Abstract
ADP influx and ADP phosphorylation may alter mitochondrial free [Ca2+] ([Ca2+]m) and consequently mitochondrial bioenergetics by several postulated mechanisms. We tested how [Ca2+]m is affected by H2PO4− (Pi), Mg2+, calcium uniporter activity, matrix volume changes, and the bioenergetic state. We measured [Ca2+]m, membrane potential, redox state, matrix volume, pHm, and O2 consumption in guinea pig heart mitochondria with or without ruthenium red, carboxyatractyloside, or oligomycin, and at several levels of Mg2+ and Pi. Energized mitochondria showed a dose-dependent increase in [Ca2+]m after adding CaCl2 equivalent to 20, 114, and 485 nM extramatrix free [Ca2+] ([Ca2+]e); this uptake was attenuated at higher buffer Mg2+. Adding ADP transiently increased [Ca2+]m up to twofold. The ADP effect on increasing [Ca2+]m could be partially attributed to matrix contraction, but was little affected by ruthenium red or changes in Mg2+ or Pi. Oligomycin largely reduced the increase in [Ca2+]m by ADP compared to control, and [Ca2+]m did not return to baseline. Carboxyatractyloside prevented the ADP-induced [Ca2+]m increase. Adding CaCl2 had no effect on bioenergetics, except for a small increase in state 2 and state 4 respiration at 485 nM [Ca2+]e. These data suggest that matrix ADP influx and subsequent phosphorylation increase [Ca2+]m largely due to the interaction of matrix Ca2+ with ATP, ADP, Pi, and cation buffering proteins in the matrix.
Introduction
Matrix free Ca2+ ([Ca2+]m) may play a major role in regulating mitochondrial function. Studies have shown a correlation between increased bioenergetics and increased [Ca2+]m (1–6). However, excess [Ca2+]m predisposes the mitochondria to form and open the permeability transition pore (mPTP) (7–11), a key factor in cell apoptosis; inhibition of mPTP formation reduces ischemia-reperfusion injury (12–17). The importance of [Ca2+]m in both physiological and pathological conditions implies a necessity to tightly regulate [Ca2+]m.
[Ca2+]m is regulated in part by voltage-dependent cation fluxes via a series of poorly identified cation channels and exchangers on the inner mitochondrial membrane (IMM) (7,8,10,18). The primary route for matrix Ca2+ uptake is via the ruthenium-red (RR)-sensitive Ca2+ uniporter (CU), whereas the principal Ca2+ efflux pathway is the Na+/Ca2+ exchanger (NCE). There may also be Ca2+ efflux through a Na+-independent Ca2+ exchanger (NICE), putatively a Ca2+/H+ exchanger (CHE) (19–22). Transport through the CU and NCE are dependent on the mitochondrial membrane potential (ΔΨm), whereas the CHE is thought to be concentration-, and not ΔΨm-dependent (21,23,24). Several independent studies have shown a correlation between a larger ΔΨm and a higher [Ca2+]m (10,22).
In measuring [Ca2+]m, we observed an increase in [Ca2+]m with no added buffer CaCl2 during state 3 respiration, a state in which ΔΨm is decreased. This paradoxical increase in [Ca2+]m implies a mechanism other than ΔΨm in modulating [Ca2+]m. We tested five mechanisms that might play a role in this phenomenon:
The first mechanism that might explain an increase in [Ca2+]m is allosteric activation of the CU by ADP to increase [Ca2+]m (25). As ADP becomes phosphorylated, allosteric activation would decrease as [ADP] falls and [Ca2+]m would return to the preexisting level. A second mechanism is that ADP phosphorylation might also induce a large matrix volume contraction, thereby raising [Ca2+]m.
A third possible mechanism is modulation of [Ca2+]m by precipitation of free Ca2+ with Pi, such as Ca3(PO4)2 (26). The complexing of Ca2+ with Pi is dependent on the product of concentrations of different species of Pi and Ca2+. Thus, a decrease in matrix [Pi] would facilitate an increase in [Ca2+]m. In this situation, matrix [Pi] would be lower during state 3 respiration, as Pi becomes phosphorylated to ADP. After phosphorylation of ADP, matrix [Pi] would increase to near the level before phosphorylation, as Ca2+ would again form a complex with Pi.
