Abstract
Objectives
To determine the effectiveness of around-the-clock (ATC) analgesic administration, with or without nurse coaching, compared to standard care with PRN dosing in children undergoing outpatient tonsillectomy.
Methods
Children 6 to 15 years were randomized to receive acetaminophen and hydrocodone (167mg/2.5mg/5ml) for 3 days after surgery: Group A (N=39) – every 4 hours PRN, with standard postoperative instructions; Group B (N=34) – every 4 hours ATC, with standard postoperative instructions, without nurse coaching; and Group C (N=40) – every 4 hours ATC, with standard postoperative instructions, with coaching. Parents completed a medication log, and recorded the presence and severity of opioid-related adverse effects and children's reports of pain intensity using a 0 to 10 numeric rating scale.
Results
No differences were found in analgesic administration or pain intensity scores between the 2 ATC groups. Therefore, they were combined for comparison with the PRN group. Children in the ATC group received more analgesic than those in the PRN group (p<0.0001). Children in the PRN group had higher pain intensity scores compared to children in the ATC group, both at rest (p=0.017) and with swallowing (p=0.017). Pain intensity scores for both groups were higher in the morning compared to the evening (p<0.0001). With the exception of constipation, scheduled analgesic dosing did not increase the frequency or severity of opioid-related adverse effects.
Discussion
Scheduled dosing of acetaminophen and hydrocodone is more effective than PRN dosing in reducing pain intensity in children following tonsillectomy. Nurse coaching does not impact parent's adherence to ATC dosing.
Keywords: pediatric pain, tonsillectomy pain, postoperative pain management, nurse coaching, acetaminophen with hydrocodone
Introduction
Current outpatient management of postoperative pain following tonsillectomy in children includes the use of oral opioid and non-opioid analgesics, largely prescribed on an as needed, pain contingent basis. However, postoperative pain following tonsillectomy has predictable characteristics (e.g., prolonged duration, relatively constant in nature, moderate to severe intensity),1-8 which suggests that scheduled dosing regimens may be efficacious. Guidelines on postoperative management of pediatric pain recommend time-contingent analgesic dosing to reduce or prevent pain before it begins.9-12 Potential benefits of this approach include the maintenance of therapeutic levels of opioids, the facilitation of routine postoperative activities (e.g., oral intake, activity, sleep), and the avoidance of delays in analgesic administration because of inaccurate parental assessment of children's pain behaviors. However, many clinicians and parents fear that regularly scheduled administration of analgesics may result in the administration of unnecessary and/or excessive amounts of analgesics, which may cause undesirable and potentially harmful adverse effects.
Three randomized clinical trials (RCTs) have evaluated the effectiveness of around-the-clock (ATC) dosing of analgesics in children at home following tonsillectomy.13-15 In the first RCT that evaluated for differences in pain intensity between children who received scheduled versus as needed (i.e., PRN) administration of acetaminophen,13 no between group differences were found in children's reports of pain intensity. Furthermore, 50% of the children who received acetaminophen ATC reported moderate levels of pain. In another RCT,14 ATC administration of acetaminophen with codeine was compared to PRN dosing in children following tonsillectomy. Despite the fact that children in the ATC group received twice the dose of analgesic medication, no between group differences were found in pain intensity scores or in the frequency of moderate-to-severe side effects over the first three postoperative days. In addition, pain scores in the ATC group were in the moderate range, which suggested that the potency of the analgesic, not the schedule of administration, was inadequate for the management of acute pain following tonsillectomy. Furthermore, the lack of efficacy of codeine in this study may be partially explained by the fact that substantial genetic variation exists in the activity of the cytochrome P450 enzyme CYP2D6, which is responsible for the metabolism of codeine into its active metabolite, morphine. In individuals with this polymorphism, codeine does not have an analgesic effect.16-17 Finally, in a recent RCT that compared ATC administration of rofecoxib to ATC administration of hydrocodone and acetaminophen,15 pain intensity scores with swallowing were significantly lower in the rofecoxib group. However, no between group differences were found in pain intensity scores assessed just prior to the administration of the analgesic and pain intensity scores were in the moderate range.
Given the limited number of studies on the effectiveness of ATC dosing of analgesics for the management of postoperative pain in children, additional research is needed. Therefore, the purposes of this RCT, in children who underwent tonsillectomy, were to determine whether ATC dosing of a weight-appropriate dose of acetaminophen with hydrocodone, with or without nurse coaching, compared to standard care with PRN dosing of the same analgesic reduced children's reports of pain intensity with and without swallowing, increased pain relief, and increased analgesic consumption. In addition, the effect of ATC dosing on the frequency and severity of opioid-related adverse effects were evaluated.
Materials and Methods
Setting and Sample
Children 6 to 15 years of age were recruited from February 2002 to January 2006 from a 297-bed regional tertiary care center that serves about 950,000 children in Central California. The study was approved by the Institutional Review Board at the study site and at the University of California, San Francisco. Eligibility criteria included no prior history of neurologic impairments (i.e., visual or hearing deficits, learning disability, or motor function deficit); no history of allergies to hydrocodone or acetaminophen; ability of the child to speak English; ability of the parent to read, write, and speak English; and access to a telephone.
