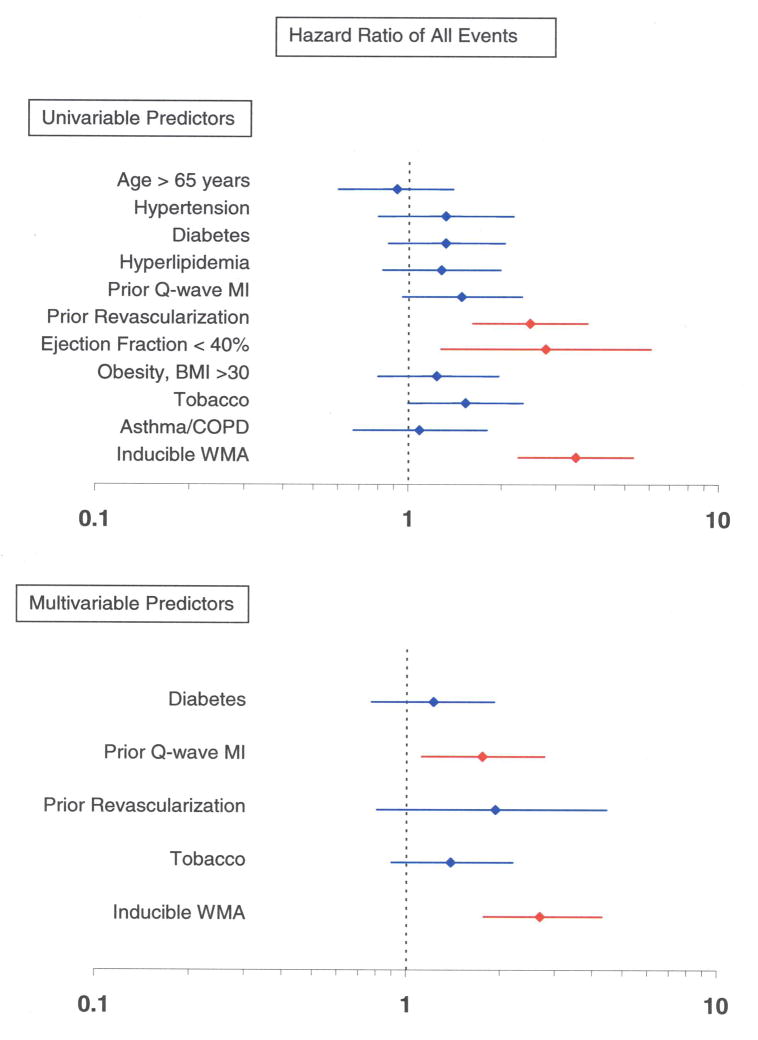
Sixty of the 221 women (27%) experienced inducible LVWM abnormalities during cardiac testing. The overall proportion of women with cardiac events in those with and without inducible LVWM abnormalities were 63.0% and 30.0% respectively (p<0.0001), and the proportion of women with hard events was 33.3% and 7.5%, respectively (p<0.0001). A Kaplan Meier analysis of the overall event rate and the hard event rate, in those with and without inducible LVWM abnormalities, is shown in Figure 1. Figure 2 displays the HRs of cardiac events in isolation (univariable) and after accounting for risk factors for coronary arteriosclerosis or cardiac events predicted in the univariable analysis (multivariable analysis).
Figure 1. Kaplan-Meier survival plots indicating the proportion of women free from cardiac events (y-axis) versus time (x-axis).
