Abstract
Purpose
To document the progression of disease in male and female members of a previously described family with X-linked dominant retinitis pigmentosa (RP) caused by a de novo insertion after nucleotide 173 in exon ORF15 of RPGR.
Methods
The clinical records of 19 members of family UTAD054 were reviewed. Their evaluations consisted of confirmation of family history, standardised electroretinograms (ERGs), Goldmann visual fields, and periodic ophthalmological examinations over a 23-year period.
Results
Male members of family UTAD054 had non-recordable to barely recordable ERGs from early childhood. The males showed contracted central fields and developed more severe retinopathy than the females. The female members showed a disease onset delayed to teenage years, recordable but diminishing photopic and scotopic ERG amplitudes in a cone-rod pattern, progressive loss and often asymmetric visual fields, and diffuse atrophic retinopathy with fewer pigment deposits compared with males.
Conclusions
This insertion mutation in the RPGR exon ORF15 is associated with a RP phenotype that severely affects males early and females by 30 years of age, and is highly penetrant in female members. Families with dominant-acting RPGR mutations may be mistaken to have an autosomal mode of inheritance resulting in an incorrect prediction of recurrence risk and prognosis. Broader recognition of X-linked RP forms with dominant inheritance is necessary to facilitate appropriate counselling of these patients.
Keywords: X-linked dominant retinitis pigmentosa, RPGR, ORF15
Introduction
Retinitis pigmentosa (RP) is a heterogeneous group of inherited disorders that typically leads to night blindness, progressive loss of peripheral vision, retinal and electrophysiological abnormalities, and legal blindness.1 Hereditary transmission patterns for RP include autosomal recessive, autosomal dominant, and X-linked.2 X-linked forms of RP (XLRP) account for only about 10–15% of RP cases, but result in some of the most severe phenotypes. Clinical symptoms typically manifest from childhood to late teens, leading to significant vision loss by the fourth decade of life. Mutations in the RP GTPase regulator (RPGR) and RP2 genes account for almost 90% of XLRP.3–5
In 1981, Heckenlively et al6 presented the initial clinical description of a family believed to have an autosomal dominant form of RP. By 1995, enough family members had been examined to make it apparent that, although males were much more severely affected than female members, females in the line of descent who carried the mutation always had the disease. Linkage analysis was performed, revealing that the mutation resided on the X-chromosome.7 This was the first report of an X-linked dominant form of RP. A more extensive genetic analysis of this family enabled the mutation to be identified as a de novo insertion A in the ORF15 exon of the RPGR gene.8
There have been few reports of a similar dominant pattern of X-linked inheritance in literature, and mutations in both RP2 and the RPGR gene have been reported.9–12 However, clinical description of individuals with RP and an X-linked dominant mode of inheritance remains sparse. Furthermore, a clear history of progression in females has not been documented. In this study, we present a detailed clinical description of family UTAD054. We considered two issues: first, whether the affected females of this family have stationary disease or whether they have RP, a clearly progressive disorder; second, whether there are female ‘carriers’ who are unaffected. Members of family UTAD054, who carry the mutation in exon ORF15 of RPGR with a follow-up of 10 years or more, were found to be affected with RP regardless of their gender. The phenotypes of males and females were compared and progression of the disease in family members was documented over time.
Materials and methods
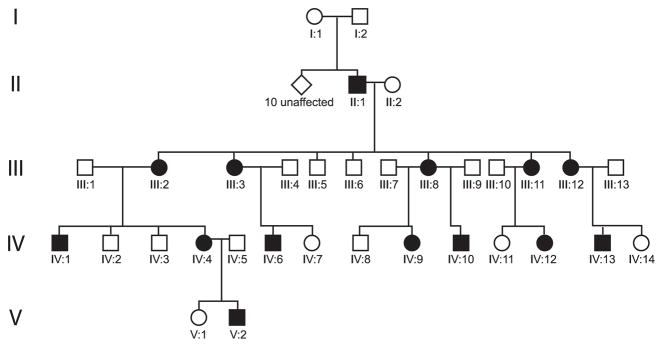
Participation in the molecular genetics analysis and initial clinical assessment was with informed consent of adults or with consent of parents of minors and with previous approval obtained from the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston. Approval for the review of follow-up data was obtained from the Medical School Institutional Review Board at the University of Michigan. The initial genetic analysis and clinical assessment of the mutation in family UTAD054 has already been described in the literature.6–8 Figure 1 shows the pedigree describing family UTAD054, with affected members coloured in black. Individuals I : 1, I : 2, and 10 of their children were unaffected with RP. Only one of their children (II : 1) developed RP linked to the de novo mutation, which was subsequently passed through generations III–V.
Figure 1.
Pedigree of the UTAD054 family. Filled symbols represent affected family members, all of whom are represented by blackened symbols and carry the mutation. Individual I : 1 carries the haplotype, but was negative for the mutation. She and I : 2 had 10 unaffected children and one affected child with a de novo mutation (individual II : 1).
For this follow-up, we reviewed original and updated medical records of 19 members of family UTAD054, including all 14 members who were known to carry the mutation. Comprehensive ophthalmic examinations were performed, including slit-lamp and external examination, fundus photography, Goldmann visual fields, measurement of final rod thresholds after dark adaptation, and standardised full-field electroretinography (ERG) as described earlier.6,13 Repeat examinations in medical records of family members span 23 years.