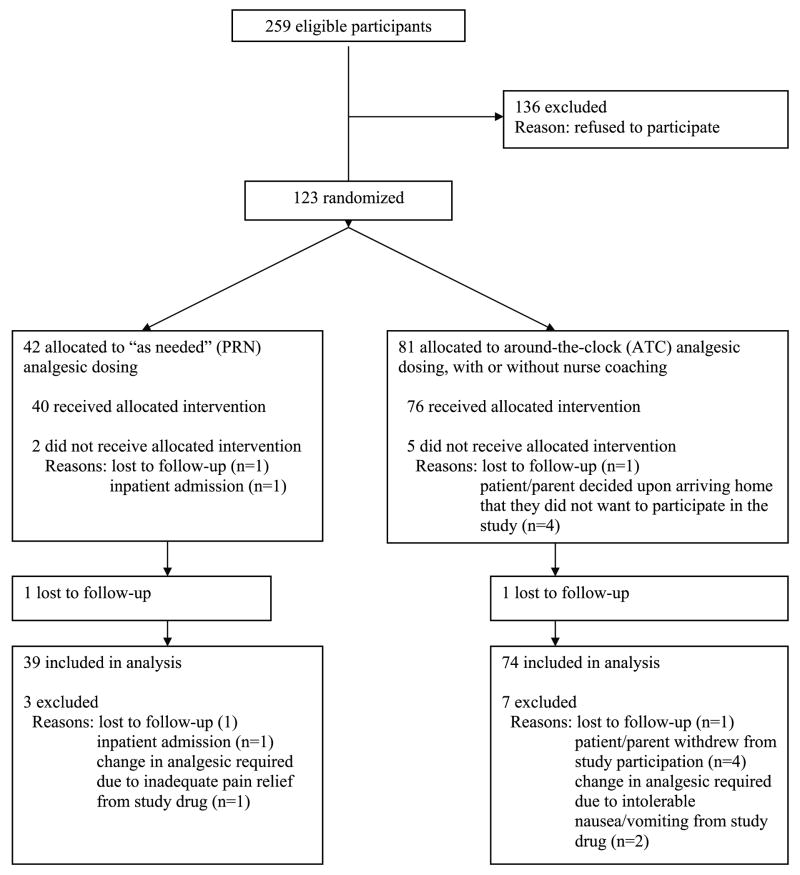
Initially, an iterative approach that considered the analysis of each of the study aims was used to determine sample size. Testing the between subject's main effects of the proposed two-way analysis of variance (ANOVA) would require the greatest sample size. Satisfying the sample size requirements of these main effects would ensure an adequate sample for the within subjects tests. In order to achieve power at or above .80 to detect a moderate effect size (f = .25, percent of explained variance approximately 7%) at an alpha level of .01, a total sample size of 240 participants was required (i.e., 80 per group). Assuming a potential drop out rate of approximately 20%, we planned to enroll 300 patients. The actual sample size differed from that originally proposed based on findings from an interim analysis to determine whether it was necessary to continue recruitment. This interim analysis demonstrated a significant difference between the treatment groups on pain intensity scores such that further data collection was unnecessary. One hundred and twenty-three children were enrolled in the study. One hundred and thirteen children completed the study and are included in this analysis (see Figure 1).
Figure 1.
CONSORT diagram showing the flow of participants through each stage of the randomized trial.
Ten children became ineligible to participate or withdrew their consent to participate after surgery and were withdrawn from the study. The minimal drop out rate did not differ between the PRN (N=3, 7.1%) and ATC groups combined (N=7, 8.6%; χ2 = 0.08, p =0.77). In the PRN group, one child was admitted postoperatively for observation due to increased edema and persistent desaturation. One child received inadequate pain relief from the study drug which necessitated a change in analgesic medication, and one child was withdrawn after their parents decided upon arriving home that they did not want to participate in the study. In the ATC group, two children developed intolerable side effects (i.e., persistent nausea and vomiting) that required a change in analgesic medication, and 5 children and/or their parents decided upon arriving home that they did not want to participate in the study. Since children who did not complete the study dropped out early on, and provided no data or incomplete data for the first postoperative day, they were not included in the analysis. Children who did not complete the study (N=10) were younger (7.2 years vs. 9.3 years; t=2.6, p<0.001) and weighed less (28.2 kg vs. 40.8 kg; t=2.4, p=0.02). No differences were found in any of the surgical or parental characteristics between children who did and did not complete the study. The refusal rate for the study was 53%. The main reasons for refusal included: parents who only wanted to give the analgesic PRN (57.3%); parents who only wanted to give the analgesic ATC (5.9%); and parents who were too busy to participate (36.8%).
Study Procedures
On admission to the outpatient surgery setting, parents of children ages 6 to 15 years who were to undergo tonsillectomy, with or without other concurrent minor procedures such as myringotomy tube placement, were approached to participate in the study. Written informed consent was obtained from all parents and written assent was obtained from children over the age of 7. Parents and children were instructed in the use of the 0 (no pain) to 10 (worst pain imaginable) numeric rating scale (NRS) prior to surgery and provided a return demonstration.
Following consent and assent, children were stratified by gender and then randomized to one of three treatment groups using an SPSS software-generated random selection process, to receive a weight appropriate dose of hydrocodone and acetaminophen elixir (i.e., approximately 0.2 mg/kg/dose of hydrocodone; maximum daily acetaminophen dose of approximately 73 mg/kg) for postoperative pain for the first 3 days after surgery as follows: PRN group - every 4 hours PRN, with standard postoperative instructions, without nurse coaching; ATC group - every 4 hours ATC, with standard postoperative instructions, without nurse coaching; or ATC + coaching group - every 4 hours ATC, with standard postoperative instructions and nurse coaching. A computer-generated randomization list was drawn up by the statistician and given to an administrative assistant who prepared two sets of serially numbered sealed envelopes, one for male participants and one for female participants, each containing the random group assignment. Once recruitment and baseline data collection were complete, the research nurse opened the envelope to reveal the participant's group assignment. Blinding of participants was not possible due to the study design.
A preprinted order sheet was completed that indicated the treatment group, the child's weight, and the standardized dose of the analgesic by weight, which was signed by the surgeon prior to the procedure and sent to the inpatient pharmacy for preparation and dispensing. The bottles were labeled with the patient's information, the name of the analgesic medication, and dosing instructions according to the patient's study group assignment. The study drug was delivered to the Day Surgery area and was provided to the parent prior to discharge home.
Surgical Procedure
No attempt was made to standardize the inhalation anesthetics used during surgery, concurrent administration of opioid analgesics, or surgical technique. However, no differences were found among the three groups in any of these parameters. All patients received local anesthetic infiltration of the tonsillar beds by the surgeon.
