Abstract
Lipophilicity/bioavailibility of Mn(III)N-alkylpyridylporphyrin-based SOD mimics has major impact on their in vivo ability to suppress oxidative stress. Meta isomers are less potent SOD mimics than ortho analogues, but are 10-fold more lipophilic and more planar. Enhanced lipophilicity contributes to their higher accumulation in cytosol of SOD-deficient E. coli, compensating for their lower potency; consequently both isomers exert similar-to-identical protection of SOD-deficient E. coli. Thus meta isomers may be as prospective therapeutics as are ortho porphyrins.
Keywords: Meta and ortho isomers of Mn(III) N-alkylpyridylporphyrins, SOD-deficient E. coli protection, accumulation of Mn porphyrins in E. coli, SOD mimics, peroxynitrite scavengers, MnTM-2-PyP, MnTE-2-PyP5+, MnTnHex-2-PyP, MnTM-3-PyP, MnTE-3-PyP
Introduction
Positively charged Mn(III) N-alkylpyridylporphyrins (Figure 1) display electrostatic and thermodynamic facilitation for the dismutation of superoxide (O2·−) and reduction of peroxynitrite (ONOO−).1–4 Some members of the series are among the most potent catalytic scavengers of these reactive species with activity towards O2·−nearly the same as that of the SOD enzymes,4 and reactivity towards ONOO−, kred exceeding 107 M−1 s−1.3 Reactive species are widely viewed as signaling molecules controlling cellular transcriptional activity.5 Thus, inhibition of the activation of redox-active transcription factors such as AP-1,6 NF-kB,7,8 and HIF-1α9–11, presumably via scavenging of reactive species, was detected by some members of the series. Mn porphyrins, such as MnTE-2-PyP, MnTnHex-2-PyP, and MnTDE-2-ImP, effectively attenuate oxidative stress in animal models of cancer,6,9–11 central nervous system disorders,8 radiation injury,12 diabetes,7,13 morphine tolerance,14 ischemia/reperfusion injuries16 etc. Recent data, however, has hinted that factors other than redox-based antioxidant potency, such as lipophilicity, size, and the overall geometry and conformational flexibility of the Mn porphyrins (MnP) should play also a significant role in their design and activity.4,15,17
Figure 1.
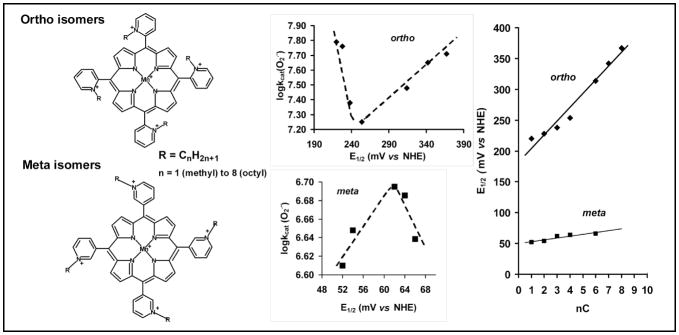
Structures of meta and ortho Mn(III) N-alkylpyridylporphyrins and the relationships between the log kcat (O2·−) [at (25±1)°C, kcat in M−1s−1] and E½ (MnIIIP/MnIIP redox couple), and E½ and the number of carbon atoms (nC) in the alkyl groups (R) of isomers.