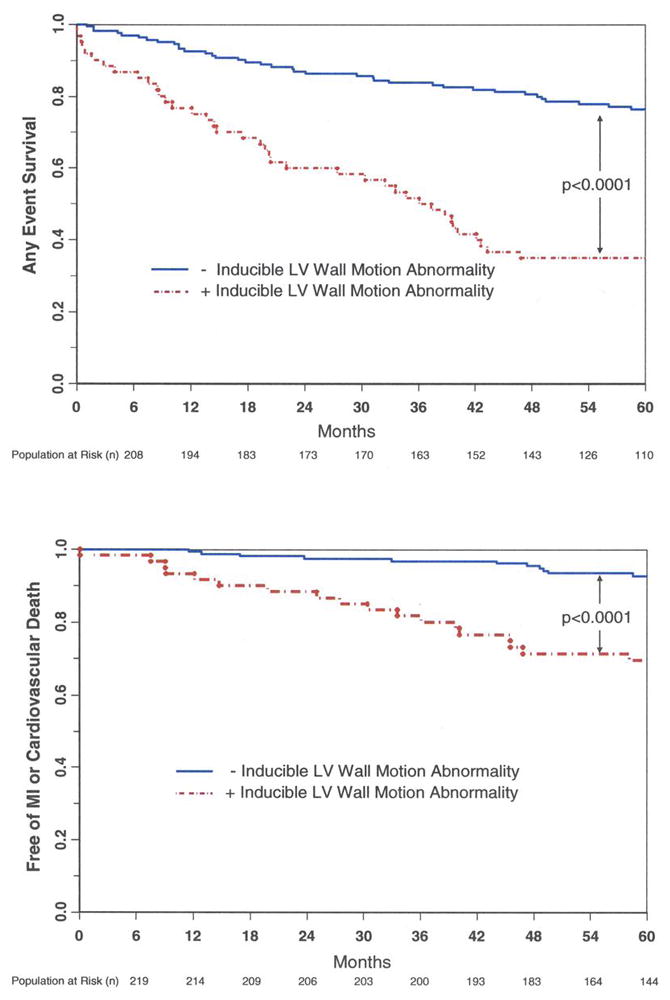
Women with inducible LVWM abnormalities are indicated by the red lines, and individuals without inducible LVWM abnormalities are indicated by the black lines. As shown, those individuals without inducible LVWM abnormalities experienced fewer (any, top graph; hard, bottom graph) cardiac events compared to individuals with inducible LVWM abnormalities.
Figure 2. Univariable and multivariable predictors for cardiac events.
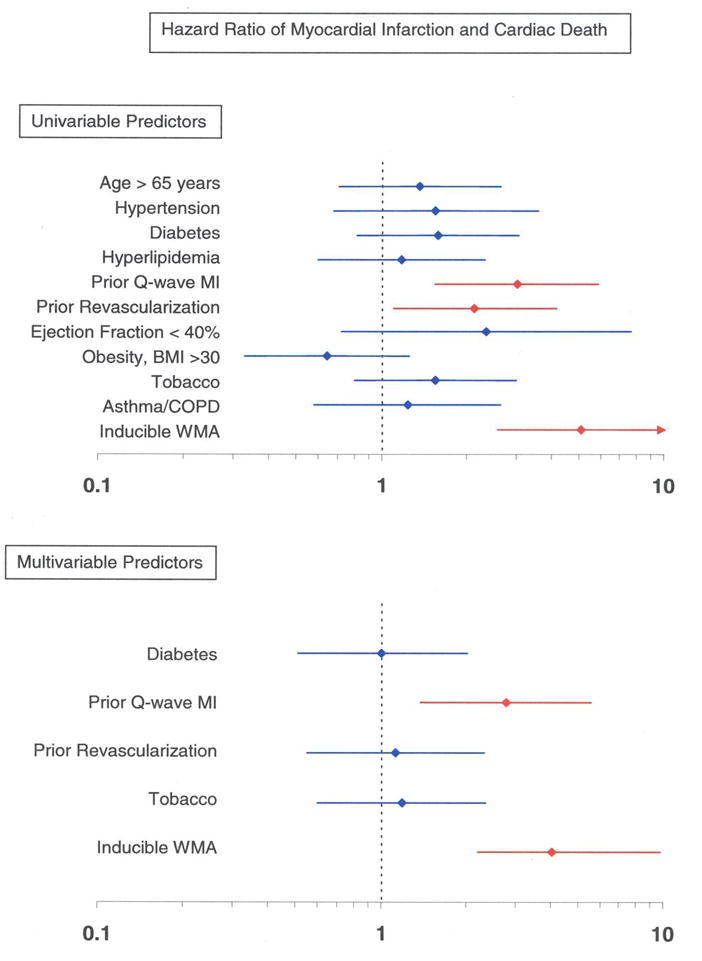
Univariable and multivariable analyses displaying hazard ratios ±95% confidence intervals (x-axis) for developing MI or cardiac death (Panel A), and any cardiac events (Panel B). This model includes risk factors for coronary arteriosclerosis and myocardial infarction. As shown, a stress induced LVWM abnormality is an independent predictor of MI and cardiac death, and any cardiac event after accounting for known risk factors for cardiac events.
One hundred and ten women in our population without a history of coronary artery disease (history of MI or prior coronary revascularization) underwent stress testing. The 5 year event rate for all cardiac events in women with inducible LVWM abnormalities but without coronary disease, was lower 27.6% vs 46.1% than those with coronary atherosclerosis (p<0.001). In women without known coronary atherosclerosis, LVWM abnormalities during testing remained an adverse predictor of any cardiac event (HR 4.0, CI 1.8 – 9.0, p=0.003), and trended towards identifying those at risk of a future hard event (HR 2.7, CI 0.7 – 11.6, p=0.14).