Results
The 14 individuals from family UTAD054 (six male and eight female), who had been found previously to carry the mutation in RPGR, were included in this study. Clinical characteristics are summarised in Table 1. Ages at first examination ranged from 7 months to 53 years for males and 9 to 44 years for females. Eight individuals (four male and four female) had follow-up information available. Maximum follow-up intervals were 15 years in males and 23 years in females.
Table 1.
Summary of male and female patients
| Patient | Age at exam | Visual acuity (OD–OS) | Fundus findings | Dark adaptation rod threshold | ERG Amplitude/Implicit Time (OD–OS) |
|---|---|---|---|---|---|
| (A) Summary of male patients | |||||
| II : 1 | 53 | 10/200–10/200 | Preserved fovea, diffuse RPE atrophy, bone spicule pigmentation, disc pallor, peripapillary atrophy, pallor and tilted disc | 4.3 OU log unit elevation | Non-recordable |
| 56 | 10/160–10/160 | NA | 4.35 log unit elevation | NA | |
| IV: 1 | 7 | 20/60–20/50 | Preserved fovea, diffuse RPE loss in equatorial region, vessel attenuation, pallor and TA | 3.05–3.35 log unit elevation (OD–OS) | Non-recordable |
| 22 | 20/50–20/50 | Bone spicule pigmentation in addition to the above | 3–3.35 log unit elevation (OD–OS) | Still non-recordable | |
| IV: 13 | 7 months | NA | Dull macular reflex, no pigment changes, vessel attenuation, slight disc pallor | NA | Non-recordable |
| 6 | 20/30–20/30 | Still no bone spicule pigmentation, mild nerve fibre layer attenuation | NA | Not repeated | |
| IV: 10 | 10 | 20/50–20/40 | Diffuse RPE atrophy, bone spicule as well as round subretinal pigment deposits, disc pallor and TA | Unreliable | Non-recordable |
| V: 2 | 9 | 20/60–20/60 | Very dull foveal reflex, diffuse RPE atrophy, TA | NA | Flicker barely detectable OU. Scotopic non-detectable. |
| IV: 6 | 11 | 20/40–20/50 | Preserved fovea, scattered clumps of round pigment and lacunar atrophy with hyperpigmented edges | 1.5–0.5 log unit elevation (OD–OS) | Non-recordable |
| 15 | 20/40–20/50 | Absent foveal reflex, macula involved, fine bone spicule pigmentation, heavy RPE granularity, vessel attenuation, ONH gliosis | Not repeated | Not repeated | |
| (B) Summary of female patients | |||||
| III : 2 | 33 | 20/30–20/30 | Preserved fovea, occasional pigment clumping, ONH drusen and temporal atrophy | Normal OU | P 50 μV/36 ms–20 μV/36 ms S 100 μV/60 ms–70 μV/60 ms M 150 μV/48 ms–100 μV/48 ms F barely recordable OU |
| 48 | 20/32–20/40 | Generalised RPE atrophy with very mild bone spicules in periphery in addition to the above | Normal OU | P NR S 135 μV/92 ms–132 μV/84 ms M 109 μV/21 ms–89 μV/24 ms F NR–NR |
|
| III : 11 | 13 | 20/30–20/30 | Fovea intact, few scattered pigment clumps and bone spicules in periphery, ONH drusen, tilted disc, peripapillary atrophy | Normal OU 1.3 OU at age 15 | P NR–NR S NR–NR M 30 μV/48 ms–NR F NR–NR |
| 30 | 20/30–20/70 | Foveal atrophy, attenuation of nasal arcades, ONH drusen, tilted disc | 3.25 log unit elevation | Virtually non-recordable by age 16 | |
| III : 12 | 10 | 20/25–20/30 | Intact fovea, no pigment deposits, blond fundus, peripapillary nerve fibre layer dropout | Normal OU | P 30 μV/40 ms–NR S 50 μV/80 ms–NR M 90 μV/60 ms–50 μV/60 ms F Barely recordable |
| 33 | 20/40–20/40 | Fovea involved, bone spicule pigmentation, diffuse RPE atrophy, TA, and vessel attenuation | 3.00 log unit elevation OU (age 28) | Non-recordable | |
| III : 3 | 44 | 20/25–20/30 | Dull foveal reflex with dark choroidal pattern and tigroid appearance, thinning of RPE in parafoveal region, fine bone spicule pigment clumps, ONH gliosis, vessel attenuation, peripapillary atrophy | 1.8–2 log unit elevation (OD–OS) | P 43 μV/40 ms–15 μV/38 ms S 100 μV/66 ms–60 μV/90 ms M NA F 18 μV–8 μV |
| III : 8 | 35 | 20/50–20/40 | Pigment clumps, bone spicules, lacunar atrophy, paving stone changes in periphery | Normal OU (1 log unit elevation) | P 29 μV/52 ms–49 μV/32 ms S 58 μV/77 ms–140 μV/70 ms M 124 μV/52 ms–226 μV/53 ms F 45.2 μV–36.8 μV |
| IV: 12 | 9 | 20/20–20/20 | Fovea and fundus intact | NA | P 110 μV/30 ms–106 μV/30 ms S 262 μV/70 ms–239 μV/74 M 319 μV/54 ms–301 μV/54 ms F 100 μV/29 ms–93 μV/30 ms |
| IV: 9 | 14 | 20/40–20/40 | Fovea intact, occasional bone spicules in periphery, round pigment spots, white granular deposits in anterior retina and adjacent areas, no disc anomaly | Unreliable | P 58 μV/38 ms–53 μV/37 ms S 62 μV/90 ms–171 μV/106 ms M 175 μV/56 ms–156 μV/58 ms F 40.8 μV–40.8 μV |
| IV: 4 | 10 | 20/40–20/40 | Parafoveal orange colour, mild vessel attenuation, TA, nerve fibre layer dropout | Normal OU | NA |
| 31 | 20/30–20/30 | Same as above | NA | P 62 μV/41 ms–60 μV/41 ms S 185 μV/91 ms–186 μV/93 ms M 214 μV/61 ms–215 μV/61 ms F 61 μV/35 ms–61 μV/36 ms |
|
F, flicker; M, dark-adapted bright/flash; NA, not available; NR, non-recordable; ONH, optic nerve head drusen; P, photopic; RPE, retinal pigment epithelium; S, scotopic; TA, temporal atrophy.