Perioperative Analgesic Administration
All children received fentanyl (0.25 mcg/kg up to a total dose of 2 mcg/kg) or morphine (0.025 mg/kg up to a total dose of 0.2 mg/kg) intravenously, as needed for pain during the immediate postoperative period in the Post Anesthesia Care Unit and Day Surgery area. Prior to discharge home, children were given the first dose of hydrocodone and acetaminophen, with instructions to administer the pain medication at home based on their group assignment. Parents were given instructions on proper dose measurement and were asked to provide a return demonstration with the first dose administered prior to discharge. Parents were told not to give their child any other pain medication. In addition, they were instructed to call the research nurse if their child experienced fever, unrelieved pain, or intolerable side effects. Parents in both of the ATC dosing groups were provided with a digital timer and instructed to set the timer for each 4-hour dosing interval as a reminder to administer the next scheduled dose.
Postoperative Instructions and Nurse Coaching Intervention
When the child was transferred to the Day Surgery area, the research nurse reviewed standard postoperative instructions with all of the parents, regardless of group assignment, using a preprinted teaching booklet that was given to the parents to take home. Information was provided on activity restrictions, school attendance, postoperative bleeding, diet, ear and throat pain, analgesic administration, possible changes in sleep patterns, expected appearance of the back of the throat, fever, and the follow-up appointment with the surgeon. Additional written instructions were included for children undergoing concurrent myringotomy tube placement.
The preprinted teaching booklet given to the parents of children in the PRN group instructed the parents to give the hydrocodone and acetaminophen every 4 hours “as needed” for pain. Whereas, the booklet that was given to the parents of children in the ATC and the ATC + coaching groups instructed the parents to give the hydrocodone and acetaminophen every 4 hours ATC. In addition, all parents were provided with a home diary and were instructed by the research nurse in how to complete the diary twice-a-day with the child's self report of pain at rest and with swallowing, pain relief, and medication intake.
For children in the ATC + coaching group, the research nurse provided a coaching intervention that included a review of the pain management teaching guide that was incorporated into the preprinted teaching booklet. Information was provided on the reported postoperative pain experience of children following tonsillectomy, the rationale for administration of a nonopioid with an opioid analgesic, the ordered dose and ATC dosing, strategies for improving children's adherence with analgesic consumption, and myths about psychological addiction.
Follow-Up Phone Calls
The research nurse made scheduled follow-up phone calls to all parents on days one and two postoperatively, to evaluate the parent's level of adherence with completing the home diary. In addition, parents in the ATC + coaching group received the coaching intervention at the time of the phone calls to promote adherence with the ATC dosing regimen. The coaching intervention included an evaluation of the child's current condition, review of the pain intensity scores, verification that the child was taking the pain medication, re-education regarding the rationale for ATC dosing, review of strategies to facilitate medication administration, and re-education about potential side effects associated with analgesic administration. At the end of the day 2 phone call, the research nurse scheduled an appointment for a home visit on the fourth day after surgery to collect the home diary and measure the amount of medication that remained in the bottle.
Instruments
Demographic characteristics of the children and parents were collected at the time of enrollment into the study. In addition, the children's medical records were reviewed to obtain surgical and anesthesia data.
Home Diary
The Home Diary obtained information on pain intensity, presence and severity of opioid-related adverse effects, and medication use. Pain intensity, with and without swallowing, was rated by the child prior to bedtime and upon awakening, using a 0 (no pain) to 10 (worst pain imaginable) NRS. Severity of opioid-related adverse effects (i.e., nausea, vomiting, constipation, daytime sedation, lightheadedness or feeling dizzy, and nightmares) were evaluated each evening using a 0 (did not have) to 4 (very severe) rating scale.
Parents completed the pain medication log on a daily basis. Parents recorded the volume (in milliliters) and the times they administered the pain medication to their child. For patients in the ATC + coaching group, the research nurse recorded in the diary the volume and the times that the parents should administer the pain medication based on the time that the first dose of the pain medication was given just prior to discharge from Day Surgery. Parents were instructed to circle the times in the diary when they administered the pain medication to their child. In the event that the dosing schedule needed to be modified (e.g., excessive sedation that resulted in a skipped dose, with subsequent breakthrough pain prior to the next scheduled dose of the analgesic), parents were instructed to revise the dosing schedule by drawing a line through the times previously written in, and entering new times at 4-hour intervals, beginning with the time of the dose just given.
Data Analysis
Because no differences were found in any of the demographic, parental, and surgical characteristics; pain intensity scores; and amount of analgesic administered between the two ATC groups, these two groups were combined and compared with the PRN group for the analyses of pain intensity scores (i.e., with and without swallowing), analgesic consumption, and side effects data.
Data were analyzed using SPSS Version 14. Descriptive statistics were performed to summarize sample characteristics. Differences between the PRN and ATC groups, in demographic, surgical, and parental characteristics, were evaluated with independent sample t tests and Chi-square analyses. Differences, over time between the PRN and ATC groups, in pain intensity scores (i.e., with and without swallowing) and analgesic consumption were determined using two-way, repeated measures analysis of variance (ANOVA) with one between subjects factor (i.e., group with two levels) and one within subjects factor (i.e., time with 4 levels [day of surgery, and postoperative days 1, 2, and 3] or 7 levels [first evening at home following surgery, POD 0 PM, and morning and evening measurement points for the first 3 days following surgery, POD 1 AM, POD 1 PM, POD 2 AM, POD 2 PM, POD 3 AM and POD 3 PM]). T-tests were performed to evaluate for differences between treatment groups in the mean pain intensity scores, with and without swallowing, at each of the 7 times, and effect sizes were calculated to evaluate the magnitude of these differences. The effect size d represents the difference between the two group means in standard deviation units.18
Each side effect was recoded into a dichotomous response (i.e., 0 = did not have symptom or had slight symptom or 1 = symptom that was moderate, severe, or very severe) to make the comparisons more clinically meaningful in terms of children who did and did not experience significant side effects. Chi-square analyses were performed to evaluate for differences, between treatment groups, in the frequency of side effects of moderate-to-severe intensity, at each time point after surgery. To evaluate the magnitude of these differences, Cohen's effect size h that used arcsine transformations of proportions was used to calculate effect sizes. For both Cohen's d for means and Cohen's h for proportions, a small effect size is equal to .20, a medium effect size is equal to .50, and a large effect size is equal to .80.18
The Cochran Q test was used to assess for changes over time in the frequency of moderate-to-severe side effects within a treatment group. For those variables in which the Cochran's Q was significant, McNemar's tests of pairwise proportions was used to determine where the significant differences were.
