Abstract
The importance of lipids in cell signaling and tissue physiology is demonstrated by many CNS pathologies that involve deregulated lipid metabolism. One such critical metabolic event is activation of phospholipase A2 (PLA2), resulting in hydrolysis of membrane phospholipids and release of free fatty acids including arachidonic acid, a precursor for important cell-signaling eicosanoids. Reactive oxygen species (ROS, a product of arachidonic acid metabolism) react with cellular lipids to generate lipid peroxides, which degrade to reactive aldehydes (oxidized phospholipid, 4-hydroxynonenal, and acrolein) that covalently bind to proteins, altering their function and causing cellular damage. Dissecting the contribution of PLA2 to lipid peroxidation in CNS injury and disorders is challenging due to multiple forms of PLA2, diverse sources of ROS and lack of specific PLA2 inhibitors. This review summarizes the role of PLA2 in CNS pathologies including stroke, spinal cord injury, Alzheimer’s, Parkinson’s, Multiple sclerosis - Experimental autoimmune encephalomyelitis and Wallerian degeneration.
Keywords: Arachidonic acid, CNS pathologies, 4-hydroxynonenal, Oxidative stress, Phosphatidylcholine, neurodegenerative disorders
INTRODUCTION
Phospholipids are important components of all mammalian cells and have a variety of biological functions: 1) formation of lipid bilayers that provide structural integrity necessary for protein function and 2) serve as precursors for various lipid second messengers. Deregulated lipid metabolism is of particular importance for CNS disorders and injuries since the brain has the highest lipid concentration after adipose tissue. Recent reviews are available on lipid signaling in CNS pathologies (1–3) and other disorders (4). This review focuses on the role of phospholipase A2 (PLA2) in CNS injury and disorders. The role of ROS in conjunction with lipoxygenases will be discussed by Dr. Jae-Hong Kim in the accompanying mini-review.
PLA2 ENZYMES OCCUR IN MULTIPLE FORMS
PLA2 enzymes cleave fatty acids at the sn-2 position of glycerophospholipids to give free fatty acids and lysophospholipids. The reaction is of particular importance when the fatty acid released from the sn-2 position is arachidonic acid (ArAc), since it can metabolized by cyclooxygenases and lipoxygenases to various bioactive eicosanoids, including prostaglandins, thromboxanes, and leukotrienes. The other PLA2 products, lysophospholipids, are also biologically active and are important in cell signaling (5, 6).
Nearly 22 different PLA2 enzymes have been identified in various mammalian tissues (5) and have been broadly classified into three families based on their calcium requirement for catalytic activity as calcium-dependent cytosolic (cPLA2) and secretory (sPLA2) types and calcium-independent forms (iPLA2). PLA2 enzymes are currently systematically classified into groups based on their nucleotide and amino acid sequence. cPLA2s (α, β, γ; group IVA, IVB and IVC) have molecular masses of 85, 114 and 61 kDa, respectively. cPLA2α requires μM Ca2+ concentrations for binding to lipid substrates, and preferentially hydrolyzes ArAc in the sn-2 position of phospholipids. cPLA2α is regulated by phosphorylation on Ser-505, Ser-727, and Ser-515 mediated by MAPK, MEK1, and calcium- and calmodulin-dependent kinase II, respectively, although the mechanism involved in regulation of cPLA2α by phosphorylation is not well understood (7). sPLA2s (groups I, II, III, V, X and XII) have low molecular weight (14–19 kDa), usually require mM calcium for catalytic activity, and lack specificity for the fatty acid in the sn-2 position. In addition to their catalytic activity as phospholipases, some sPLA2 forms can stimulate a variety of biological responses through binding to a high affinity transmembrane PLA2 receptor. iPLA2s (group VI) have molecular masses of 84–88 kDa (8), are not selective for ArAc, and play an important role in cell cycle regulation through membrane phospholipid remodeling (9) . Platelet activating factor acetylhydrolases comprise PLA2 groups VII and VIII, have molecular masses of 26–45 kDa, and can also cleave short chain oxidized fatty acids (up to nine carbons) in the sn-2 position of phosphatidylcholine (PC) or phosphatidylethanolamine. Lipoprotein-PLA2 (Lp-PLA2) is a 45 kDa group VIIA PLA2 found in blood circulation and is associated with apo-B100 of LDL. Elevated levels of Lp-PLA2 are associated with coronary heart disease, stroke and dementia (10). Group VII and VIII PLA2s may serve a protective role by removing fatty acids damaged as a result of oxidative stress. All major groups of PLA2 are present in the central nervous system (2, 11).