Normal ERG values: P 151 (±41) μV/32 (±5) ms|S 337 (±100) μV/66 (±14) ms|M 471 (±160) μV/45 (±13) ms | F>55 μV.
Visual acuity
In young males, visual acuities at initial presentation ranged from 20/40 to 20/60 and remained above 20/60 in the first two decades of life. The only visual acuity data available for males later in life were from the proband, who was in his fifties and legally blind (10/160). For females, best-corrected visual acuity ranged from 20/20 to 20/40 between ages 10 and 20. Visual acuities remained largely stable beyond the age of 20 years, except in III : 11, who had preserved right eye visual acuity but an asymmetric decrease in her left eye visual acuity to 20/70.
Refractive error
Myopia has been noted to be a feature of XLRP,1,14 and was a characteristic of the majority of affected family members. Excluding the legally blind proband (II : 1), three of the five remaining males had moderate-to-high myopia at their initial visits. Only one male (IV: 13) was found to have no myopia on his follow-up examination at 6 years of age. All five females for whom refraction data were available were found to have mild-to-high myopia.
Funduscopic appearance
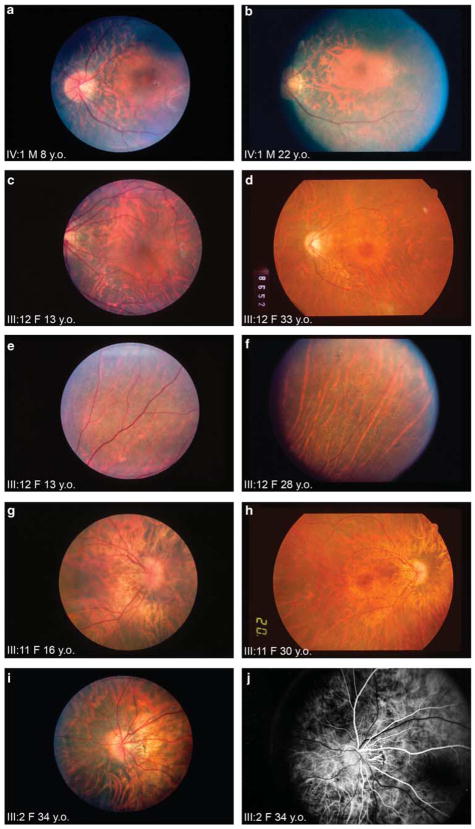
All males except for the 7-month-old infant had atrophy of the retinal pigment epithelium. Bone spicule pigmentation was a relatively uncommon finding on initial examination, as it was seen only in two males, aged 10 and 53 years. Two additional males developed bone spicule pigmentation on follow-up at ages 15 and 22 years. Foveal preservation was the rule rather than the exception (Figure 2a–b), with only two young patients (7 months and 9 years) having examinations suggesting foveal involvement. On follow-up examination, one patient who had a healthy-appearing fovea at age 11 developed foveal atrophy by age 15. Vascular attenuation was noted as early as 7 months and was a finding in half of the males carrying the mutation. Optic disc pallor was noted in all male patients and temporal atrophy of the disc was noted in half.
Figure 2.
(a and b) Fundus photographs, left eye, male patient IV: 1 at 8 and 22 years of age, respectively. There is preservation of the fovea at both time points, otherwise diffuse hypopigmentation is seen throughout the retina. Vascular attenuation and temporal atrophy of the optic disc are present. This patient did not initially have bone spicule-like pigmentation at the age of 8 years, but developed them in the peripheral retina by the age of 22 years (not illustrated). Figures 2c and d, fundus photographs left eye, female patient III : 12 at 13 and 33 years of age, respectively. At the age of 13 years, her fovea appears intact, optic disc is pink, although some nerve fibre layer dropout is seen. At the age of 33 years, she has foveal bulls-eye atrophy and regional attenuation of retinal and choroidal vessels. Figures 2e and f, fundus photographs right eye, female patient III : 12 at 13 and 28 years of age, respectively. Each photograph shows the same supranasal peripheral field of the retina. There is significant retinal vascular attenuation between the two time-points. In addition, at 28 years of age (Figure 2e), patient III : 12 developed generalised atrophy and pigment deposits not seen earlier (Figure 2f). Figures 2g and h, fundus photographs right eye, female patient III : 11 at 16 and 30 years of age, respectively. At the age of 16 years (Figure 2g 30° view), there is a foveal reflex and her optic nerve is pink, whereas there is obvious attenuation of the nasal vascular arcades. At the age of 30 years (Figure 2h, 50° view), foveal atrophy, optic disc pallor, and additional attenuation of retinal and choroidal vessels are evident. Figures 2i and j, fundus photograph and fluorescein angiogram left eye, female patient III : 2 at 34 years of age. Optic disc pallor, peripapillary nerve head drusen, and temporal atrophy are noted on both. Fluorescein angiography shows that papillary vessels extend across the empty space in the temporal region, reinforcing the observation that temporal atrophy, rather than a congenital tilted disc, is present.