In our seminal work published in 1998,18 the magnitude and the importance of the lipophilicity for the in vivo SOD-like efficacy of MnP, as well as the remarkable gain in lipophilicity (at the expense of activity) by the mere shift of the alkyl group from an ortho onto a meta position could only have been retrospectively speculated. Recently, we were able to overcome the methodological difficulties associated with the determination of the partition coefficients between n-octanol and water, POW. The POW, as opposed to TLC retention factor, Rf, is a common and practical indicator of drug lipophilicity which allows comparison of MnP to other drugs of similar target (Table 1).17 We observed that, whereas Rf is linearly related to log POW, small differences in Rf translate into considerable difference in log POW.17 We further showed that a ~10-fold gain in lipophilicity is achieved by either (1) moving the alkyl groups on meso pyridyl substituents from ortho to meta positions, or (2) by increasing the length of alkyl chains by one CH2 group (Table 1).17 Thus, MnTnHex-2-PyP and MnTnOct-2-PyP are, respectively, ~13,000- and ~460,000- fold more lipophilic than MnTE-2-PyP. Whereas these compounds share roughly the same antioxidant capacity (as given by their log kcat values), the 120- and 3000-fold increase in in vivo efficacy of MnTnHex-2-PyP12,16,20,22,23 and MnTnOct-2-PyP20,21(relative to MnTE-2-PyP) in animal and cellular models of oxidative stress seems to be due, at least in part, to their increased lipophilicity and therefore increased cellular accumulation. Among several different classes of metal compounds studied (Mn cyclic polyamines, Mn salens, different MnP and MnCl2 as a control), only MnTnHex-2-PyP protects ataxia telangiectasia cells against radiation damage in a first study to show that both bioavailability and antioxidant capacity are essential for in vivo drug efficacy.23 In a rabbit model of cerebral palsy MnTnHex-2-PyP, and not hydrophilic MnTE-2-PyP, rescued puppies.23 Due to the exceptional antioxidant potential of ortho isomeric Mn N-alkylpyridylporphyrins in dismuting O2·− and reducing ONOO−, we1–4 and others24 have been extensively studying them and analogues ortho-substituted corroles.25
Table 1.
The lipophilicity expressed as TLC retention factor, Rf (Sigma/Aldrich silica-gel plates with KNO3-saturated H2O:H2O:acetonitrile=1:1:8)17 and partition between n-octanol and water, POW17, kcat for O2·− dismutation (at (25 ± 1)°C, 0.05 M phosphate buffer, pH 7.8), 2,18,19, and this work and the metal-centered reduction potential for MnIIIP/MnIIP redox couple, E½, in mV vs NHE (in 0.05 M phosphate buffer, pH 7,8, 0.5mM MnP, 0.1 M NaCl).2,18,19 and this work
| Porphyrin | Rf | log POW | E½ | log kcat |
|---|---|---|---|---|
| MnTM-2-PyP | 0.03 | −7.86 | +220 | 7.79 |
| MnTE-2-PyP | 0.06 | −6.89 | +228 | 7.76 |
| MnTnPr-2-PyP | 0.11 | −5.93 | +238 | 7.38 |
| MnTnBut-2-PyP | 0.19 | −5.11 | +245 | 7.25 |
| MnTnHex-2-PyP | 0.38 | −2.76 | +314 | 7.48 |
| MnTnHep-2-PyP | 0.46 | −2.10 | +342 | 7.65 |
| MnTnOct-2-PyP | 0.49 | −1.24 | +367 | 7.71 |
| MnTM-3-PyP | 0.05 | −6.96 | +52 | 6.61 |
| MnTE-3-PyP | 0.10 | −5.98 | +54 | 6.65 |
| MnTnPr-3-PyP | 0.22 | −5.00 | +62 | 6.69 |
| MnTnBut-3-PyP | 0.40 | −4.03 | +64 | 6.69 |
| MnTnHex-3-PyP | 0.55 | −2.06 | +66 | 6.64 |
The ~10-fold increase in the lipophilicity of the meta as compared to ortho isomers may compensate for their inferior antioxidant potency making them prospective therapeutics. Further, the rotational flexibility of the meta isomers when compared to conformational rigidity of the ortho isomers may affect favorably their cellular uptake and sub-cellular biodistribution. We thus decided to revisit meta isomers to better describe the factors that contribute to the in vivo efficacy of these seemingly different MnP. For in vivo studies we employed our simple and convenient O2·−-specific model that relies on the aerobic growth of SOD-deficient E. coli. Thus far, it has always been a reliable predictor of a prospective drug candidate.18,20
Results and Discussion
Two new meta isomers, MnTE-3-PyP and MnTnPr-3-PyP, were synthesized and characterized by elemental analysis, uv/vis spectroscopy, ESI-MS, electrochemistry (E½), lipophilicity (Rf and POW) and SOD-like activity (kcat) (Table 1 and 1S). There are no significant differences in the uv/vis spectral properties of new compounds (see Experimental and Table 1S) relative to other meta analogues.19 The ESI-MS spectral pattern, given in Experimental, was similar to that described previously for the ortho methyl and ethyl analogues.26 Elemental analyses (Experimental) are consistent with the hydrated species.