All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest complete set of data across groups. A p value of less than 0.05 was considered statistically significant.
Results
Sample Characteristics
As shown in Table 1, no differences were found in any of the demographic, parental, and surgical characteristics between the PRN and ATC groups, except for the ethnic background of the child's mother.
TABLE 1.
Differences in Demographic and Surgical Characteristics Between the PRN and ATC Groups
| Characteristic | PRN Group (n=39) |
ATC Groups Combined (n=74) |
Statistics |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (months) | 114.1 (34.8) | 111.2 (29.5) | t = 0.44, p = .66 |
| Weight (kilograms) | 41.3 (16.7) | 40.5 (15.9) | t = 0.27, p = .79 |
| Total surgery time (minutes) | 34.0 (9.4) | 34.4 (10.2) | t = 0.28, p = .46 |
| Total length of stay (hours) | 5.8 (1.5) | 5.8 (1.2) | t = 0.13, p = .90 |
| % (n) | % (n) | ||
| Gender | |||
| Male | 43.6% (17) | 43.2% (32) | χ2 = 0.001, p =1.00 |
| Female | 56.4% (22) | 56.8% (42) | |
| Mother's ethnicity | |||
| White | 38.5% (15) | 44.6% (33) | χ2 = 9.44, p = 0.02 |
| Hispanic | 35.9% (14) | 47.3% (35) | |
| African American | 15.4% (6) | 2.7% (2) | |
| Other | 10.3% (4) | 2.7% (2) | |
| No Response | 0.0% (0) | 2.7% (2) | |
| Father's ethnicity | |||
| White | 25.6% (10) | 35.1% (26) | χ2 = 5.39, p = 0.15 |
| Hispanic | 43.6% (17) | 48.6% (36) | |
| African American | 20.5% (8) | 6.8% (5) | |
| Other | 10.3% (4) | 6.8% (5) | |
| No Response | 2.7% (2) | ||
| Surgery type | |||
| Tonsillectomy (T) | 10.3 (4) | 14.9 (11) | χ2 = 0.47, p = .57 |
| T and adenoidectomy | 89.7 (35) | 85.1 (63) | |
| Intraoperative anti-emetic(s) | |||
| Decadron | 100.0 (39) | 95.9 (71) | χ2 = 1.62, p = .55 |
| Metoclopramide | 53.8 (21) | 50.5 (37) | χ2 = 0.15, p = .84 |
| Ondansetron | 30.8 (12) | 25.7 (19) | χ2 = 0.33, p = .66 |
| Two or more anti-emetic drugs given intraoperatively | 59.5 (25) | 55.6 (45) | χ2 = 0.75, p = .41 |
| One or more anti-emetic doses given after surgery | 16.7 (7) | 18.5 (15) | χ2 = 1.00, p=.50 |
PRN, as needed; ATC, around the clock; SD, standard deviation.
Analgesic Consumption
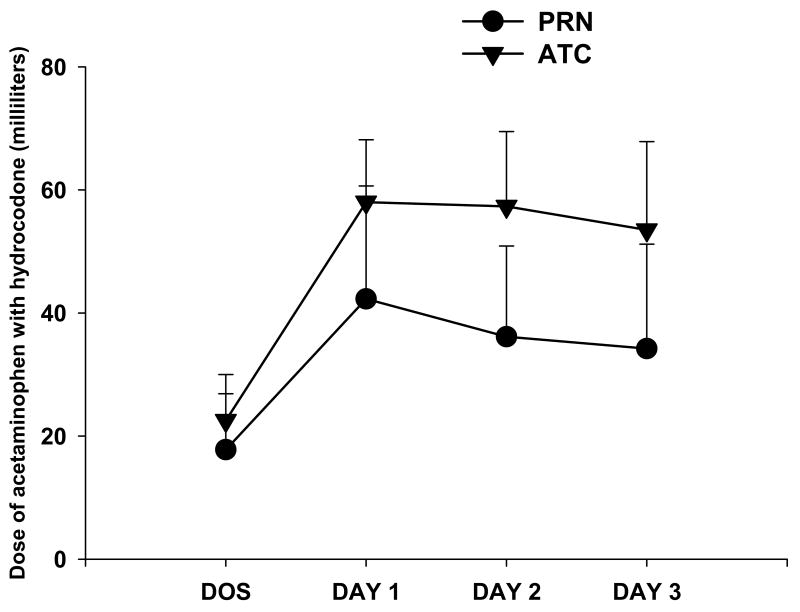
Figure 2 illustrates the changes, over time, in analgesic consumption between the PRN and ATC treatment groups. The two-way ANOVA demonstrated a significant main effect of group (F(1,102)=49.67, p<0.0001), a significant main effect of time (F(3,306)=212.55, p<0.0001), and a significant group × time interaction (F(3,306)=15.45, p<0.0001). While no between group differences in analgesic consumption occurred on the day of surgery, the ATC group had greater analgesic consumption compared to the PRN group on each of the first three postoperative days. These differences in analgesic consumption were substantiated through analyses of the volume of medication that remained in the bottles that was measured on the day of the home visit. The two groups differed significantly from each other in the total number of day time doses (ATC: 9.34 ± 0.92, PRN: 6.21 ± 2.61, t = -9.24, p < 0.0001) and in the total number of night time doses (ATC: 8.84 ± 1.07, PRN: 4.26 ± 2.28, t = -14.48, p < 0.0001). Two-way ANOVAs for changes, over time, in analgesic consumption between the ethnic groups for the child's mother and father found no significant between group differences for the PRN or ATC treatment groups.