All eight females with the mutation, except a 9-year-old patient, had abnormalities of the retinal pigment epithelium, ranging from pigment clumping to diffuse retina pigment epithelial atrophy. Bone spicule-like pigmentation, although not typically present in teenage years, was found in half of the females decades later on reexamination (Figures 2e and f). Two females who did not have foveal atrophy at their initial examinations at ages 10 and 13 years developed atrophy by ages 33 and 30, respectively (Figures 2c and d, and 2g and h). An additional female patient whose initial examination was at age 44 years was also found to have foveal atrophy. Two patients showed optic nerve head drusen and atrophy and three patients had nerve fibre layer atrophy, one of whom also showed signs of optic nerve gliosis (Figures 2i and j).
Dark adaptation rod thresholds
Dark adaptation rod thresholds were available from three males at their initial visit (Table 1a) and were elevated even at young ages. Both males with follow-up examinations continued to have elevated rod thresholds (Table 1a). In contrast, five of the six female patients (age range 10–35 years), who had reliable dark adaptation testing during their initial visits, seemed to have normal dark adaptation rod thresholds of one log unit or less (Table 1b). The only exception was III : 3, who at age 44 had an elevation of 1.8 log units OD and two log units OS. On follow-up, two female patients who had only 0.6 log unit elevations at ages 10 and 13 years had log unit elevations of 3 and 3.25 at ages 33 and 30, respectively. All females in this family showed a relative preservation of rod thresholds until they reached middle age, suggestive of a cone-rod pattern of degeneration.
Electroretinograms
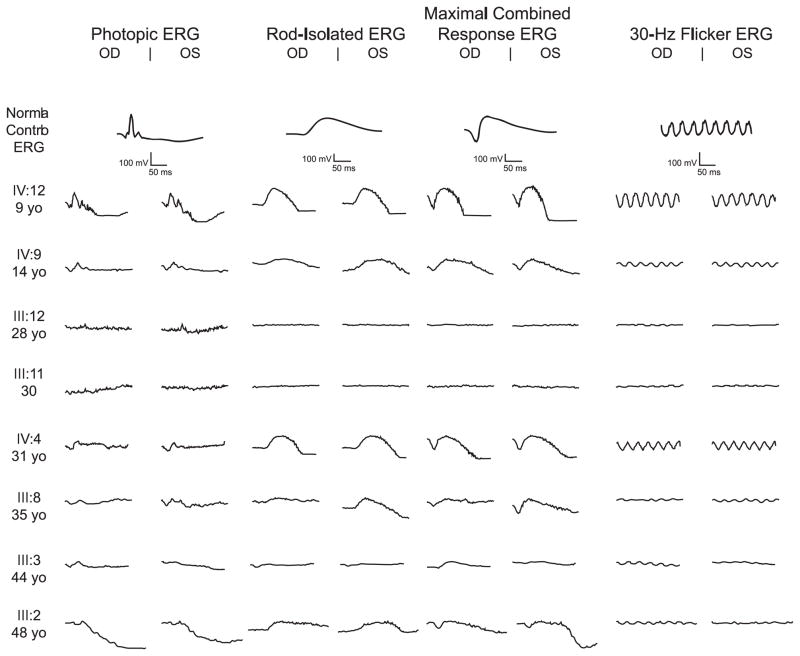
Electroretinograms (ERGs) were performed on 13 of the 14 family members. One 9-year-old boy (V: 2) had barely detectable flicker ERGs and an undetectable scotopic ERG. The remaining five males had ERGs that were already non-recordable at the first visit, with the earliest performed at age 7 months. In contrast, most females had recordable ERGs. ERGs of the eight females with the mutation, as well as that of a normal control, are shown in Figure 3, with amplitudes given in Table 1b. ERG amplitudes were significantly reduced from normal in all patients. Two of three females in whom follow-up ERGs were attempted (III : 2, III : 11, and III : 12) had non-recordable ERGs at ages 16 and 33; female III : 2 at the age of 48 years had non-recordable photopic ERGs in the presence of residual scotopic ERGs, again suggestive of a cone-rod pattern.
Figure 3.
Photopic and scotopic electroretinogram (ERG) amplitudes for affected females. A control ERG is placed first for comparison. ERGs from females are less severely affected than those from males, although amplitudes are generally diminished and ERGs on three females became unrecordable.