We examined the relationship between the number of segments that experienced deterioration in LVWM and future events. Overall, 3970 segments were assessed during testing. In regards to the number of segments that experienced deterioration in LVWM, the estimated proportion of women without a hard event at 5 years was 64.2% versus 79.6%, respectively, for those individuals experiencing deterioration in LVWM in 1 or 2 segments versus ≥ 3 segments. (p=0.20). Similarly, for individuals with inducible LVWM abnormalities involving 1 coronary artery territory (as defined by the American Heart Association) (6), the estimated proportion of women without a hard event at 5 years was 71.1% versus 73.3% in women with an inducible LVWM abnormality in 2 or 3 coronary territories (p=0.86).
The 36 individuals that did not achieve a heart rate response of 80% predictive for age without an inducible LVWM abnormality averaged 58 ± 10 (range 45–72) years in age and exhibited a resting LVEF of 58 ± 14 (range 20–76)%. Over 5 years, this group of individuals experienced 4 hard events of which 1 was a cardiac death; their 5-year overall estimated percentage of women having a hard event rate was 11%. This 5-year percentage was similar to the 7% 5-year event percentage of hard events in individuals without inducible LVWM abnormalities that achieved 80% predicated heart rate response for age, and substantially lower than the 5-year event percentage of 33% in individuals with inducible LVWM abnormalities. The same women experienced 13 total cardiac events (for an overall percentage of events of 36%) which was similar to individuals without inducible LVWM abnormalities that achieved 80% predicted heart rate response for age (29%), and not as elevated (5-year proportion of those with events of 63%) as individuals with inducible LVWM abnormalities.
Discussion
The results of this study indicate that in women poorly suited for noninvasive imaging with DSE, the presence of DCMR inducible LVWM abnormalities forecasts a high rate of cardiovascular events including future MI and cardiac death (Table 1). Second, there is a high negative predictive value of DCMR wall motion stress test results in women (Figure 1). Women who obtain > 80% predictive heart rate response for age during pharmacologic stress without inducible LVWM abnormalities exhibit an event free survival of 1.2% per year for MI and cardiac death for the 5 years following the stress test. Third, the results of DCMR stress are useful in determining cardiac risk in women after accounting for known clinical predictors of adverse cardiac risk (Figure 2).
Despite the fact that more women than men die annually of cardiac events related to coronary artery disease, women are under reported in studies assessing the prognostic utility of cardiovascular testing methods (14). In addition, with transthoracic echocardiography, due to poor acoustic windows, or radionuclide scintigraphy, due to attenuation artifacts, it is often difficult to obtain adequate imaging test results in women (15,16). Studies have demonstrated a higher diagnostic accuracy of DCMR in detection of LVWM abnormalities compared to DSE (17), and there are several additional studies that have highlighted the utility of DCMR stress to forecast cardiac prognosis (1,2,12,18). To date, however, there have been limited data on the prognostic utility of DCMR for determining cardiac prognosis in women with suspected coronary artery disease (19). Given the need to identify cardiac prognosis in women, and the difficulty in obtaining image results in women with echocardiography or radionuclide scintigraphy, this study was designed to address the prognostic utility of DCMR in women.
The women enrolled in this study exhibited an average age similar to those referred for cardiovascular stress testing (14). Also, they exhibited poor acoustic windows for transthoracic echocardiography. As shown in Table 1 and in Figure 1, the proportion of women with events and the event free survival of those women with and without DCMR inducible LVWM abnormalities during testing is similar to populations previously reported undergoing DCMR noninvasive cardiac stress testing that predominately included men (1,2). Women without inducible LVWM abnormalities during testing experienced a favorable prognosis for the 5 years following testing. The post-stress proportion of women with events of 1.2% per year is similar to that reported in previous DCMR stress testing comprised primarily of men (1,2). Also, as shown in Table 2, the hemodynamic response to cardiovascular stress is similar to that reported previously in individuals undergoing DCMR stress testing (4). In addition, the prognosis of women undergoing DCMR stress testing in our study compares similarly to that of other large prognostic studies of DSE and radionuclide scintigraphy of women in whom imaging results could be obtained (14,20).
Well established, existing risk factors for cardiac events, such as prior MI, diabetes, hyperlipidemia, and hypertension were prevalent in the women participating in this study, and were notably disproportionally distributed between women with and without inducible LVWM abnormalities and cardiac events. To address whether the presence of risk factors in the 2 study groups influenced our results, we performed multivariable regression analysis to determine the prognostic utility of DCMR stress results after accounting for the presence of these risk factors. After this multivariable analysis (Figure 2), inducible LVWM abnormalities identified during DCMR independently predicted future cardiac events in women.