A fourth possible mechanism is that the increased [Ca2+]m results from basic physicochemical differences, i.e., different binding affinities (Kd) of ADP and ATP for Ca2+ (27). These differences in Kd predict an increase in [Ca2+]m whenever the mitochondrial ADP/ATP ratio increases, and vice versa. Since ATP, ADP, and Pi bind variably to other cations (e.g., Mg2+ and H+) as well, a change in concentration of these ions may also result in a significant change of free ATP, ADP, and Pi (ATP4−, ADP3−, and H2PO4−) and thus alter the buffering capacity for Ca2+.
A fifth possible mechanism is that the altered bioenergetic state during state 3 respiration leads to release of matrix Ca2+ stores by an unknown mechanism.
To explore which of these possible mechanisms might underlie the large change in [Ca2+]m during transition to and from state 3 respiration, we measured matrix [Ca2+]m as a function of extramatrix free [Ca2+] ([Ca2+]e) by increasing buffer [CaCl2]. We also examined changes in [Ca2+]m at a higher and lower buffer [Pi] and [Mg2+], and assessed the effect of Δ[ADP] on matrix volume. Experiments were done with or without the CU blocker RR, the F1F0-ATPase blocker oligomycin (OMN), and the ADP/ATP carrier (AAC) blocker carboxy-atractyloside (CATR). Na+/Ca2+ exchange (NCE) was inactive and was eliminated as a factor because there was no Na+ present in the experimental buffer.
Materials and Methods
Fluorescence measurements
Fluorescence spectrophotometry was used to measure matrix free Ca2+, NADH, pH, and ΔΨm (Qm-8, Photon Technology, Birmingham, NJ) (28–30). Isolated mitochondria (5 mg/ml) were incubated for 20 min at room temperature (25°C) with 5 μM indo-1 AM to measure [Ca2+]m or with 5 μM BCECF AM to measure pHm (Invitrogen, Carlsbad, CA) followed by suspension in 25 ml isolation buffer and repeated centrifugation at 8000 × g. The AM form of the dyes is taken up into the matrix where it is deesterified and retained. The dye-loaded pellet was resuspended in 0.5 ml isolation buffer, and protein concentration was measured again and diluted to 12.5 mg mitochondrial protein/ml. In other mitochondria, background autofluorescence (AF), which at 456 nm represents NADH (redox state), was measured and ΔΨm was determined using rhodamine (Rh) 123. Mitochondria were kept on ice for the duration of the studies. All studies were conducted at room temperature. Please refer to the Supporting Material for detailed information on methods to assess matrix and extramatrix [Ca2+]m and Mg2+, redox state, matrix pH, ΔΨm, matrix volume, and respiration.
Experimental groups and protocol
Guinea pig heart mitochondria were isolated (see Supporting Material) as described previously (31,32) and diluted (33). Isolated mitochondria were divided into seven treatment groups: control (CON, 5 mM Pi), high Pi (HP, 10 mM Pi), low Pi (LP, 1 mM Pi), RR at start (RRS), RR later, after CaCl2 addition (RRL), and OMN or CATR, each given at t = −120 s (Fig. 1). The CON mitochondria were suspended (0.5 mg/ml) in experimental buffer containing (in mM) 130 KCl (EMD Biosciences, San Diego, CA), 5 K2HPO4, 20 MOPS, 0.016 bovine serum albumin, and 0.04 EGTA, pH 7.15 (adjusted with KOH). To adjust for osmolarity, the HP and LP experimental buffers were adjusted to contain 123 mM KCl and 10 mM K2HPO4 (HP) or 136 mM KCl and 1 mM K2HPO4 (LP). MOPS, BSA, and EGTA concentrations and pH were the same for all groups. In the RRS group, 25 μM RR was added to the CON experimental buffer before energizing mitochondria. In the RRL group, 25 μM RR was added after the addition of CaCl2. In the OMN and CATR groups, 100 μM OMN or 1.3 μM CATR was added before energizing mitochondria. In selected studies, 1 mM MgCl2 was present while the CaCl2 and ADP were added (see Supporting Material).
Figure 1.