Goldmann visual field testing
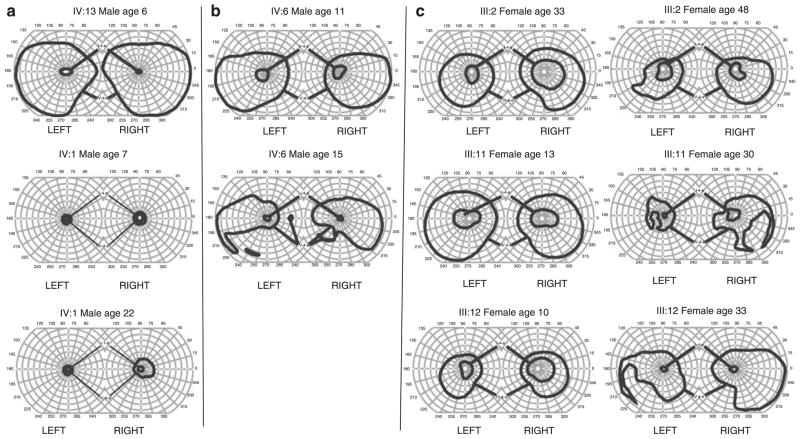
Visual fields were available for 13 of the 14 affected family members. Individual IV: 1 (male), at age 7 years, already had severe bilateral constriction of the IV-4-e isopter field to a maximum diameter of approximately 20 (Figure 4a). However, most males had relatively full visual fields outlined by the IV-4-e isopter at early ages; IV: 13, IV: 10, and IV: 6 showed preservation of the IV-4-e isopter fields bilaterally at ages 6, 10, and 11 years, respectively. Regardless of the extent of the IV-4-e or V-4-e isopter field in a particular family member, I-4-e and II-4-e visual fields were severely restricted to a maximum diameter of 10–20° in all five males (age range 7–53 years) in whom visual fields were performed. Furthermore, with advancing age, IV-4-e/V-4-e isopter fields decreased in area. Male IV: 6 showed further temporal and inferior constriction to his sensitivity for the V-4-e isopter when it was repeated at the age of 15 years (Figure 4b), and male II : 1 at the age of 53 years showed minimal detection of a target centrally (data not shown). Nevertheless, even with the progressive decrease in isopter area, preservation of the central visual field was noted in males as far as age 53 years (II : 1).
Figure 4.
(a) Top panel shows the visual field OD from IV:13 at 6 years of age. The visual field for IV-4-e is full. In contrast, the middle panel shows the visual field OD from IV: 1 at 7 years of age. Visual fields for both IV-4-e and I-4-e stimuli are severely restricted. Although virtually all males show a severely constricted I-e visual field between 10° and 20° in maximum diameter, there is variability in the initial visual field deficit for the IV-4-e isopter. The bottom panel shows visual fields of patient IV: 1 at the age of 22 years. Despite considerable constriction of visual fields in this patient from a very young age, his central vision remained preserved into early adulthood. (b) The top panel shows visual fields for the V-4-e and II-4-e isopters from family member IV: 6 at 11 years of age. There is superior restriction of fields bilaterally. At 11 years of age, the patient was unable to visualise the I-4-e isopter, hence the II-4-e isopter is shown in the top panel. Even the II-4-e stimulus visual field was significantly contracted to 20° full diameter. The bottom panels show visual fields from the same individual at 15 years of age when the patient could visualise the I-4-e isopter, but as with other males, that field was severely constricted. In addition, a significant loss to the inferior visual field (V-4-e) had occurred bilaterally. (c) Each panel shows the visual field IV-4-e and I-4-e from a female family member at initial evaluation and at follow-up. I-4-e fields are markedly reduced, although they are still larger than those in affected males. IV-4-e fields are constricted to a lesser degree but morphologically normal. There are varying degrees of regional scotomata in the IV-4-e isopter field on follow-up. I-4-e isopter fields are globally constricted.
Although females seemed to have a greater preservation of the visual field area than males, their fields often showed regional scotomata. Initial maximal diameters of I-4-e visual fields in females were generally larger (50–110°) than in males (10–20°), but did restrict with time as they progressed from their teens to their thirties and forties. Figure 4c shows the visual fields from three female family members in whom an initial and follow-up set of visual fields were performed. In each case, there was progressive regional loss of portions of the visual field corresponding to the IV-4-e isopter, as well as a more global constriction of the visual field corresponding to the I-4-e isopter.
Discussion
There have been intermittent reports of X-linked females expressing features of dominant RP. It is well known that females in families with X-linked recessive RP occasionally express partial, often asymmetric ‘carrier’ phenotypes.15–20 The heterogeneity seen in affected carriers in XLRP has been ascribed to lyonisation, the phenomenon in which female carriers randomly inactivate one of their two X-chromosomes, resulting in mosaicism of cells expressing either the normal or mutated X-chromosome.21,22 Radial fundus autofluorescence patterns and a patchy distribution of rod and cone sensitivities observed in the retinas of heterozygotes have been put forth as evidence of the heterogeneous composition of the retinas of female carriers.23,24 Lyonisation mosaicism could explain the occasional severely affected female in families with X-linked recessive RP, but cannot account for the complete and severe penetrance in females from families with X-linked dominant RP, such as UTAD054.
The disease process affecting females in family UTAD054 is fundamentally different than that observed in ‘carrier’ females from families with X-linked recessive RP, in whom disease phenotypes are (usually) more mild and relatively stationary.20 As one would expect with an X-linked dominant disorder, females in this family experienced delayed onset of deterioration but ultimately manifested the full phenotype as measured by clinical examination and testing of visual fields, dark adaptation thresholds, and ERGs. Our report on family UTAD054 represents the only detailed clinical description of a family with an RPGR X-linked dominant RP with an extended period of observation (as far as 23 years). The few other reports available in literature contain only brief clinical descriptions and little or no follow-up data, but their presence suggests that family UTAD054 is not alone. Rozet et al12 identified 14 families in which carrier females also had findings consistent with RP, sometimes equally affected as their male counterparts. All 14 families had linkage analysis data supporting localisation to the RP3 region, with 9 of the 14 families (and all five of the families whose female members were affected as equally as males) having mutations identified in exon ORF15 of RPGR. Banin et al10 reported on one family with a missense mutation in exon 8 of the RPGR gene in whom carrier females, as well as males, also consistently showed loss of visual acuity, visual field, and decreased ERG ampltudes. In their study on RPGR and RP2 gene mutations, Neidhardt et al11 identified three families with affected females and a presumed autosomal dominant pattern that were subsequently found to have mutations in ORF15. Most recently, Al-Maskari et al9 reported on two English families with a missense mutation in exon 5 of the RPGR gene, in which both females and males showed severe deterioration in visual acuity, visual field, and high myopia with age.