Dependence of the MnIIIP/MnIIP reduction potential (E½) and the SOD activity (kcat) on the number of C atoms in the N-alkylpyridyl chains
The lengthening of the ortho alkyl groups influences dramatically solvation and stericity of the porphyrin, which, in turn, affects E½ and kcat, as previously discussed in details.19 Briefly, as the alkyl chain grows from 1 to 8 carbon atoms, due to the increasingly less solvated, thus more exposed positive charges on pyridyls, the E½ grows approximately linearly from +220 to +367 mV vs NHE (Table 1). However, the dependence of kcat on the number of carbons reveals a delicate balance between two antagonistic factors: the increase of steric hindrance, i. e. crowding near the Mn site, which would decrease kcat; and the increase of the MnIIIP/MnIIP reduction potential (E½), which is a surrogate for the driving-force of the electron transfer and would increase kcat. Accordingly, kcat first decreases from methyl to butyl as the steric hindrance to the approach of O2·− to the Mn site increases, but then increases as the favorable thermodynamics for the dismutation reaction (E½) predominates (Table 1, Figure 1).19 The maximum change in E½ is 147 mV, and in kcat 0.54 log units (Table 1).
When the alkyl side chains are located in the meta positions, farther from the porphyrin core, free rotation of the pyridyl groups is allowed, and the steric and the solvation effects are, thus, much less pronounced. Consequently, the maximum change in E½ for the meta series is only 14 mV, and for kcat only 0.08 log units (Figure 1). As the alkyl chain lengthens, E½ continuously increases (Table 1, Figure 1). For small alkyl side chains, the favorable thermodynamics dominates and an initial small increase in kcat is observed. As the alkyl chains lengthen further (from n-butyl on), the steric hindrance prevails over the increase in E½, and kcat starts to decrease. As a result of such trends the SOD activity of some members of the meta series is close to the respective analogues in the ortho series; this happens for example for the n-propyl and n-butyl compounds where the SOD activities of the respective ortho and meta isomers approach each other (Figure 1, Table 1). It is worthwhile noting, however, that while the kcat of the ortho n-propyl and ortho n-butyl compounds are only about 4.9- and 3.6-fold higher than that of their meta analogues, the lipophilicity of the meta isomers remains 10-fold bigger throughout the series (Table 1).17 Therefore, due to the interplay of antioxidant potency and lipophilicity some members of the meta series may be of identical or higher efficacy than ortho isomers in in vivo models of oxidative stress. To explore such assumption we studied here the accumulation and the efficacy of both isomeric series in protecting SOD-deficient E. coli.
SOD-deficient E. coli (JI132) growth. Efficacy of MnP
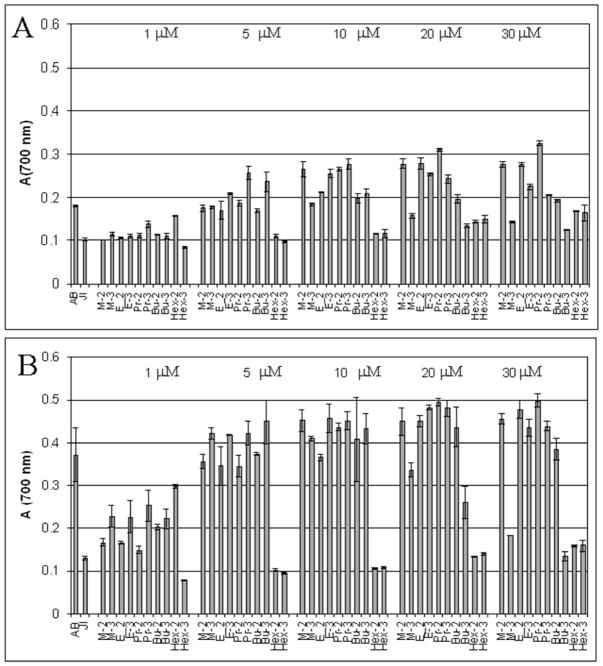
The JI132 strain lacks two cytoplasmic SODs, FeSOD and MnSOD, and thus displays phenotypic defects attributable to the damage of important cellular components by superoxide.27–30 Among those phenotypic deficiencies is the inability to grow aerobically in minimal glucose medium.29 Only compounds that are potent, cell-permeable SOD mimics, can exert protection, thus restoring the aerobic growth in minimal medium.31 Our data show that ortho and meta isomers protect SOD-deficient E. coli with nearly equal efficacy (Figure 2). The meta and ortho ethyl, n-propyl and n-butyl porphyrins appear of nearly identical efficacy at higher concentrations (10 and 20 μM), but meta analogues and most so the more lipophilic n-propyl and n-butyl porphyrins are more efficacious than ortho analogues at lower concentrations (1 and 5 μM), presumably due to their faster cellular uptake (Figure 2, and 1S). The structural flexibility of the meta isomers, that allows them to change their shape and size while crossing the membranes, is difficult to quantify but likely contributes to their high efficacy. Our data suggest that the shorter and the more planar methyl analogue, MnTM-3-PyP becomes somewhat less efficient at 10–30 μM levels presumably due to the enhanced unfavorable interactions with nucleic acids.18 The more lipophilic MnTnBu-3-PyP, that also possesses significant surfactant character and can thus disturb the cell membranes, becomes toxic at concentrations ≥20 μM. MnTnHex-3-PyP of even stronger surfactant character is toxic at all concentrations studied; its ortho analogue is only slightly less toxic (Figure 2A); this is in agreement with our previous E. coli study on ortho Mn(III) N-alkylpyridylporphyrins.18
Figure 2.