cPLA2 IN CEREBRAL ISCHEMIA
in vitro
A number of studies have demonstrated up-regulation of PLA2 in a variety of cerebral ischemia models. Organotypic hippocampal slice cultures were subjected to oxygen glucose deprivation (OGD) and re-oxygenation, an in vitro model for cerebral ischemia. PLA2 activity increased by 2-fold as compared to controls in the hippocampal pyramidal cell layer immediately after 35 min of OGD and remained elevated at 24 h re-oxygenation. Use of PLA2 inhibitors (AACOCF3 to inhibit cPLA2 and iPLA2, BEL for iPLA2, and LY311727 for sPLA2) indicated that PLA2 activation after OGD was attributable to cPLA2. Inhibition of cPLA2 also attenuated OGD-induced neuronal death, indicating involvement of cPLA2 in ischemic injury (5). Recent studies showed that release of lysosomal cathespins caused CA1 hippocampal neuronal death through NMDA-mediated calcium influx, activation of cPLA2 , ArAc release and subsequent ROS production in rat hippocampal slices subjected OGD (12).
FOREBRAIN ISCHEMIA
cPLA2 gene expression was examined in rat brain by in situ hybridization. In normal rat brain, faint signals were detected in hippocampal CA1–CA3 neurons and dentate granule cells. After 10 min transient forebrain ischemia in male Wistar rat, the cPLA2 gene was intensely expressed in dentate granule cells, which remain viable after transient ischemia, at 12 and 24 h of reperfusion. Expression in CA1–CA3 was slightly increased at 6 and 12 h but returned to control levels by 24 h (5).
Increased cPLA2 immunoreactivity compared to shams was detected in astrocytes in the CA1 region at 24 h after 30 min of 4-vessel occlusion, a time before histologically evident neuronal damage. Intense labeling for cPLA2 was observed throughout the hippocampal CA1 region at 72 h after ischemia, at which time necrosis of CA1 neurons is observed. cPLA2 was selectively expressed in activated microglia and astrocytes in areas of neurodegeneration. cPLA2 activity and protein expression were increased in hippocampal extracts at 72 h reperfusion (5).
FOCAL CEREBRAL ISCHEMIA
At 24 h after permanent occlusion of the MCA, monocytes and macrophages were strongly cPLA2 immunoreactive and were primarily localized to the core of the infarct. cPLA2 immunoreactive astrocytes were present in the penumbra region (5).
The PLA2 inhibitor quinacrine attenuated neurological deficits and decreased infarction following 2 h of transient focal cerebral ischemia (6). These studies provide evidence that PLA2 contributes to ischemic injury, however since quinacrine is a general PLA2 inhibitor, these results do not indicate which PLA2 form was involved in the ischemic injury (6). Indirect evidence has been provided by studies demonstrating that inhibition of ArAc metabolism attenuated cerebral ischemia-induced oxidative injury, blood-brain barrier dysfunction, edema, infarction, and hippocampal neuronal death (5).
Transgenic mice lacking cPLA2 were generated by targeted disruption of its gene. Following transient focal cerebral ischemia, cPLA2 deficient mice had smaller infarcts and fewer neurological deficits compared to wild type (5, 8), demonstrating a role for cPLA2 in ischemic injury. We have not observed any changes in total cPLA2 protein expression after transient focal cerebral ischemia (13), consistent with other studies wherein cPLA2 mRNA expression did not show any changes at 3 d after ischemia (14). In our studies the antibody was not specific for phosphorylated cPLA2 and changes in phospho-cPLA2 probably would not have been detected (13). Aβ induced phospho-cPLA2 changes were evident in AD models (15, 16). A knockout animal may not provide a clear understanding of the normal role of a specific gene product, as the overall phenotype can result from primary gene loss as well as adaptive responses during development and maturation (17). It should be noted that mouse strains C57BL/6J and 129/SV used for transgenic studies have a naturally occurring mutation in the gene for sPLA2 IIA (5, 8), and thus the cPLA2 knockout mice were deficient in both cPLA2 and sPLA2 IIA. Transgenic mice expressing the human sPLA2 IIA gene have been developed and are available from Taconic, but this mouse strain apparently has not yet been used in stroke research to assess the role of sPLA2 IIA.
UNILATERAL HYPOXIA-ISCHEMIA
Following 15 min unilateral hypoxia-ischemia in 21-d-old male Wistar rat, cPLA2 immunoreactivity was selectively higher in reactive glia in the hippocampal CA1 region undergoing delayed neuronal death at 3 and 7 d after injury. Immunoreactivity for cPLA2 was low in all regions of the control uninjured hemisphere (5).
cPLA2 expression studies present the anomaly that mRNA was expressed mainly in neurons, whereas the protein expression was induced in glia but was undetectable in neurons. These differences cannot be explained by strain differences since all these studies used Wistar rats (5). Recent studies show induction of cPLA2 by Aβ in cortical neuronal cultures indicating neurons do express cPLA2 protein, however the contribution from residual non-neuronal cells in these cultures cannot entirely be ruled out (15).
sPLA2 IN CEREBRAL ISCHEMIA
FOREBRAIN ISCHEMIA
PLA2 activities significantly increased in cytosolic, mitochondrial and microsomal fractions after global cerebral ischemia in gerbil, the majority of which was calcium dependent, 14 kDa characteristic of sPLA2. Similar results were found in our studies in forebrain ischemia. PLA2 activities significantly increased in mitochondrial and membrane fractions after 2 h of reperfusion. Maximum activity was achieved at mM Ca2+, indicating that the membrane and mitochondrial fractions predominantly contain sPLA2 (18). Quinacrine also attenuated CA1 neuronal death in transient forebrain ischemia in gerbil (6).