To date, the only similar report of dominant XLRP not involving an RPGR mutation has come from Pomares et al25, who characterised a family with an intronic point mutation in RP2 that results in aberrant splicing and a truncated protein. All males had uniformly high aberrant RP2 transcripts and a clear RP phenotype, whereas three of the four carrier females had variably high levels of aberrant transcripts and two of the carrier females had mild-to-moderate progressive deterioration in fundus appearance, visual field, and ERG. Pomares et al categorised this family as having a ‘semi-dominant’ phenotype and suggested that skewed X-inactivation could be the cause. As this is the first report of such an inheritance pattern associated with RP2, the hypothesis of skewed X-inactivation needs to be tested in other semi-dominant families with RP2 mutations, as they are discovered. Pelletier et al5 have already studied the X-inactivation pattern in 55 women from 28 RPGR mutation families in which several carrier females complained of severe visual impairment. Of those tested, 54 out of 55 had a random inactivation of the X-chromosome and only one of the 55 had skewed X-inactivation. Furthermore, when the other four carriers in her family were tested, they were found to have random patterns of X-inactivation. Thus, skewed inactivation may represent a rare event in these families and is not likely to represent the primary reason behind severe phenotypes in females with RPGR mutations.
The fact that nearly all reports of a dominant pattern of inheritance have been associated with mutations in the RPGR gene argues that some specific aspect of RPGR mutations may lead to higher penetrance in females. In general, such a phenotypic difference can be attributed to at least two causes. First, the penetrance and expressivity of the phenotype may be modified by the presence of a modifier allele in a completely different gene that is inherited. Interactions between the mutation in RPGR and additional modifiers are likely to be important determinants of the phenotype. We have earlier shown that RPGR exists in multiprotein complexes in photoreceptors26 and hypothesise that a partial loss of function mutation in the protein partners can affect the penetrance and expressivity of the disease.27 Recently, we identified a hypomorphic mutation in the ciliary protein RPGR-interacting protein 1-like (RPGRIP1 L/NPHP8), which significantly affects its interaction with RPGR and is associated with the penetrance of the retinal degeneration phenotype in patients with syndromic ciliopathies.28 We hypothesise that single allelic mutations may affect this mechanism and could occur on a dominant basis in females. Second, the effect of the specific RPGR–ORF15 mutation per se is such that the mutant protein behaves in a dominant gain-of-function manner and results in a more severe phenotype in both males and females. Support for this hypothesis comes from an earlier demonstration by Hong et al29 that a naturally occurring RPGR variant with an in-frame deletion in exon ORF15 in transgenic mice is associated with more severe progression of the disease in the RPGR knockout as well as wild-type background. Moreover, two canine models of XLRP carrying mutations in RPGR–ORF15 have also been reported to show discordant phenotypes, with one being more severely affected than the other.30 With a predicted dominant, gain-of-function mutation, the random inactivation of some abnormal X-chromosomes in carrier females may provide some initial protection compared with males who possess only the mutant version. However, this may not be sufficient to ameliorate the disease phenotype over time. An additional analysis of animal models will be required to completely understand the phenotypic heterogeneity.
The high number of female members of family UTAD054 with severe disease is clearly different than the more sporadic nature of affected carriers with X-linked recessive RP, but how does it compare with other diseases that are classified as being inherited in an X-linked dominant manner? A recent review of X-linked disorders discussed the utility of considering penetrance and severity in the discussion of such diseases.31 Of the 32 X-linked disorders reviewed in that study, there was a bimodal distribution of penetrance in carrier females, with most disorders having either 0–10% or 90–100% penetrance of the mutation. Interestingly, the severity index (which the authors defined as the proportion of all symptomatic individuals with a severe phenotype) was above 70% in only three of these disorders, all of which were classically considered X-linked dominant. Family UTAD054 then resembles other X-linked dominant disorders because there is complete penetrance of a severe RP phenotype in 100% of adult female mutation carriers. There are several reports of XLRP families with mutations in RPGR in which penetrance is high in heterozygote females, but with a large variance in severity.24,32–35 Part of the difficulty in extrapolating penetrance and severity from other reports in the literature is because of limitations in the number of family members available for examination and subsequent follow-up. The large size of family UTAD054 and the extended period of time in which we could follow its members enabled us to document the progressive nature of their disease, in contrast to the more stable carrier phenotype associated with the X-linked recessive disease. It would be interesting to see how many female heterozygotes in other studies progressed in severity over time as the affected female members of family UTAD054 did.