Given the challenge of managing women with suspected, but not known, coronary atherosclerosis, we performed a separate analysis to assess the utility of DCMR in this population. Given the lower prevalence of ischemic heart disease in women without known coronary arteriosclerosis, there were less cardiac events appreciated overall. Among the 110 women, only 9 hard events occurred: 5 of the hard events occurred in the 20 women with inducible ischemia. For this reason, the results of this study indicate a trend (p=0.14) toward the utility of DCMR wall motion results predicting future MI and cardiac death in women without known coronary arteriosclerosis. We were able to determine however, that inducible ischemia is an adverse predictor of all cardiac events. These results demonstrate that the identification of a DCMR induced LVWM abnormality provides additional prognostic information for identifying cardiac risk in women with known or suspected ischemic heart disease.
Previous studies of cardiac prognosis after DCMR stress have demonstrated a trend toward an association between the number of left ventricular (LV) myocardial segments with inducible ischemia and more frequent adverse events (1,21,22). Our results in women demonstrate a similar trend. However, as a safety precaution we note that our stress testing protocol was terminated at the earliest detection of a wall motion abnormality, thus potentially limiting our ability to identify all myocardial regions at risk (4). A study with more participants may discern the relationship between number of LV myocardial segments with inducible LVWM abnormalities and incremental cardiac risk in women.
Two important aspects of the study population merit further discussion. First, as shown in Table 1, the average body mass index of the participants enrolled in this study was elevated (classified as obese by the World Health Organization) (23). Obesity is increasing in prevalence in many developing and developed industrialized nations (23). Likely, the elevated body mass index accounts for the reason why many of the women were referred after failed DSE due to poor acoustic windows. High spatial and temporal resolution images are acquired during DCMR that allow for early recognition of inducible LVWM abnormalities (4,5). In this study, all myocardial segments were visualized throughout the course of DCMR testing. The results of this study indicate that in relatively obese women DCMR is a suitable testing modality for accurately identifying cardiac risk.
Second, the LVEF in this particular study averaged 59 ± 11%. This is not surprising given that many elderly women with cardiac disorders such as heart failure exhibit a preserved LVEF (24,25). Recently, our group identified that the presence of inducible LVWM abnormalities during dobutamine stress in patients with a LVEF < 40% did not offer prognostic information above and beyond the resting LVEF (25). In this study, only 14 participants exhibited a resting LVEF < 40%. It is important to recognize that data from the current study indicate that the presence of DCMR inducible LVWM abnormalities is predictive of adverse cardiac events in women whom the majority exhibit a LVEF > 40%. We remain uncertain as to the prognostic utility of dobutamine inducible LVWM abnormalities in women with a LVEF < 40%.
Our study has the following limitations. First, our study utilized visual assessments of LVWM. Newer imaging protocols that involve simultaneous assessments of LVWM, myocardial perfusion, and late gadolinium enhancement may yield improved results in certain patient populations (27). Second, we are uncertain of the prevalence of LV hypertrophy in our participants. LV hypertrophy is a known predictor of adverse cardiac events (28,29).
Conclusions
Inducible ischemia of any myocardial segment during DCMR predicts cardiac death and MI in women, while women with no evidence of ischemia have a good mid-term prognosis. Thus, DCMR is a suitable noninvasive stress imaging modality for identifying cardiac risk in women that may be at risk in adverse cardiac events, and DCMR stress results exhibit high prognostic utility similar to that previously identified in men.
Acknowledgments
Research supported in part by National Institution of Health Grants R01 HL076438, P30 AG21332, and M01 RR007122
We appreciate the expert assistance of Deanna Carr in preparation of the manuscript.