The aerobic growth of SOD-deficient E. coli JI 132 in 5 amino acid minimal medium (absorbance at 700 nm) in the presence of ortho(2) and meta(3) analogues shown here at 14th (A) and 18th hour (B)of growth. The growth of SOD-proficient E. coli AB1157 is shown also. Bars represent mean ± S.E.
Toxicity of MnP to wild type E. coli, AB1157
Further assessment of MnP toxicity was accomplished by monitoring their effect on the growth of AB1157, in complete, M9CA medium. None of the Mn porphyrins exerted toxicity at concentrations at which they protected the SOD-deficient JI132 strain (Figure 2S. At higher concentrations, however, the same trend in toxicity, as observed with the growth of JI132, was seen with AB1157 in M9CA medium. MnTM-3-PyP, and more so MnTnBu-3-PyP, exerted toxicity at ≥100 μM (Figure 2AS). Both hexyl analogues accumulate to the highest degree in cytosol and membranes (Figure 3) and exert toxicity at concentrations ≥ 1 μM (Figure 2BS). No toxicity of Mn porphyrins to AB1157 (alkyl = methyl to n-butyl) was observed in 5 amino acid minimal medium at 20 μM levels (Figure 3S).
Figure 3.
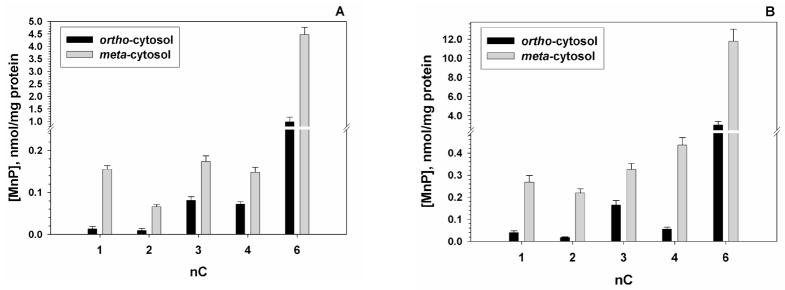
Accumulation of isomeric Mn(III) N-alkylpyridylporphyrins in cytosolic fractions of wild AB1157 (A) and SOD-deficient JI132 strain of E. coli (B). E. coli was incubated 1 hour with 5 μM MnP in M9CA medium. Bars represent mean ± S.E. Accumulation in membranes is shown in Supplemental Material, Figures 6S–9S.