Group IIA sPLA2s have been studied the most because of their involvement in inflammatory processes (19, 20). Expression of sPLA2 II mRNA in rat brain after 20 min of transient forebrain occlusion showed biphasic increases at 1–6 h and 7 and 20 d post-ischemia (5 and references cited therein).
FOCAL CEREBRAL ISCHEMIA
sPLA2 IIA mRNA showed biphasic increases in expression at 30 min of reperfusion after 60 min of focal cerebral ischemia and from 12 h to 3 d, which declined to sham levels by 7 d and increased again at 14 d. The increase in sPLA2 IIA mRNA occurred primarily in the ischemic cortex, while the increases at 1 and 3 d were observed mainly in the penumbra. sPLA2 IIA protein was expressed mainly in the penumbra at 3 d post-ischemia and was localized in GFAP-positive astrocytes but not in microglia (14).
We have also observed a 4-fold increase in sPLA2 IIA mRNA in the ipsilateral cortex compared to contralateral cortex after 1 h of middle cerebral artery occlusion (MCAO) and 24 h of reperfusion in spontaneously hypertensive rats (13). sPLA2 protein expression was significantly increased in ipsilateral over 7 d reperfusion after 1 h of MCAO (21).
In permanent MCAO, PLA2 activity was maximum at 8 h and was increased by 2-fold as compared to contralateral cortex. The sPLA2 inhibitor indoxam significantly reduced infarct volume. In vitro, sPLA2 IIA caused complete neuronal death in cultured rat cortical neurons but had no effect on survival of astrocytes (5 and references cited therein).
PLA2 IN SPINAL CORD INJURY (SCI)
The initial event after SCI is depolarization and opening of voltage-dependent ion channels, and consequent massive release of neurotransmitters including glutamate. The accumulation of intracellular calcium initiates mitochondrial dysfunction, and activation of nitric oxide synthase (NOS) and PLA2. The resulting generation of free radicals (comprised of different species including reactive nitrogen species (RNS), ROS and other radicals) and subsequent lipid peroxidation is believed to be a major pathway of secondary injury in SCI (22). PLA2 activity and cPLA2 expression were significantly increased after SCI; cPLA2 expression was confined to neurons and oligodendrocytes. Mepacrine, a PLA2 inhibitor, attenuated spinal neuronal death in vitro and PLA2-induced demyelination in vivo (22).
PLA2 IN NEURODEGENERATIVE DISEASES
mRNA expression of pro-inflammatory sPLA2 IIA was up-regulated in Alzheimer’s Disease (AD) brains compared to non-dementia elderly brains. sPLA2 IIA immunoreactive astrocytes in AD hippocampus were associated with Aβ plaques (23). Aβ and NMDA induced cPLA2 phosphorylation and ArAc release via activation of NADPH oxidase, ROS generation, and activation of ERK1/2 in cortical neuronal cultures (15, 24). Aβ induced mitochondrial dysfunction through iPLA2 and cPLA2 in cultured cortical astrocytes (16).
Free radical generation and lipid peroxidation play a significant role in Parkinson’s Disease (PD). One of the factors responsible for this is believed to be phospholipases activation in substantia nigra, supported by the fact that cPLA2 deficient mice are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrohydropyridine (MPTP) induced neurotoxicity (6), an animal model for PD.
EXPERIMENTAL AUTOIMMUNE ENCEPHALOMYELITIS (EAE) can be induced in animals by immunizing against myelin antigens (25) and is an animal model for multiple sclerosis (MS) due to similarities in its histopathology and clinical course. Recent studies demonstrated a key role for cPLA2 in EAE (25, 26). cPLA2, which can be induced by the cytokine tumor necrosis factor-α (TNF-α) (27), was highly expressed in EAE lesions. Blocking cPLA2 showed a remarkable decrease in both the onset and progression of the disease (25), indicating that cPLA2 has a significant role in both the induction and effector phases of EAE. A second study showed that cPLA2 null mice were resistant to EAE (26). Treatment of EAE rats with the nonapeptide sPLA2 inhibitor CHEC-9 significantly attenuated sPLA2 activity, EAE symptoms, and ED-1 positive microglia/macrophages (28). Recently, MS patients were shown to have elevated sPLA2 activity in the urine (28). These studies suggest that both cPLA2 as well as sPLA2 inhibition may be treatment options for MS.