Figure 2.
Changes, over time, in the dose of acetaminophen (167 mg/5 ml) and hydrocodone (2.5 mg/5 ml) administered to patients in the PRN (n = 39) and ATC (n = 74) groups. All values are plotted as means ± standard deviations. On the x-axis, DOS refers to the day of surgery, followed by the first 3 postoperative days.
Pain Intensity Scores
Table 2 shows the mean pain intensity scores with and without swallowing, over time for the two groups of children. Paired t-tests demonstrated that the PRN group had significantly higher mean pain intensity scores at rest on the second evening (t=2.23, p=0.028) and the second morning after surgery (t=2.33, p=0.002). Pain intensity scores with swallowing were significantly higher in the PRN group beginning on the first morning after surgery (t=2.11, p=0.037) until the third evening after surgery (t=2.599, p=0.011; second evening after surgery, t=2.05, p=0.43 and second morning after surgery, t=2.99, p=0.003).
TABLE 2.
Differences in Mean Pain Intensity Scores, With and Without Swallowing, Between the PRN and ATC Groups
| Pain Measures | PRN Group (N=39) | ATC Group (N=70) | Statistics | Effect Size |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Pain intensity scores without swallowing | ||||
| Average of 7 assessments | 3.9 (2.2) | 3.0 (1.7) | F(1,107)=5.85, p=0.02 |
d=0.47 |
| POD 0 PM | 3.5 (2.6) | 2.8 (2.2) | t=1.55, p=0.12 | d=0.31 |
| POD 1 AM | 4.3 (2.8) | 3.7 (2.4) | t=1.07, p=0.56 | d=0.21 |
| POD 1 PM | 3.7 (3.0) | 2.6 (2.3) | t=2.23, p=0.03 | d=0.44 |
| POD 2 AM | 5.0 (2.9) | 3.2 (2.9) | t=3.22, p=0.002 | d=0.62 |
| POD 2 PM | 3.7 (2.7) | 2.8 (2.4) | t=1.82, p=0.07 | d=0.36 |
| POD 3 AM | 4.1 (2.7) | 3.1 (2.3) | t=1.92, p=0.06 | d=0.38 |
| POD 3 PM | 2.9 (2.1) | 2.6 (2.4) | t=0.80, p=0.43 | d=0.16 |
| Pain intensity scores with swallowing | ||||
| Average of 7 assessments | 4.2 (2.4) | 3.2 (1.2) | F(1,107)=5.83, p=0.02 |
d=0.47 |
| POD 0 PM | 3.8 (2.8) | 3.4 (2.4) | t=0.87, p=0.39 | d=0.17 |
| POD 1 AM | 4.9 (2.8) | 3.8 (2.5) | t=2.11, p=0.04 | d=0.41 |
| POD 1 PM | 4.0 (3.3) | 2.9 (2.5) | t=2.05, p=0.04 | d=0.40 |
| POD 2 AM | 5.0 (2.9) | 3.3 (2.8) | t=2.99, p=0.003 | d=0.57 |
| POD 2 PM | 4.2 (2.9) | 2.9 (2.4) | t=2.60, p=0.01 | d=0.51 |
| POD 3 AM | 4.2 (3.0) | 3.3 (2.5) | t=1.56, p=0.12 | d=0.31 |
| POD 3 PM | 3.2 (2.5) | 2.7 (2.3) | t=1.-2, p=0.30 | d=0.21 |
POD, postoperative; AM, morning; PM, evening; PRN, as needed; ATC, around the clock; SD, standard deviation.
For both the PRN and ATC groups, the average pain intensity scores with and without swallowing were higher in the morning compared to the evening (F(1,110)=28.99, p<0.0001 and F(1,110)=35.98, p<0.0001, respectively). Post hoc analyses of the average pain intensity scores without swallowing indicated significant differences between the groups at all time points except those at POD 0 PM, POD 1 AM, and POD 3 PM, with corresponding effect sizes ranging from d=0.036 to d=0.62. For the average pain intensity scores with swallowing, post hoc analyses indicated significant differences between the PRN and ATC groups at all time points except those at POD 0 PM, POD 3 AM, and POD 3 PM, with corresponding effect sizes ranging from d=0.17 to d=0.57.
Between Treatment Group Comparisons of Side Effects
On each postoperative evening, the following side effects were assessed: daytime sedation, lightheadedness, feeling dizzy, nightmares, nausea, vomiting, and constipation. As shown in Table 3, no significant between group differences were found in the proportion of children with moderate-to-severe symptoms for any of the side effects. Effect sizes were small for most side effects at each of the measured times, with the exception of nightmares which approached a medium effect size on postoperative day 2 (h=-0.35) and 3 (h=-0.40), and daytime sedation (h=-0.38) and nausea (d=-0.36), which approached a medium effect size on postoperative day 3.
TABLE 3.