Accumulation of porphyrins
Accumulation of MnPs in cytosolic (Figure 3) and membrane fractions (Figure 5S) was assessed in both AB 1157 and JI132 E. coli. Both strains show similar MnP accumulation profile, but on average the absolute uptake of MnPs is higher in the JI132 strain; this is clearly visible with the hexyl analogues and may be driven by a general E. coli attempt to accumulate nutrients or biologically relevant compounds that are neither produced within nor readily available. Since the SOD-deficient E. coli strain lacks two cytosolic superoxide dismutases, accumulation of SOD mimics in the cytosol is essential for protecting SOD-deficient mutant. The levels of porphyrins within cytosol and membrane (Figures 3 and 5S) were quantified by using calibration curves for ortho and meta isomeric porphyrins, where area below porphyrin Soret band was plotted vs porphyrin concentration (Figure 4S). Two major findings, depicted in Figures 3 are: (1) Compared to ortho analogues, meta isomers accumulate to significantly higher levels in the cytosol of both strains; due to the more planar structure (conformationally flexible) and higher lipophilicity they cross membrane easier, which apparently overcomes the inferior thermodynamics of meta isomers for O2·− dismutation, contributing at least in part to their high efficacy in substituting for deficiency in both cytosolic SODs. Consequently, the efficacy of meta and ortho ethyl and n-propyl analogues in E. coli study are nearly identical. (2) Ortho and meta hexyl analogues, which possess strong micellar character, accumulate much more than any other porphyrin in cytosol and membranes (Figures 3 and 5S), which seems to compromises membrane structure and its functions.
Although meta and para isomers have similar E½ and SOD activity, the systematic experimentation with the para isomers has remained on hold. Due to their conformational flexibility para isomers adopt planar structures and thus interact extensively with nucleic acids,15 which not only results in high toxicity, but also prevents the approach of superoxide to the Mn site;15 this in turn suppresses their effects in protecting SOD-deficient E. coli.15 The bulkiness of the ortho and meta isomers prevents such associations to a greater degree.18 In lights of the finding in the ortho and meta series described here, longer alkyl para analogues may be bulky enough to overcome possible nucleic acid intercalation and further studies with bulkier para isomers may thus be warranted.
Experimental Section
Experimental details are given in Supplemental Information. General. All chemicals are of highest purity and obtained from same commercial sources as indicated before. Porphyrins. Elemental analysis (Atlantic MicroLab, Norcross, GA): H2TE-3-PyPCl4·11H2O. (C48H72N8O11Cl4) Found: C, 53.82; H, 6.21; N, 10.89; Cl, 13.44 Calculated: C, 53.43; H, 6.73; N, 9.39; Cl, 13.14. H2TnPr-3-PyPCl4·16H2O (C52H90N8O16Cl4) Found: C, 50.98; H, 5.78; N, 9.32; Cl, 11.75 Calculated: C, 50.98; H, 7.40; N, 9.15; Cl, 11.58. MnTE-3-PyPCl5·8.5H2O (MnC48H61N8O8.5Cl5) Found: C, 51.74; H, 5.13; N, 9.93; Cl, 16.53 Calculated: C, 51.56; H, 5.50; N, 10.02; Cl, 15.85. MnTnPr-3-PyPCl5·10H2O (MnC52H76N8O10Cl5) Found: C, 51.39; H, 6.01; N, 9.22; Cl, 14.31 Calculated: C, 51.05; H, 6.43; N, 9.16; Cl, 14.49. Compounds are ≥98% pure. Electrospray ionization mass spectrometric analyses (ESI-MS) of 5 μM MnP in a H2O-acetonitrile (1:1, v/v; containing 0.1 % v/v HFBA) were done as described elsewhere.26 The m/z peak assignments are consistent with the following species in the given order: [MnT(alkyl)-3-PyP5+ + 2HFBA−]3+/3), [MnT(alkyl)-3-PyP5+ + HFBA− − 2H+]2+/2, [MnT(alkyl)-3-PyP5+ + 2HFBA− − H+]2+/2 and [MnT(alkyl)-3-PyP5+ + 3HFBA−]2+/2. MnTE-3-PyPCl5: Found 404.7 499.3, 606.3, 713.6, Calculated: 404.6, 499.3, 499.3, 606.3, 713.5; MnTnPr-3-PyPCl5, Found: 423.7, 527.5, −, 741.5, Calculated: 423.4, 527.6, 634.6, 741.6. UV/vis spectra were recorded in H2O at room temperature (25 ± 1°C). The λ(nm) of Soret bands and the corresponding molar absorptivities, ε (log values in parentheses) are: H2TE-3-PyP 417.0(5.52); H2TnPr-3-PyP 417.0(5.55); MnTE-3-PyP 460.0(5.19); MnTnPr-3-PyP 460.0 (5.14). Other absorption maxima and related ε are in Table 1S. Cyclic voltammetry was performed as described elsewhere.26 SOD-like activity. Determination of the kcat(O2·−) using cyt c assay was performed as previously described in details.1,18
In vivo studies. E. coli growth
Escherichia coli studies on SOD-deficient JI132 and wild type SOD-proficient strain, AB1157 were performed as described.31Accumulation of Mn porphyrins in E. coli. The wild type AB1157 and JI132 were grown in flasks in 10 mL of M9CA medium to a density corresponding to A700 ~ 0.6. Five μM Mn porphyrins were then added, and the cells were kept on a shaker for additional 60 minutes. Cells were then rapidly washed with ice-cold PBS, resuspended to a total volume of 1.0 ml and disrupted by sonication. Cytosolic and membrane fractions were separated by centrifugation. The membrane fractions were solubilized in 10% sodium dodecylsulfate for 24 hours and then centrifuged at high speed to remove the unsolubilized material. Spectra were recorded and the absorbances of MnP in cytosolic and membrane fractions measured at their Soret bands.19 Protein levels were measured by Lowry method.32 Details on the calculations of the accumulation of porphyrins in cytosolic and membrane fractions are given in Supplemental Information along with related Figures 4S–9S.