REACTIVE OXYGEN SPECIES (ROS) CONTRIBUTE TO STROKE INJURY
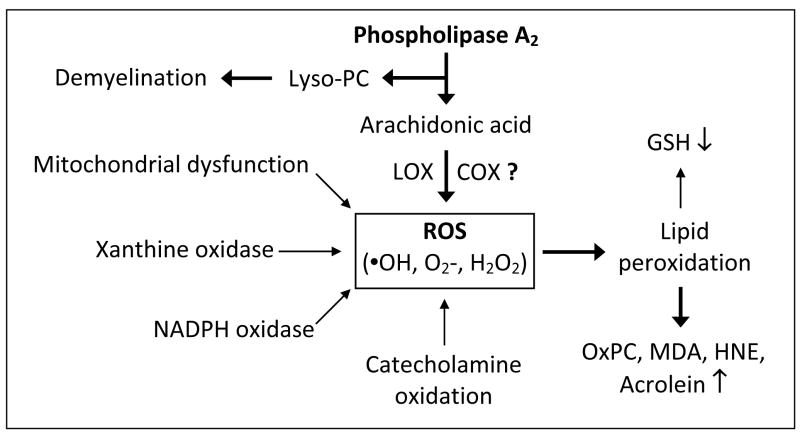
ROS including hydrogen peroxide (H2O2) and superoxide radical (O2•− ) are produced by a number of cellular oxidative metabolic processes including xanthine oxidase, NADPH oxidase, metabolism of ArAc by cyclooxygenases (COX) and lipoxygenases (LOX) (29, 30), and the mitochondrial respiratory chain. Cardiolipin is an exclusive inner mitochondrial phospholipid essential for mitochondrial electron transport (31). Hydrolysis of cardiolipin by mitochondrial sPLA2 could disrupt the mitochondrial respiratory chain, resulting in increased ROS generation. ROS can also be formed non-enzymatically, for example by autoxidation of catecholamines (18). Sources of ROS and the involvement of PLA2 are summarized in Fig. 1. Recent literature suggests that COX-2 does not directly produce ROS during ArAc oxidative metabolism, but does form free radicals (i.e., tyrosyl radicals on the protein and carbon-centered radicals on ArAc) (32). ROS production was elevated in a stroke model but was not attenuated in COX-2 deficient mice nor by the COX-2 inhibitor NS398 (33). Mice lacking the nox2 subunit of O2•− -producing NADPH oxidase (33) exhibited reduced ROS generation, implicating NADPH oxidase as a significant source of ROS in the stroke model. Although there are many reports in the literature on ROS production by COX, this may be due to secondary ROS generation induced by various eicosanoids. However, in the above cited studies (33), COX-2 inhibition or knockout would be expected to attenuate any secondary ROS production due to COX-2 metabolites. This apparent incongruity could be due to the specific time points chosen. The studies of Kunz, et al., showed increased ROS generation over 2 h to 3 days. ROS production after COX-2 inhibition by NS398 or in COX-2 null mice was determined at 2 h and 3 day, which does not preclude the possibility of COX-2 dependent ROS generation during the intermediate 2–48 hr reperfusion times. NS398 did reduce the ROS generation in hippocampal slices subjected to OGD as measured by DHE fluorescence, leaving open the idea that COX-2 may at least indirectly contribute to ROS generation (12).
Fig. 1.
Contribution of PLA2 to ROS formation and lipid peroxides. COX-2 role in ROS generation is under question (33).
Endogenous defenses that detoxify ROS include enzymatic systems such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px), and non-enzymatic antioxidants such as ascorbic acid, vitamin E, β-carotene, and glutathione (GSH) (5). Neurons may be particularly vulnerable to free radical damage since they contain low levels of GSH (34). While ROS are proposed to play important roles in coordinating and regulating a number of cellular signaling pathways (redox signaling), oxidative stress results when the formation of ROS exceeds the capacity of anti-oxidant defense systems (35). Transgenic mouse models have provided evidence that ROS contribute to ischemic brain injury. Mice over-expressing either SOD1 or GSH-Px-1 showed significantly smaller infarcts compared to wild type counterparts following focal cerebral ischemia. Conversely, ischemic injury was increased in mice deficient in SOD1 (35).
ROS AND RNS INITIATE LIPID PEROXIDATION
The highly reactive hydroxyl radical (•OH) is formed from H2O2 in the presence of divalent metal ions, especially Fe2+ and Cu2+, via the Fenton reaction. Once formed, •OH reacts almost instantaneously with many cellular components, including polyunsaturated fatty acids of membrane lipids. Nitric oxide (•NO) is formed by enzymatic oxidation of L-arginine to citrulline by nitric oxide synthases (NOS) and serves as an important regulator of vascular response and neuronal signaling (36). O2•− does not directly induce lipid peroxidation, but can react with •NO to form peroxynitrite (ONOO ), a strong oxidant that can initiate lipid peroxidation.