Differences in the Proportion of Children With Moderate-to-Severe Side Effects Between the PRN and ATC Groups
| Time Point | PRN Group | ATC Group | Statistics | Effect Size |
|---|---|---|---|---|
| Daytime | ||||
| Sedation | ||||
| POD 0 PM | 42.1% (16/38) | 35.6% (26/73) | χ2 = 0.45, p = .54 | h = 0.12 |
| POD 1 PM | 15.4% (6/39) | 16.4% (12/73) | χ2 = 0.02, p = 1.00 | h = -0.03 |
| POD 2 PM | 17.9% (7/39) | 16.4% (12/73) | χ2 = 0.04, p = 1.00 | h = 0.05 |
| POD 3 PM | 7.7% (3/39) | 21.4% (15/70) | χ2 = 3.43, p = .10 | h = -0.38 |
| Lightheadedness, Feeling Dizzy | ||||
| POD 0 PM | 30.8% (12/39) | 27.8% (20/72) | χ2 = 0.11, p = 0.83 | h = 0.07 |
| POD 1 PM | 12.8% (5/39) | 21.9% (16/73) | χ2 = 1.38, p = .31 | h = -0.24 |
| POD 2 PM | 7.7% (3/39) | 12.3% (9/73) | χ2 = 0.57, p = .54 | h = -0.13 |
| POD 3 PM | 10.5% (4/38) | 17.1% (12/70) | χ2 = 0.85, p = .41 | h = -0.17 |
| Nightmares | ||||
| POD 0 PM | 5.3% (2/38) | 3.0% (2/67) | χ2 = 0.34, p = .62 | h = 0.10 |
| POD 1 PM | 2.6% (1/39) | 4.2% (3/72) | χ2 = 0.19, p = 1.00 | h = -0.06 |
| POD 2 PM | 0.0% (0/38) | 2.8% (2/72) | χ2 = 1.07, p = .54 | h = -0.35 |
| POD 3 PM | 0.0% (0/38) | 4.3% (3/70) | χ2 = 1.67, p = .55 | h = -0.40 |
| Nausea | ||||
| POD 0 PM | 38.5% (15/39) | 30.6% (22/72) | χ2 = 0.71, p = .41 | h = 0.17 |
| POD 1 PM | 23.1% (9/39) | 23.6% (17/72) | χ2 = 0.004, p = 1.00 | h = -0.02 |
| POD 2 PM | 10.3% (4/39) | 19.2% (14/73) | χ2 = 1.50, p = .29 | h = -0.26 |
| POD 3 PM | 12.8% (5/39) | 27.1% (19/70) | χ2 = 2.99, p = .10 | h = -0.36 |
| Vomiting | ||||
| POD 0 PM | 34.2% (13/38) | 24.7% (18/73) | χ2 = 1.13, p = .37 | h = 0.20 |
| POD 1 PM | 7.7% (3/39) | 5.5% (4/73) | χ2 = 0.21, p = .69 | h = 0.08 |
| POD 2 PM | 5.1% (2/39) | 9.6% (7/73) | χ2 = 0.68, p = .49 | h = -0.19 |
| POD 3 PM | 2.6% (1/39) | 8.6% (6/70) | χ2 = 1.50, p = .42 | h = -0.26 |
| Constipation | ||||
| POD 0 PM | 5.3% (2/38) | 5.8% (4/69) | χ2 = 0.013, p = 1.00 | h = -0.04 |
| POD 1 PM | 13.2% (5/38) | 15.2% (10/66) | χ2 = 0.08, p = 1.00 | h = -0.06 |
| POD 2 PM | 15.8% (6/38) | 18.3% (13/71) | χ2 = 0.11, p = 0.80 | h = -0.05 |
| POD 3 PM | 17.9% (7/39) | 22.1% (15/68) | χ2 = 0.27, p = 0.80 | h = -0.10 |
POD, postoperative day; PM, evening; PRN, as needed; ATC, around the clock
In calculating the effect sizes, the differences in proportions were always calculated left to right, as PRN group – ATC group.
Within Treatment Group Comparisons of Side Effects
Daytime sedation
For children in both the PRN and ATC groups, significant decreases in the frequency of moderate-to-severe daytime sedation were found over the four postoperative assessments (Cochran Q = 18.19, 12.95, respectively, both p < 0.01). For children in the PRN group, daytime sedation was higher on the day of surgery compared to each of the 3 days following surgery (p=0.006, 0.035, and 0.001, respectively). For children in the ATC group, daytime sedation was higher on the day of surgery compared to the first 2 postoperative days (p=0.001 and 0.013, respectively).
Lightheadedness, feeling dizzy
For children in both the PRN and ATC groups, significant decreases in the frequency of moderate-to-severe lightheadedness/feeling dizzy were found over the four postoperative assessments (Cochran Q = 13.64, 7.92, respectively, both p<0.05). For children in the PRN group, lightheadedness occurred more frequently on day of surgery compared to the first 3 days after surgery (p=0.0.016, 0.012, and 0.039, respectively). For children in the ATC group, lightheadedness occurred more frequently on day of surgery compared to the second postoperative day (p=0.019).
Nightmares
For children in both the PRN and ATC groups, no differences in the frequency of moderate-to-severe nightmares were found over the four postoperative assessments (Cochran Q = 2.00, p = .57; Cochran Q = 0.75, p = .86, respectively).
Nausea
For children in the PRN group, significant decreases in the frequency of moderate-to-severe nausea were found over the four postoperative assessments (Cochran Q = 13.00, p = 0.005). Nausea occurred more frequently on the day of surgery compared to the second (p=0.007) and third (p=0.021) postoperative days. For children in the ATC group, no significant differences in the frequency of moderate-to-severe nausea were found over the four postoperative assessments (Cochran Q = 3.05, p = .38).
Vomiting
For children in both the PRN and ATC groups, significant decreases in the frequency of moderate-to-severe vomiting were found over the four postoperative assessments (Cochran Q = 27.15, 15.91, respectively both p < 0.01). For children in both the PRN and ATC groups, vomiting occurred more frequently on the day of surgery compared to each of the first 3 postoperative days (PRN group: p=0.002, 0.003, and < 0.0001; ATC group: p=0.001, 0.035, and 0.013). p=0.001 and 0.013, respectively).
Constipation
For children in the PRN group, no differences in the frequency of moderate-to-severe constipation were found over the four postoperative assessments (Cochran Q = 4.16, p = .25). For children in the ATC group, significant increases in the frequency of moderate-to-severe constipation were found over the four postoperative assessments (Cochran Q = 8.73, p = 0.03), with constipation occurring more frequently on each of the first 3 postoperative days compared to the day of surgery (p=0.031, 0.039, and 0.003, respectively).