Abbreviations Page
- CI
Confidence Interval
- DCMR
Dobutamine Cardiovascular Magnetic Resonance
- DSE
Dobutamine Stress Echocardiography
- ECG
Electrocardiographic
- HR
Hazard Ratio
- LV
Left Ventricular
- LVEF
Left Ventricular Ejection Fraction
- LVWM
Left Ventricular Wall Motion
- MI
Myocardial Infarction
Footnotes
No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106(18):2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 2.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: Adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115(13):1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 3.Klem I, Greulich S, Heitner JF, et al. Value of cardiovascular magnetic resonance stress perfusion testing for the detection of coronary artery disease in women. Am Coll Cardiol Img. 2008;1:436–445. doi: 10.1016/j.jcmg.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100(16):1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton CA, Link KM, Salido TB, Thomas MS, Epstein FH, Hundley WG. Is imaging at intermediate doses necessary during dobutamine stress magnetic resonance imaging? J Cardiovasc Magn Reson. 2001;3(4):297–302. doi: 10.1081/jcmr-100108582. [DOI] [PubMed] [Google Scholar]
- 6.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 7.Lawson MA, Blackwell GG, Davis ND, Roney M, DellItalia LJ, Pohost GM. Accuracy of biplane long-axis left ventricular volume determined by cine magnetic resonance imaging in patients with regional and global dysfunction. Am J Cardiol. 1996;77(12):1098–1104. doi: 10.1016/s0002-9149(96)00140-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen CG, Li L, Chen LL, et al. Incremental doses of dobutamine induce a biphasic response in dysfunctional left-ventricular regions subtending coronary stenoses. Circulation. 1995;92(4):756–766. doi: 10.1161/01.cir.92.4.756. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert J, White H. Universal Definition of Myocardial Infarction. J Am Coll Cardiol. 2007;50(22):2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 11.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 12.Rerkpattanapipat P, Morgan TM, Neagle CM, Link KM, Hamilton CA, Hundley WG. Assessment of preoperative cardiac risk with magnetic resonance imaging. Am J Cardiol. 2002;90(4):416–419. doi: 10.1016/s0002-9149(02)02501-8. [DOI] [PubMed] [Google Scholar]
- 13.McNeill AJ, Fioretti PM, el-Said SM, Salustri A, Forster T, Roelandt JR. Enhanced sensitivity for detection of coronary artery disease by addition of atropine to dobutamine stress echocardiography. Am J Cardiol. 1992;70(1):41–46. doi: 10.1016/0002-9149(92)91387-j. [DOI] [PubMed] [Google Scholar]
- 14.Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111(5):682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 15.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiograph. J Am Coll Cardiol. 1997;30(3):595–606. doi: 10.1016/s0735-1097(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 16.Wallis JW, Miller TR, Koppel P. Attenuation Correction in Cardiac SPECT without a Transmission Measurement. J Nucl Med. 1995;36(3):506–512. [PubMed] [Google Scholar]
- 17.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI - Comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 18.Wahl A, Paetsch I, Roethemeyer S, Klein C, Fleck E, Nagel E. High-dose dobutamine-atropine stress cardiovascular MR imaging after coronary revascularization in patients with wall motion abnormalities at rest. Radiology. 2004;233(1):210–216. doi: 10.1148/radiol.2331030463. [DOI] [PubMed] [Google Scholar]
- 19.Makaryus AN, Shaw LJ, Mieres JH. Diagnostic strategies for heart disease in women: An update on imaging techniques for optimal management. Cardiol Rev. 2007;15(6):279–287. doi: 10.1097/CRD.0b013e318156e9cd. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LJ, Vasey C, Sawada S, Rimmerman C, Marwick TH. Impact of gender on risk stratification by exercise and dobutamine stress echocardiography: long-term mortality in 4234 women and 6898 men. Eur Heart J. 2005;26:447–456. doi: 10.1093/eurheartj/ehi102. [DOI] [PubMed] [Google Scholar]
- 21.Hundley WG, Rerkpattanapipat P, Little WC, Link KM, Morgan TM. Relation of cardiac prognosis to segment location with apical left ventricular ischemia. Am J Cardiol. 2003;92(10):1206–1208. doi: 10.1016/j.amjcard.2003.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Elhendy A, Mahoney DW, Khandheria BK, Paterick TE, Burger KN, Pellikka PA. Prognostic significance of the location of wall motion abnormalities during exercise echocardiography. J Am Coll Cardiol. 2002;40(9):1623–1629. doi: 10.1016/s0735-1097(02)02338-0. [DOI] [PubMed] [Google Scholar]
- 23.Hyde R. Europe battles with obesity. Lancet. 2008;371(9631):2160–2161. doi: 10.1016/s0140-6736(08)60936-8. [DOI] [PubMed] [Google Scholar]
- 24.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2004;43(3):317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 25.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41(2):217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 26.Dall’Armellina E, Morgan TM, Mandapaka S, et al. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index. J Am Coll Cardiol. 2008;52(4):279–286. doi: 10.1016/j.jacc.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebker R, Jahnke C, Manka R, et al. Additional value of Myocardial perfusion imaging during dobutamine stress magnetic resonance for the assessment of coronary artery disease. Circ Cardiovasc Imaging. 2008;1:122–130. doi: 10.1161/CIRCIMAGING.108.779108. [DOI] [PubMed] [Google Scholar]
- 28.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141(3):334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 29.Levy D, Garrison RJ, Savage DD, Anderson Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199012133232413. [DOI] [PubMed] [Google Scholar]