LIPID PEROXIDATION PRODUCTS CAN ALTER CELLULAR FUNCTION
Peroxidation of lipids can disrupt the organization of the membrane, causing changes in fluidity and permeability, inhibition of metabolic processes, and alterations of ion transport (37). Damage to mitochondria induced by lipid peroxidation can lead to further ROS generation. In the presence of oxygen radicals, double bonds of unsaturated fatty acids of phospholipids can get oxidized. Scission of the oxidized polyunsaturated fatty acids results in formation of phospholipid aldehydes such as oxidized phosphatidylcholine (OxPC), and aldehyde cleavage fragments including malondialdehyde (MDA), 4-hydroxynonenal (HNE), and acrolein (38, 39). These aldehydes in turn covalently bind to proteins and alter their function. Among all α,β-unsaturated aldehydes, acrolein is by far the strongest electrophile and shows the highest reactivity with proteins. Overproduction of lipid peroxides and aldehyde products can cause depletion of GSH through detoxification by GSH-Px and glutathione S-transferase (18).
The presence of OxPC on the apoptotic cell surface has been shown using EO6 monoclonal antibodies that exclusively bind to OxPC and OxPC-protein adducts (40). Oxidation of PC to OxPC on apoptotic cells may enhance pro-inflammatory signals (41, 42). The presence of OxPC has been demonstrated in multiple sclerosis brain using EO6 monoclonal antibodies (40). Although multiple sclerosis (a brain disorder) and stroke (brain injury) have specific differences, a common denominator is inflammatory response and ROS production.
LIPID PEROXIDATION IN CEREBRAL ISCHEMIA
GLOBAL CEREBRAL ISCHEMIA
Transient forebrain ischemia in gerbil (which lacks circle of Willis and therefore collateral blood flow) is characterized by delayed hippocampal CA1 neuronal death. Neurodegeneration is evident by 3 d reperfusion and neuronal death culminates by 6 d (43). Five min of forebrain ischemia and 6 h reperfusion in gerbil resulted in significantly increased levels of MDA, HNE and lipid hydroperoxides in the cortex, striatum and hippocampus and thus preceded the onset of neuronal death (44). These increases persisted over 4 d reperfusion in the ischemia-vulnerable hippocampus.
In another study (39 and references cited therein) using 3 min global cerebral ischemia, a transient increase in nuclear HNE immunoreactivity was observed in hippocampal CA1 neurons with little or no reactivity in the cytosol. In contrast, cytoplasmic immunoreactivity increased markedly from 8 h to 7 d reperfusion. At 7 d, HNE immunoreactivity was observed within reactive astrocytes in the CA1 region. Similar results were found in our studies in 10 min forebrain ischemia and 24 h of reperfusion of gerbil. Immunohistochemical studies showed localization of HNE in the ischemia susceptible hippocampal CA1 region (5). HNE immunoreactivity was strongest in the nuclei, with significant labeling also in cytoplasm. Little or no HNE immunoreactivity was observed in shams.
Lipid peroxidation was assessed using thiobarbituric acid assay in rat brains after 30 min forebrain ischemia and up to 72 h reperfusion. Lipid peroxide levels were unchanged during ischemia and 1 h reperfusion but increased between 8 and 72 h of recirculation in the ischemia-vulnerable hippocampus, with the greatest increase at 48 h (5 and references cited therein).
HNE immunoreactivity was examined post mortem in a group of patients who had global cerebral ischemia (cardio-respiratory attack) and subsequently died. There was a significant increase in the number of HNE-positive neurons and glia in the hippocampus of the ischemic group compared to age and sex matched controls (45).
FOCAL CEREBRAL ISCHEMIA
Relative generation of OxPC in the ischemic hemisphere vs contralateral region was assessed by Western blot after transient focal cerebral ischemia and 1 day reperfusion. Our studies show that there was an increase in OxPC-modified protein (~15 kDa) in the ischemic cortex compared to contralateral cortex (Fig. 2). Presently the identity of the modified 15-kDa protein is unknown (40).
Fig. 2.
(A) Dot blot of POVPC standard (1-palmitoyl-2-(5’-oxo)-valeryl-sn-glycero-3-phosphorylcholine) and lipid extracts from contra- and ipsi-cortex after MCAO/24 h reperfusion using EO6 antibodies. Free OxPC in brain extracts was very low. (B) Standard curve generated from pixel density of POVPC. (C) Western blot of OxPC-protein adduct in ipsi-cortex after tMCAO.