Discussion
Analgesic research for home pain management in children following tonsillectomy has endeavored to identify the type of analgesic and dosing frequency that is most effective. Acetaminophen and hydrocodone is a widely accepted standard treatment for moderate to severe acute postoperative pain in children, but it has not been well-studied as an analgesic for post-tonsillectomy pain relief. We hypothesized that pediatric patients who received regularly scheduled acetaminophen and hydrocodone elixir following tonsillectomy would report less pain than those who received the same opioid analgesic on a PRN basis.
Findings from this study suggest that ATC dosing of acetaminophen with hydrocodone was more effective than PRN dosing in reducing children's pain, with and without swallowing, in the first three days following tonsillectomy. For children who received scheduled dosing of acetaminophen with hydrocodone, on average across the 7 assessment times, the mean pain intensity scores with and without swallowing were 3 on a 0 to 10 NRS, compared to mean pain intensity scores of 4 for children who received as needed analgesic dosing. On average, the differences in mean pain intensity ratings between the PRN and ATC groups at rest and with swallowing were 0.93 and 1.01 (range between 0.36 to 1.86 and 0.44 to 1.69, respectively, across the 7 assessment times). The equivalent of a one-unit decrement on a NRS was reported as the threshold for a clinically meaningful change in pain intensity in children with acute pain.19 In an adult population with acute postoperative pain, an equivalent change of 1.3 units on a NRS was the threshold for minimal pain relief when the baseline pain intensity was moderate.20 A smaller absolute difference in mean pain intensity scores may be a more clinically meaningful difference for a child with mild to moderate pain compared to a child with severe pain. While pain intensity scores with and without swallowing were significantly lower in the ATC group compared to the PRN group, these differences are relatively small and of modest clinical significance. However, the finding that the biggest difference between the two groups (with and without swallowing) occurs on the second morning after surgery provides direction for the design of future studies and clinical recommendations. In addition, future studies need to determine a more optimal analgesic regimen for the management of postoperative pain in children following tonsillectomy.
For children in both treatment groups, pain intensity scores were significantly higher in the morning compared to evening ratings. Over the first 3 postoperative days, children in the scheduled dosing group received, on average, 9 night time doses of the analgesic, compared to only 4 night time doses in the PRN group. While interruption of analgesic dosing during the night may account for higher pain scores upon awakening in the PRN group, a similar pattern was noted for children in the ATC group. Other factors that may contribute to increased pain in the morning that gradually improves during the day include dryness of the mouth, muscle spasm, increased edema with supine positioning, and poor sleep quality. The potential factors that contribute to this pain pattern suggest an opportunity to provide other interventions (in addition to scheduled analgesic administration) that may further improve the child's pain experience at home following tonsillectomy (e.g., elevating the head of the bed, offering fluids during the night).
Ethnicity is an important consideration with respect to children's pain which can influence the child's pain expression, as well as the family caregiver's perception and awareness, and ultimately how they treat their child's pain.21 While self-report ratings of ethnicity were obtained for the child's mother and father, each parent's level of acculturation was not assessed in this study. While a larger proportion of African-American mothers were in the PRN group and mothers were the primary caregivers at home, no ethnic differences were found in the dose of analgesic administered in either the PRN or ATC groups for the first 3 days at home after tonsillectomy. This finding suggests that parent's ethnicity did not have a significant impact on analgesic administration.
With the exception of constipation, scheduled analgesic dosing did not increase the frequency or severity of opioid-related adverse effects. However, our study was not powered to detect small effect sizes which were present for most adverse effects at each of the measurement times. The trend towards increased daytime sedation and nausea for children in the ATC group on the third postoperative day suggests that scheduled analgesic dosing beyond the first 48 hours after surgery may contribute to an increased potential for these opioid-related adverse effects. Of note, 22% of the children in both treatment groups, reported moderate-to-severe nausea on the third postoperative day which is a common but unpleasant adverse effect associated with opioid administration.
This RCT is the second to evaluate the effectiveness of nurse coaching with scheduled analgesic dosing regimens for the management of post-tonsillectomy pain in children. As in our previous study,14 regardless of whether or not nurse coaching was provided, parents assigned to the ATC dosing groups demonstrated high levels of adherence with the administration schedule. In addition to a review of the medication log in the home diary, adherence with the analgesic regimen was verified through measurement of the volume of medication that remained in the bottle. The replication of the finding that parents do not require additional coaching has important clinical implications. Findings from these two studies suggest that written instructions on ATC dosing and the use of a timer are sufficient interventions to promote adherence to a prescribed scheduled analgesic dosing regimen that can easily be implemented in pediatric surgery centers throughout the country.
More than a decade of published data has documented the limitations of a PRN approach to analgesic dosing for children following tonsillectomy. Findings from this study demonstrate the efficacy and relatively low incidence of opioid-related side effects with the ATC approach to analgesic dosing in children at home in the early postoperative recovery following tonsillectomy, particularly for the first 48 hours after surgery. Given the predictable characteristics of postoperative pain following tonsillectomy (i.e., prolonged duration, relatively constant nature, moderate to severe intensity), scheduled dosing of analgesics should be considered during the early postoperative period, with transition to an “as needed” approach as the child's pain intensity and analgesic requirements decrease. Parents need to be educated about regular analgesic administration in order to improve patient outcomes. Although adherence with the scheduled dosing regimen was excellent for participants in our study, the high refusal rate, with the primary reason reported being parental desire to use PRN dosing, suggests that the practice of regularly scheduled analgesic administration is not considered standard of care by consumers. The frequent dosing that is required with currently available short-acting analgesics suitable for pediatric patients is a significant drawback particularly during the night. Appropriate education about the benefits of scheduled dosing needs to become the standard of care for the home management of postoperative pain in pediatric patients.