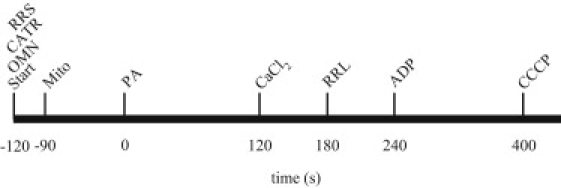
Time line for adding substances to experimental buffer. PA, pyruvic acid; RRS and RRL, ruthenium red at start or later; ADP, adenosine diphosphate; CATR, carboxyatractyloside; OMN, oligomycin; CCCP, carbonylcyanide m-chlorophenylhydrazone.
Experiments were initiated at t = −120 s, when OMN, CATR, and RR were added to comprise the OMN, CATR, and RRS groups; at t = −90 s, mitochondria were added (Fig. 1). At t = 0 s, pyruvic acid (PA, 0.5 mM) was added, followed by either of two concentrations of CaCl2 (10 or 25 μM in deionized H2O) or vehicle (0 CaCl2) at t = 120 s. At t = 180 s, RR was added to the RRL group. At t = 240 s, ADP (250 μM) was added, followed by 4 μM of the protonophore carbonylcyanide m-chlorophenylhydrazone (CCCP) at t = 400 s to maximally depolarize the IMM. All buffers and reagents, including substrates, were Na+-free to prevent NCE activation. Inactivity of the NCE was verified by comparing data from experiments with and without added CGP-37157, a specific mitochondrial NCE inhibitor (data not shown). When no drug or CaCl2 was added, the appropriate vehicle was added to the mitochondrial suspension. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless noted otherwise.
Statistical analyses
All data are presented as the mean ± SE. Repeated-measures ANOVA followed by a post hoc analysis using Student-Newman-Keuls' test was performed to determine statistically significant differences between and within groups. Data for analysis were collected at the times noted above. A P value of <0.05 (two-tailed) was considered significant.
Results
Extramatrix and matrix free [Ca2+] and inhibition of CU
Adding 0, 10, or 25 μM CaCl2 to the mitochondrial suspension caused a rapid, concentration-dependent increase in [Ca2+]e to initial values of 20 ± 3, 114 ± 13, and 485 ± 40 nM (Fig. 2) due to the presence of 40 μM of EGTA. This was followed by a slower, steady decline in [Ca2+]e as Ca2+ was transported into mitochondria via the CU; this was most prominent after adding 25 μM CaCl2. Transport of Ca2+ through the CU was confirmed in the RR groups because RR blocked the subsequent decrease in [Ca2+]e. Adding ADP did not significantly affect [Ca2+]e in the absence of RR. In the presence of RR there was a significant decrease in [Ca2+]e when ADP was added.
Figure 2.
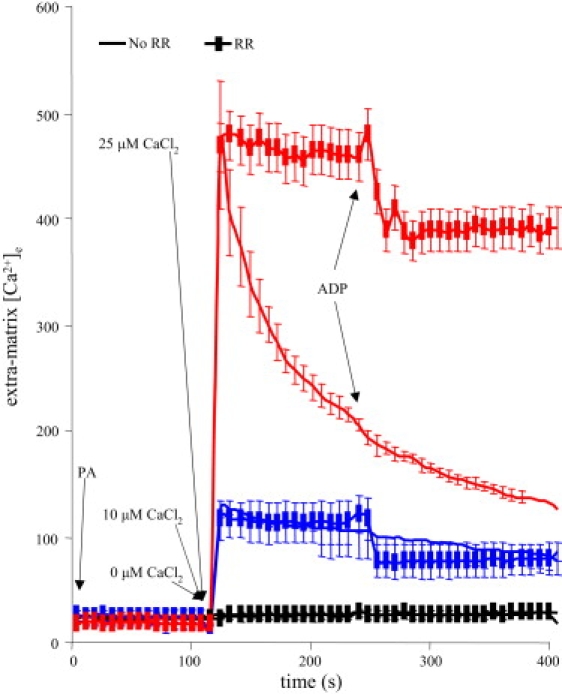
Extramatrix free [Ca2+] ([Ca2+]e) over time measured using indo-1 (in the presence of 40 μM EGTA). Adding CaCl2 increased [Ca2+]e in a concentration-dependent manner. Adding 25 μM CaCl2 increased [Ca2+]e up to 485 nM, which then slowly declined as Ca2+ was taken up into the matrix. Adding 10 μM CaCl2 increased [Ca2+]e to 114 nM with a lesser and slower subsequent Ca2+ uptake. RR blocked matrix uptake of Ca2+ through the CU so that [Ca2+]e remained constant. ADP caused a small decrease in [Ca2+]e only in the presence of RR.