The proteasome is a large 700 kDa complex that performs the majority of protein degradation and is responsible for removal of most oxidized, aggregated, or damaged proteins. Activity of the proteasome complex decreased following transient focal cerebral ischemia in mice, without a corresponding decrease in protein expression of the proteasome subunits. Levels of HNE-modified proteasome complex subunits increased as early as 1 h reperfusion, suggesting that the loss of proteasome activity was due to the modification by HNE. GSH-Px-deficient mice showed further increased levels of HNE-modified proteasome subunits (46).
Using an antibody to HNE-modified proteins, no HNE immunoreactivity was detected at 1 h, but was detectable in neurons within the infarcted zone at 3 h and in the boundary between infarcted and non-infarcted zones over 6–48 h of reperfusion following 3 h of focal cerebral ischemia in rat (39 and references cited therein). Expression of Bcl-2 attenuates ischemic injury. In transient forebrain ischemia, the hippocampal CA1 neurons destined to die cease making Bcl-2 protein (47), and it is conceivable that increased levels of HNE-modified proteins are due, at least in part, to lack of Bcl-2 expression.
In permanent focal cerebral ischemia in rat, HNE immunoreactivity increased in the ipsilateral hemisphere 4 h after induction of MCAO. HNE immunoreactivity extended beyond the area of ischemic damage, suggesting that HNE-modified proteins accumulated in tissue prior to development of infarction (5 and references cited therein). In another study, MDA and conjugated diene levels were significantly elevated in ipsilateral compared to contralateral cortex following 60 min of permanent MCAO (5).
EPC-K1 (a phosphodiester of vitamin C and E that inhibits PLA2 activity and lipid peroxidation) reduced spatial learning deficits following 20 min transient global ischemia (4-vessel occlusion) in male Wistar rat. In another study, EPC-K1 significantly decreased both cerebral lipid peroxidation and infarct in transient focal cerebral ischemia, indicating the contribution of lipid peroxidation to ischemic brain injury (5).
To date, studies on aldehyde products in ischemic brain have focused on HNE and have shown increased lipid peroxidation in several models of cerebral ischemia. Recent studies suggested acrolein as a novel biochemical marker for stroke diagnosis; however acrolein formation was attributed to the polyamine oxidase pathway (48, 49).
LIPID PEROXIDATION IN NEURODEGENERATIVE DISEASES
A number of studies demonstrated increased lipid peroxidation in AD, supporting a role for oxidative damage in AD (50). Recent studies demonstrated increased levels of HNE and acrolein in the brain tissue from patients affected by mild cognitive disorder and early AD, indicating that lipid peroxidation occurs early in the pathogenesis of AD (50). ROS may also play a role in amyloid deposition in AD as oxidizing conditions cause protein cross-linking and aggregation of Aβ peptides, and also contribute to tau protein aggregation (2).
Multiple sclerosis (MS) is an inflammatory demyelinating autoimmune disease affecting the CNS, but its underlying cause remains elusive. In MS, the immune system attacks the myelin sheath of nerve cell fibers in the brain and spinal cord. Thiobarbituric acid reactive substances and F2-isoprostane levels were shown to be elevated in CSF of MS patients, and HNE was associated with MS lesions, indicative that lipid peroxidation also occurs in MS (1).
Wallerian/anterograde degeneration results when a nerve fiber is cut or crushed and the part distal to the injury (i.e. the part of the axon separated from the neuron's cell nucleus) degenerates, a process in which PLA2 plays an important role in myelin breakdown and phagocytosis (51). Wallerian degeneration occurs after axonal injury in both the peripheral nervous system (PNS) and CNS. The axonal degeneration is followed by degradation of the myelin sheath and infiltration by macrophages. The macrophages, accompanied by Schwann cells, serve to clear the debris from the degeneration. PNS is much faster and efficient at clearing myelin debris in comparison to CNS, and Schwann cells are the primary cause of this difference. PLA2 expressed during the early stage of Wallerian degeneration hydrolyzes PC in myelin to LPC and ArAc. LPC can induce further myelin breakdown while eicosanoids derived from ArAc stimulate inflammatory responses.
Increased lipid peroxidation has been reported in Parkinson’s Disease, Huntington’s Disease and amyotrophic lateral sclerosis, but involvement of PLA2 has not yet been demonstrated (1).