Supplementary Material
Acknowledgments
I.K., J.S.R. and I.B.H. acknowledge support by NIAID U19AI67798-01 and W. H. Coulter Translation Partners Grant Program. I.S. thanks NIH/NCI DCCC Core Grant (5-P30-CA014236-33), and L.B. grant MB03/07 from Kuwait University and HSC Research Core Facility grant GM01/01 and technical assistance of Milini Thomas.
Abbreviations Charges are omitted throughout text
- MnTalkyl-2(or 3)-PyP5+
Mn(III) meso-tetrakis(N-alkylyridinium-2 or 3-yl)porphyrin
- M, AEOL10112
alkyl being methyl
- E, AEOL10113
ethyl
- nPr
n-propyl
- nBu
n-butyl
- nHex
n-hexyl
- nHep
n-heptyl
- nOct
n-octyl, 2 and 3 relate to ortho and meta isomers respectively
- AEOL10150
MnTDE-2-ImP, Mn(III) tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin
- Rf
porphyrin path/solvent path
- TLC
thin-layer chromatographic retention factor
Footnotes
Supplemental Information is available on J. Med. Chem. web site.
References
- 1.Rebouças JS, DeFreitas-Silva G, Idemori YM, Spasojević I, Benov L, Batinić-Haberle I. The impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins. Protection of SOD-deficient E. coli via alternative mechanism where Mn porphyrin acts as a Mn-carrier. Free Radic Biol Med. 2008;45:201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batinić-Haberle I, Benov L, Spasojević I, Hambright P, Crumbliss AL, Fridovich I. The Relationship Between Redox Potentials, Proton Dissociation Constants of Pyrrolic Nitrogens, and in Vitro and in Vivo Superoxide Dismutase Activities of Manganese(III) and Iron(III) Cationic and Anionic Porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 3.Ferrer-Sueta G, Vitturi D, Batinić-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of Manganese Porphyrins with Peroxynitrite and Carbonate Radical Anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 4.DeFreitas-Silva G, Rebouças JS, Spasojević I, Benov L, Idemori YM, Batinić-Haberle I. SOD-like activity of Mn(II) β-octabromo-meso-tetrakis(N-methylpyridinium-3-yl)porphyrin equals that of the enzyme itself. Arch Biochem Biophys. 2008;477:105–112. doi: 10.1016/j.abb.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutterdige JMC. Biosciences. Oxford University Press; 2007. Free Radicals in Biology and Medicine. [Google Scholar]
- 6.Zhao Y, Chaiswing L, Oberley TD, Batinić-Haberle I, StClair W, Epstein CJ, StClair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;I:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 7.Tse H, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation/reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–47. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Sheng H, Sakai H, Yang W, Fukuda S, Salahi M, Day BJ, Huang J, Paschen W, Batinić-Haberle I, Crapo JD, Pearlstein RD, Warner DS. Sustained treatment is required to produce long-term neuroprotective efficacy from a metalloporphyrin catalytic antioxidant in focal cerebral ischemia. Free Radic Biol Med. 2009 in press. [Google Scholar]
- 9.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of oxygenation, free radicals and stress granules. Cancer Cell. 2005;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 10.Rabbani ZN, Spasojević I, Vasquez-Vivar J, Haberle S, Dewhirst MW, Vujaskovic Z, Batinić-Haberle I. Anti-tumor Effects of Mn(III) Ortho Tetrakis N-ethylpyridylporphyrin, MnTE-2-PyP5+ in Mice Model of Breast Tumor Via Suppression of Tumor Oxidative Stress. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.07.001. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moeller BJ, Batinić-Haberle I, Spasojević I, Rabbani ZN, Anscher MS, Vujaskovic Z, Dewhirst MW. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Rad Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jian C, Batinić-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide-dismutase (SOD) in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piganelli JD, Flores SC, Cruz C, Koepp J, Young R, Bradley B, Kachadourian R, Batinić-Haberle I, Haskins K. A Metalloporphyrin Superoxide Dismutase Mimetic (SOD Mimetic) Inhibits Autoimune Diabetes. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 14.Batinić-Haberle I, Ndengele MM, Cuzzocrea S, Rebouças JS, Spasojević I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking morphine tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebouças JS, Spasojević I, Tjahjono DH, Richaud A, Mendez; F, Benov L, Batinić-Haberle I. Redox modulation of oxidative stress by Mn porphyrin-based therapeutics: The effect of charge distribution. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]