In summary, we provide a detailed clinical description of a large XLRP family with an RPGR–ORF15 mutation in which both female and male members of this family develop severe forms of the disease that progressively worsened with time. The identification and study of additional families with X-linked dominant inheritance is important for several reasons. Over the long term, clarifying the mechanism(s) that determine penetrance and severity in female members of families with XLRP will allow a better understanding of the pathogenesis of XLRP. Of more immediate concern, families with dominant-acting RPGR mutations may be mistaken as having an autosomal mode of inheritance resulting in an incorrect prediction of recurrence risk and errors in prognosis. As RPGR mutations are believed to account for over 70% of families with XLRP, our report is relevant to many patients.3,4,36 A broader recognition that XLRP has both a dominant and recessive inheritance mechanism is not merely a matter of semantics, but has important implications for genetic counselling in such families.
Summary.
What was known before
X-linked retinitis pigmentosa has typically been associated with a severe phenotype in males and a variable, often more mild phenotype in females. The wide range of severity among carrier females was felt to be related to random inactivation of the normal X-chromosome.
What this study adds
Some forms of X-linked retinitis pigmentosa can be associated with a highly penetrant phenotype which can inevitably affect all carrier females in a family. These appear to be associated with mutations particularly in RPGR. Without careful attention, they may be mistaken as autosomal dominant retinitis pigmentosa.
Acknowledgments
This study was funded by the Foundation for Fighting Blindness; NIH EY007961. We thank James Friedman, Naheed Khan and Randall Wallach for their helpful discussion in the preparation of this article.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Heckenlively JR. Retinitis Pigmentosa. Lippincott: Philadelphia; 1988. [Google Scholar]
- 2.Wang DY, Chan WM, Tam PO, Chiang SW, Lam DS, Chong KK, et al. Genetic markers for retinitis pigmentosa. Hong Kong Med J. 2005;11(4):281–288. [PubMed] [Google Scholar]
- 3.Breuer DK, Yashar BM, Filippova E, Hiriyanna S, Lyons RH, Mears AJ, et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet. 2002;70(6):1545–1554. doi: 10.1086/340848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharon D, Sandberg MA, Rabe VW, Stillberger M, Dryja TP, Berson EL. RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am J Hum Genet. 2003;73(5):1131–1146. doi: 10.1086/379379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelletier V, Jambou M, Delphin N, Zinovieva E, Stum M, Gigarel N, et al. Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: genotype-phenotype correlations and impact on genetic counseling. Hum Mutat. 2007;28(1):81–91. doi: 10.1002/humu.20417. [DOI] [PubMed] [Google Scholar]
- 6.Heckenlively JR, Rosales T, Martin D. Optic nerve changes in dominant cone-rod dystrophy. Doc Ophthal Proc Series. 1981;27:183–192. [Google Scholar]
- 7.McGuire RE, Sullivan LS, Blanton SH, Church MW, Heckenlively JR, Daiger SP. X-linked dominant cone-rod degeneration: linkage mapping of a new locus for retinitis pigmentosa (RP 15) to Xp22.13-p22.11. Am J Hum Genet. 1995;57(1):87–94. [PMC free article] [PubMed] [Google Scholar]
- 8.Mears AJ, Hiriyanna S, Vervoort R, Yashar B, Gieser L, Fahrner S, et al. Remapping of the RP15 locus for X-linked cone-rod degeneration to Xp11.4-p21.1, and identification of a de novo insertion in the RPGR exon ORF15. Am J Hum Genet. 2000;67(4):1000–1003. doi: 10.1086/303091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Maskari A, O’Grady A, Pal B, McKibbin M. Phenotypic progression in X-linked retinitis pigmentosa secondary to a novel mutation in the RPGR gene. Eye. 2009;23(3):519–521. doi: 10.1038/eye.2008.427. [DOI] [PubMed] [Google Scholar]
- 10.Banin E, Mizrahi-Meissonnier L, Neis R, Silverstein S, Magyar I, Abeliovich D, et al. A non-ancestral RPGR missense mutation in families with either recessive or semi-dominant X-linked retinitis pigmentosa. Am J Med Genet A. 2007;143A(11):1150–1158. doi: 10.1002/ajmg.a.31642. [DOI] [PubMed] [Google Scholar]
- 11.Neidhardt J, Glaus E, Lorenz B, Netzer C, Li Y, Schambeck M, et al. Identification of novel mutations in X-linked retinitis pigmentosa families and implications for diagnostic testing. Mol Vis. 2008;14:1081–1093. [PMC free article] [PubMed] [Google Scholar]
- 12.Rozet JM, Perrault I, Gigarel N, Souied E, Ghazi I, Gerber S, et al. Dominant X linked retinitis pigmentosa is frequently accounted for by truncating mutations in exon ORF15 of the RPGR gene. J Med Genet. 2002;39(4):284–285. doi: 10.1136/jmg.39.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmor M, Aguirre G, Arden G, Berson E, Birch D, Boughman J, et al. Retinitis pigmentosa: a symposium on terminology and methods of examination. Ophthalmology. 1983;90:126–131. [PubMed] [Google Scholar]