CONCLUSIONS AND FUTURE PERSPECTIVES
The published findings, so far, reveal increases in cPLA2 and sPLA2 in stroke (5) and neurodegenerative diseases (Table 1). Gene knockout studies have demonstrated the role of cPLA2 (17) in ischemic injury, MS-EAE (26) and Parkinson’s disorder (6). The high expression of cPLA2 in myelin disorders such as MS-EAE (25) and Wallerian degeneration (51), and resistance to MPTP-induced Parkinsonism in cPLA2 null mice suggests potential for cPLA2 pharmacological interventions. While iPLA2s have an important role in regulation of the cell cycle by membrane phospholipid remodeling (9), their role in CNS pathology remains to be investigated. A neuronal sPLA2 receptor has been identified and is highly expressed in brain (52), but its physiological function, ligand interactions and possible role in neurodegeneration has yet to be elucidated. PLA2 activating protein that greatly stimulates sPLA2 activity and is expressed in brain is another factor that remains to be explored (5). A number of group IIA sPLA2 inhibitors were developed (53) but most of these are yet to be tested in CNS pathologies.
Table 1.
Role of PLA2 in CNS pathologies
| CNS pathology | Role of PLA2 |
|---|---|
| Alzheimer Disease | Upregulation of PLA2, increased lipid peroxidation (6, 50, 54). sPLA2 IIA expression increased in AD brains (23). Aβ induced mitochondrial dysfunction through iPLA2 and cPLA2. Aβ induced cPLA2 phosphorylation via NADPH oxidase and ROS production (15, 16). |
| Parkinson’s Diseases | cPLA2 knock out mice showed protection against MPTP toxicity (6). |
| Multiple Sclerosis-Experimental Autoimmune Encephalomyelitis (MS-EAE) | cPLA2 is highly expressed in EAE (6). cPLA2-deficient mice are resistant to EAE (26). sPLA2 activity increased and inhibition by CHEC-9 blocks inflammation (28). |
| Wallerian degeneration | PLA2 plays an important role in myelin breakdown and phagocytosis. PLA2 expressed during the early stage of Wallerian degeneration hydrolyzes PC in myelin to LPC and ArAc (51). LPC can aggravate the inflammatory responses that can further up-regulate cPLA2 in a positive feedback manner. |
| Transient focal cerebral ischemia (Stroke) | Activation of PLA2 and increased sPLA2 expression (5, 13, 14). cPLA2 knock out mice showed protection (5 and references cited therein). CDP-choline attenuated sPLA2 (13). PLA2 inhibitor quinacrine reduced the infarction size (6). |
| Spinal Cord Injury | cPLA2 expression, PLA2 activity were increased (22); Mepacrine reduced PLA2-induced neuronal death. The studies did not directly assess the role of endogenous PLA2 in neuronal injury after SCI. Major part of the studies conducted injecting PLA2 or mellitin to spinal cord of normal rats and showed increases in TNF-α, IL-1β and HNE. |
Acknowledgments
This work was supported by grants from NIH/NINDS (NS42008), American Heart Association Greater Midwest Affiliate Grant-in-Aid (0655757Z), UW-School of Medicine and Public Health Research Committee (161-PRJ13MX), UW-School of Medicine and Public Health, UW-Graduate school and UW-Neurological Surgery Department and laboratory resources provided by William S. Middleton VA Hospital (to RMA). EO6 antibodies were generously provided by Dr Joseph Witztum, Univ. of California-San Diego, La Jolla, CA.
References
- 1.Adibhatla RM, Hatcher JF. Role of lipids in brain injury and diseases. Future Lipidol. 2007;2:403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. In: Quinn PJ, Wang X, editors. Lipids in Health and Disease. Subcellular Biochemistry. Vol. 49. Springer-Verlag; New York: 2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adibhatla RM, Hatcher JF. An odyssey of plaque to stroke: a lipid perspective. 2008 www.athero.org.