- 16.Saba H, Batinić-Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kos I, Rebouças JS, DeFreitas-Silva G, Vujaskovic Z, Dewhirst MW, Spasojević I, Batinić-Haberle I. The effect of lipophilicity of porphyrin-based antioxidants. Comparison of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2009;47:72–78. doi: 10.1016/j.freeradbiomed.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batinić-Haberle I, Benov L, Spasojević I, Fridovich I. The Ortho Effect Makes Manganese (III) Meso-Tetrakis(N-methylpyridinium-2-yl)Porphyrin (MnTM-2-PyP) a Powerful and Potentially Useful Superoxide Dismutase Mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 19.Batinić-Haberle I, Spasojević I, Stevens RD, Hambright P, Fridovich I. Manganese(III) meso-tetrakis ortho N-alkylpyridylporphyrins. Synthesis, characterization and catalysis of O2•− dismutation. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 20.Okado-Matsumoto A, Batinić-Haberle I, Fridovich I. Complementation of SOD-deficient Escherichia Coli by manganese porphyrin mimics of superoxide dismutase activity. Free Radic Biol Med. 2004;37:401–10. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Wise-Faberowski L, Warner DS, Spasojević I, Batinić-Haberle I. The effect of lipophilicity of Mn (III) ortho N-alkylpyridyl- and diortho N, N′-imidazolylporphyrins in two in-vitro models of oxygen and glucose deprivation -induced neuronal death. Free Radic Res. 2009;43:329–339. doi: 10.1080/10715760902736283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollard J, Rebouças JS, Durazo A, Kos I, Fike F, Panni M, Gralla EB, Valentine JS, Batinić-Haberle I, Gatti RA. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia telangiectasia cells. Free Radic Biol Med. 2009;47:250–260. doi: 10.1016/j.freeradbiomed.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan S, Batinić-Haberle I. MnTnHex-2-PyP in rabbit cerebral palsy model. unpublished. [Google Scholar]
- 24.Szabó C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano VG, van Duzer JH, Williams W, Salzman AL, Groves JT. Pathogenic Role of Peroxynitrite in the Development of Diabetes and Diabetic Vascular Complications: Studies with FP15, a Novel Potent Peroxynitrite Decomposition Catalyst. Mol Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 25.Saltsman I, Botoshansky M, Gross Z. Facile synthesis of ortho-pyridyl-substituted corroles and molecular structures of analogues porphyrins. Tetrahedron Lett. 2008;49:4163–4166. [Google Scholar]
- 26.Rebouças JS, Spasojević I, Batinić-Haberle I. Quality of Mn-porphyrin-based SOD mimics and peroxynitrite scavengers for preclinical mechanistic/therapeutic purposes. J Pharm Biomed Anal. 2008;48:1046–1049. doi: 10.1016/j.jpba.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imlay JA. Cellular defenses against superoxide and hydrogen petoxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 29.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messner KR, Imlay JA. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999;274:10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- 31.Batinić-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Emanuela Mazzon E, Di Paola R, Radi R, Spasojević I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two different models of oxidative stress injuries, SOD-specific E. coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.