- 14.Fishman GA, Weinberg AB, McMahon TT. X-linked recessive retinitis pigmentosa. Clinical characteristics of carriers. Arch Ophthalmol. 1986;104(9):1329–1335. doi: 10.1001/archopht.1986.01050210083030. [DOI] [PubMed] [Google Scholar]
- 15.Bird AC. X-linked retinitis pigmentosa. Br J Ophthalmol. 1975;59(4):177–199. doi: 10.1136/bjo.59.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoare GW. Choroido-retinal dystrophy. Br J Ophthalmol. 1965;49(9):449–459. doi: 10.1136/bjo.49.9.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falls HF, Cotterman CW. Choroidoretinal degeneration: a sex-linked form in which heterozygous women exhibit a tapetal-like retinal reflex. Arch Ophthalmol. 1948;40:685–703. [Google Scholar]
- 18.Jacobson SG, Yagasaki K, Feuer WJ, Roman AJ. Interocular asymmetry of visual function in heterozygotes of X-linked retinitis pigmentosa. Exp Eye Res. 1989;48(5):679–691. doi: 10.1016/0014-4835(89)90009-2. [DOI] [PubMed] [Google Scholar]
- 19.Berson EL, Rosen JB, Simonoff EA. Electroretinographic testing as an aid in detection of carriers of X-chromosome-linked retinitis pigmentosa. Am J Ophthalmol. 1979;87(4):460–468. doi: 10.1016/0002-9394(79)90231-9. [DOI] [PubMed] [Google Scholar]
- 20.Grover S, Fishman GA, Anderson RJ, Lindeman M. A longitudinal study of visual function in carriers of X-linked recessive retinitis pigmentosa. Ophthalmology. 2000;107(2):386–396. doi: 10.1016/s0161-6420(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 21.Lyon MF. Gene action in the X-chromosome of the mouse (Mus. musculus. L) Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 22.Jay B. X-linked retinal disorders and the Lyon hypothesis. Trans Ophthalmol Soc UK. 1985;104(Pt 8):836–844. [PubMed] [Google Scholar]
- 23.Wegscheider E, Preising MN, Lorenz B. Fundus autofluorescence in carriers of X-linked recessive retinitis pigmentosa associated with mutations in RPGR, and correlation with electrophysiological and psychophysical data. Graefes Arch Clin Exp Ophthalmol. 2004;242(6):501–511. doi: 10.1007/s00417-004-0891-1. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson SG, Buraczynska M, Milam AH, Chen C, Jarvalainen M, Fujita R, et al. Disease expression in X-linked retinitis pigmentosa caused by a putative null mutation in the RPGR gene. Invest Ophthalmol Vis Sci. 1997;38(10):1983–1997. [PubMed] [Google Scholar]
- 25.Pomares E, Riera M, Castro-Navarro J, Andres-Gutierrez A, Gonzalez-Duarte R, Marfany G. An intronic single point mutation in RP2 causes semi-dominant X-linked Retinitis Pigmentosa. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-3208. [DOI] [PubMed] [Google Scholar]
- 26.Khanna H, Hurd TW, Lillo C, Shu X, Parapuram SK, He S, et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem. 2005;280(39):33580–33587. doi: 10.1074/jbc.M505827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40(4):443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 28.Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, et al. A common allele in RPGRIP1 L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009 May 10; doi: 10.1038/ng.366. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong DH, Pawlyk BS, Adamian M, Li T. Dominant, gain-of-function mutant produced by truncation of RPGR. Invest Ophthalmol Vis Sci. 2004;45(1):36–41. doi: 10.1167/iovs.03-0787. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Acland GM, Wu WX, Johnson JL, Pearce-Kelling S, Tulloch B, et al. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet. 2002;11(9):993–1003. doi: 10.1093/hmg/11.9.993. [DOI] [PubMed] [Google Scholar]
- 31.Dobyns WB, Filauro A, Tomson BN, Chan AS, Ho AW, Ting NT, et al. Inheritance of most X-linked traits is not dominant or recessive, just X-linked. Am J Med Genet A. 2004;129(2):136–143. doi: 10.1002/ajmg.a.30123. [DOI] [PubMed] [Google Scholar]
- 32.Weleber RG, Butler NS, Murphey WH, Sheffield VC, Stone EM. X-linked retinitis pigmentosa associated with a 2-base pair insertion in codon 99 of the RP3 gene RPGR. Arch Ophthalmol. 1997;115(11):1429–1435. doi: 10.1001/archopht.1997.01100160599013. [DOI] [PubMed] [Google Scholar]
- 33.Bauer S, Fujita R, Buraczynska M, Abrahamson M, Ehinger B, Wu W, et al. Phenotype of an X-linked retinitis pigmentosa family with a novel splice defect in the RPGR gene. Invest Ophthalmol Vis Sci. 1998;39(12):2470–2474. [PubMed] [Google Scholar]
- 34.Andreasson S, Breuer DK, Eksandh L, Ponjavic V, Frennesson C, Hiriyanna S, et al. Clinical studies of X-linked retinitis pigmentosa in three Swedish families with newly identified mutations in the RP2 and RPGR-ORF15 genes. Ophthalmic Genet. 2003;24(4):215–223. doi: 10.1076/opge.24.4.215.17228. [DOI] [PubMed] [Google Scholar]
- 35.Andreasson S, Ponjavic V, Abrahamson M, Ehinger B, Wu W, Fujita R, et al. Phenotypes in three Swedish families with X-linked retinitis pigmentosa caused by different mutations in the RPGR gene. Am J Ophthalmol. 1997;124(1):95–102. doi: 10.1016/s0002-9394(14)71649-6. [DOI] [PubMed] [Google Scholar]
- 36.Vervoort R, Wright AF. Mutations of RPGR in X-linked retinitis pigmentosa (RP3) Hum Mutat. 2002;19(5):486–500. doi: 10.1002/humu.10057. [DOI] [PubMed] [Google Scholar]