- 4.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 5.Adibhatla RM, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: Their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 7.Tucker DE, Gijon MA, Spencer DM, Qiu Z-H, Gelb MH, Leslie CC. Regulation of cytosolic phospholipase A2α by hsp90 and a p54 kinase in okadaic acid-stimulated macrophages. J Leukocyte Biol. 2008 doi: 10.1189/jlb.0308197. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green JT, Orr SK, Bazinet RP. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J Lipid Res. 2008;49:939–944. doi: 10.1194/jlr.R700017-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Balboa MA, Perez R, Balsinde J. Calcium-independent phospholipase A2 mediates proliferation of human promonocytic U937 cells. FEBS J. 2008;275:1915–1924. doi: 10.1111/j.1742-4658.2008.06350.x. [DOI] [PubMed] [Google Scholar]
- 10.Adibhatla RM, Hatcher JF. Integration of cytokine biology and lipid metabolism in stroke. Front Biosci. 2008;13:1250–1270. doi: 10.2741/2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: Implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Windelborn JA, Lipton P. Lysosomal release of cathepsins causes ischemic damage in the rat hippocampal slice and depends on NMDA-mediated calcium influx, arachidonic acid metabolism, and free radical production. J Neurochem. 2008;106:56–69. doi: 10.1111/j.1471-4159.2008.05349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao F. CDP-choline significantly restores the phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP-phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281:6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- 14.Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He Y, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 15.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid β peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JCM. Phospholipases A2 mediate Amyloid-β peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res. 2003;44:109–117. doi: 10.1194/jlr.m200298-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Adibhatla RM, Hatcher JF, Dempsey RJ. Phospholipase A2, hydroxyl radicals and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5:647–654. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes L, Hernandez M, Nieto ML, Crespo MS. Biological effects of group IIA secreted phosholipase A2. FEBS Lett. 2002;531:7–11. doi: 10.1016/s0014-5793(02)03401-4. [DOI] [PubMed] [Google Scholar]
- 20.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2007.1012.1007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu NK, Zhang YP, Titsworth WL, Jiang XR, Han SK, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- 23.Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. Secretory PLA2-IIA: A new inflammatory factor for Alzheimer's disease. J Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-1183-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 26.Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui J, Shen MWH, DeClercq CM, Miyashiro JS, Carito BA, Thakker P, Simmons DL, Leonard JP, Shimizu T, Clark JD. Cytosolic phospholipase A2α-deficient mice are resistant to experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronke M, Adam-Klages S. Role of caspases in TNF-mediated regulation of cPLA2. FEBS Lett. 2002;531:18–22. doi: 10.1016/s0014-5793(02)03407-5. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham TJ, Yao L, Oetinger M, Cort L, Blankenhorn EP, Greensteinm JI. Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-1183-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 30.Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Kirkland RA, Adibhatla RM, Hatcher JF, Franklin JL. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience. 2002;115:587–602. doi: 10.1016/s0306-4522(02)00512-2. [DOI] [PubMed] [Google Scholar]
- 32.Jiang J, Borisenko GG, Osipov A, Martin I, Chen R, Shvedova AA, Sorokin A, Tyurina YY, Potapovich A, Tyurin VA, Graham SH, Kagan VE. Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenase-2-transfected PC12 cells. J Neurochem. 2004;90:1036–1049. doi: 10.1111/j.1471-4159.2004.02577.x. [DOI] [PubMed] [Google Scholar]
- 33.Kunz A, Anrather J, Zhou P, Orio M, Iadecola C. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- 34.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor JM, Crack PJ. Impact of oxidative stress on neuronal survival. Clin Exp Pharmacol Physiol. 2004;31:397–406. doi: 10.1111/j.1440-1681.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 36.Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Nigam S, Schewe T. Phospholipase A2s and lipid peroxidation. Biochim Biophys Acta. 2000;1488:167–181. doi: 10.1016/s1388-1981(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 38.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 40.Qin J, Goswami R, Balabanov R, Dawson G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85:977–984. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- 41.Bratton DL, Henson PM. Autoimmunity and apoptosis: refusing to go quietly. Nat Med. 2005;11:26–27. doi: 10.1038/nm0105-26. [DOI] [PubMed] [Google Scholar]
- 42.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adibhatla RM, Hatcher JF, Dempsey RJ. Lipids and lipidomics in brain injury and diseases. AAPS J. 2006;8:E314–E321. doi: 10.1007/BF02854902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Candelario-Jalil E, Mhadu NH, Al-Dalain SM, Martinez G, Leon OS. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res. 2001;41:233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 45.McCracken E, Graham DI, Nilsen M, Stewart J, Nicoll JA, Horsburgh K. 4-Hydroxynonenal immunoreactivity is increased in human hippocampus after global ischemia. Brain Pathol. 2001;11:414–421. doi: 10.1111/j.1750-3639.2001.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy MS. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2000;20:1467–1473. doi: 10.1097/00004647-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Graham SH, Nakayama M, Zhu RL, Jin KL, Stetler RA, Simon RP. Apoptosis repressor genes Bcl-2 and Bcl-X-long are expressed in the rat brain following global ischemia. J Cereb Blood Flow Metab. 1997;17:2–10. doi: 10.1097/00004647-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Adibhatla RM, Hatcher JF, Sailor KA, Dempsey RJ. Polyamines and CNS injury: Spermine and spermidine decrease following transient focal cerebral ischemia in spontaneously hypertensive rats. Brain Res. 2002;938:81–86. doi: 10.1016/s0006-8993(02)02447-2. [DOI] [PubMed] [Google Scholar]
- 49.Tomitori H, Usui T, Saeki N, Ueda S, Kase H, Nishimura K, Kashiwagi K, Igarashi K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 50.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in mild cognitive impairment and early Alzheimer's disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 51.De S, Trigueros MA, Kalyvas A, David S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24:753–765. doi: 10.1016/s1044-7431(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 52.Nicolas JP, Lin Y, Lambeau G, Ghomashchi F, Lazdunski M, Gelb MH. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J Biol Chem. 1997;272:7173–7181. doi: 10.1074/jbc.272.11.7173. [DOI] [PubMed] [Google Scholar]
- 53.Reid RC. Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 54.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]