Several limitations of this study need to be acknowledged. The analgesic dose and dosing interval were not adjusted based on an assessment of the patient's response. The use of a commercially available combination of acetaminophen with an opioid limited our ability to provide additional rescue dose(s) of analgesic if needed, given that the patient was prescribed an optimal/ceiling dose of acetaminophen. However, only one child needed a change in analgesic medication. Pain intensity ratings were only obtained twice a day, in the morning upon awakening and in the evening before going to bed, rather than at regular intervals during the day or in conjunction with analgesic administration. Pain intensity ratings were for present pain intensity with and without swallowing, and did not evaluate ratings of worst pain during the day or night. The generalizability of the study findings is limited to postoperative pain management in school-age children for the first three days at home following tonsillectomy.
In summary, the findings from this study support the use of scheduled dosing of acetaminophen with hydrocodone during the early postoperative recovery at home in an outpatient pediatric population undergoing tonsillectomy, particularly for the first 48 hours. Fear of opioid-related side effects should not be used as a reason not to administer ATC therapeutic weight-based opioid dosing to children at home following tonsillectomy. Constipation should be anticipated with scheduled opioid dosing and prophylactic measures implemented. Further studies are warranted to determine whether the benefits of scheduled analgesic dosing can be achieved in other outpatient pediatric surgical populations.
Acknowledgments
This study was supported by a grant from the National Institute of Nursing Research (NR04826).
Funding Source: This study was supported by a grant from the National Institute of Nursing Research (NR04826).
References
- 1.Gedaly-Duff V, Ziebarth D. Mothers' management of adenoid-tonsillectomy pain in 4- to 8-year olds: A preliminary study. Pain. 1994;57:293–299. doi: 10.1016/0304-3959(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 2.Hamers JPH, Abu-Saad HH. Children's pain at home following (adeno) tonsillectomy. Eur J Pain. 2002;6:213–219. doi: 10.1053/eujp.2001.0326. [DOI] [PubMed] [Google Scholar]
- 3.Homer JJ, Swallow J, Semple P. Audit of pain management at home following tonsillectomy in children. J Laryngol Otol. 2001;115:205–208. doi: 10.1258/0022215011907208. [DOI] [PubMed] [Google Scholar]
- 4.Idvall E, Holm C, Runeson I. Pain experiences and nonpharmacological strategies for pain management after tonsillectomy: A qualitative interview study of children and parents. J Child Health Care. 2005;9:196–207. doi: 10.1177/1367493505054417. [DOI] [PubMed] [Google Scholar]
- 5.Jones DT, Yoon JM, Licameli G. Effectiveness of postoperative follow-up telephone interviews for patients who underwent adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133:1091–1095. doi: 10.1001/archotol.133.11.1091. [DOI] [PubMed] [Google Scholar]
- 6.Sutters KA, Miaskowski C. Inadequate pain management and associated morbidity in children at home after tonsillectomy. J Pediatr Nurs. 1997;12:178–185. doi: 10.1016/S0882-5963(97)80075-9. [DOI] [PubMed] [Google Scholar]
- 7.Warnock FF, Lander J. Pain progression, intensity and outcomes following tonsillectomy. Pain. 1998;75:37–45. doi: 10.1016/S0304-3959(97)00202-9. [DOI] [PubMed] [Google Scholar]
- 8.Wiggins SA, Foster RL. Pain after tonsillectomy and adenoidectomy: “Ouch it did hurt bad”. Pain Manag Nurs. 2007;8:156–165. doi: 10.1016/j.pmn.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Acute Pain Management Guideline Panel. Acute pain management: Operative or medical procedures and trauma Clinical Practice Guideline. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services; 1992. AHCPR Pub. No. 92-0032. [Google Scholar]
- 10.American Academy of Pediatrics Committee on Psychological Aspects of Child and Family Health and American Pain Society Task Force on Pain in Infants, Children, and Adolescents. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics. 2001;108:793–797. doi: 10.1542/peds.108.3.793. [DOI] [PubMed] [Google Scholar]
- 11.American Pain Society Principles of analgesic use in the treatment of acute pain and cancer pain. 5th. Glenview, Il: American Pain Society; 2003. [Google Scholar]
- 12.American Society of Anesthesiologists Task Force on Acute Pain Management: Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004;100:1573–1581. doi: 10.1097/00000542-200406000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Romsing J, Hertel S, Harder A, et al. Examination of acetaminophen for outpatient management of postoperative pain in children. Paediatr Anaesth. 1998;8:235–9. doi: 10.1046/j.1460-9592.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 14.Sutters KA, Miaskowski C, Holdridge-Zeuner D, et al. A randomized clinical trial of the effectiveness of scheduled oral analgesic dosing for the management of postoperative pain in children following tonsillectomy. Pain. 2004;110:49–55. doi: 10.1016/j.pain.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Bean-Lijewski JD, Kruitbosch SH, Hutchison L, et al. Post-tonsillectomy pain management in children: Can we do better? Otolaryngol Head Neck Surg. 2007;137:545–551. doi: 10.1016/j.otohns.2007.06.731. [DOI] [PubMed] [Google Scholar]
- 16.Williams DG, Patel A, Howard RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002;89:839–45. doi: 10.1093/bja/aef284. [DOI] [PubMed] [Google Scholar]
- 17.Fagerlund TH, Braaten O. No pain relief from codeine? An introduction to pharmacogenomics. Acta Anaesthesiol Scan. 2001;45:140–49. [PubMed] [Google Scholar]
- 18.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 19.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant differenc in visual analog pain score for children. Ann Emerg Med. 2001;37:28–31. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- 20.Cepeda MS, Africano JM, Polo R, et al. What decline in pain intensity is meaningful to patients with acute pain? In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain, Progress in Pain Research and Management. Vol. 24. Seattle, USA: IASP Press; pp. 601–609. [DOI] [PubMed] [Google Scholar]
- 21.Finley GA, Kristjansdottir O, Forgeron PA. Cultural influences on the assessment of children's pain. Pain Res Manage. 2009;14:33–37. doi: 10.1155/2009/763031. [DOI] [PMC free article] [PubMed] [Google Scholar]