Adding CaCl2 caused a concentration-dependent increase in matrix [Ca2+]m (Fig. 3), which took place via the CU, as verified again by the nearly complete block in response to RR (Fig. 3 C). After adding 10 μM CaCl2 (initially equivalent to 114 nM [Ca2+]e), [Ca2+]m increased from 80 ± 5 nM to 183 ± 7 nM. After adding 25 μM CaCl2 (initially equivalent to 485 nM [Ca2+]e), [Ca2+]m increased from 80 ± 5 to 518 ± 44 nM. Note that [Ca2+]m (Fig. 3) remained at a steady-state level, whereas [Ca2+]e (Fig. 2) continued to decrease in the CON group from its initial value of 485 nM [Ca2+] (after adding 25 μM CaCl2).
Figure 3.
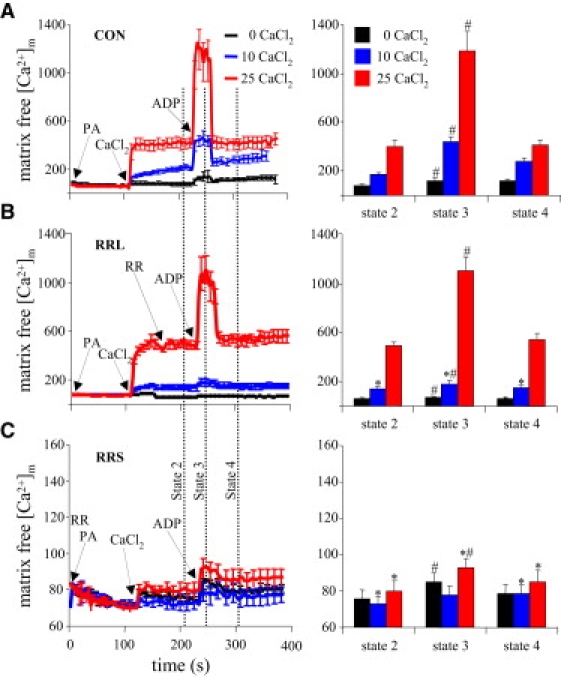
Effects of ADP and RR on [Ca2+]m over time, measured using indo-1 AM. Dynamic, time-dependent changes (left column) and mean changes (right column) taken at the vertical dotted lines in the left column are displayed. (A) Adding 10 and 25 μM CaCl2 in energized mitochondria (after PA) caused abrupt, graded increases in [Ca2+]m to steady-state values. Adding ADP caused an abrupt but transient rise in [Ca2+]m above the values established with added CaCl2. (B) Adding RR after 25 μM CaCl2 (RRL) did not blunt the rise in [Ca2+]m induced by adding ADP. (C) Adding RR before CaCl2 (RRS) blocked Ca2+ uptake, but the ADP-induced increase in [Ca2+]m remained evident, although it was much smaller (note scale) after adding 25 μM CaCl2. In the right column, for P < 0.05, # indicates state 3 versus states 2 and 4 respiration; ∗ indicates RRL and RRS versus the CON group at the same respiration state and CaCl2.
Adding ADP caused a proportional increase in [Ca2+]m in the CON, RRS, and RRL groups (Fig. 3), i.e., the magnitude of increase was dependent on the [Ca2+]m before ADP was added. The effect of ADP was absent in the RR group with 10 μM added CaCl2, as [Ca2+]m was low (80 ± 5 nM). ADP addition increased [Ca2+]m only a little (Fig. 3 C), probably because adding CaCl2 after RR did not increase [Ca2+]m. The effect of ADP on increasing [Ca2+]m was similar in the RRL and CON groups and smaller in the RRS group after adding CaCl2. There was an attenuated increase of [Ca2+]m (25 μM Ca2+; 485 nM [Ca2+]e), though not a significant one, in the RRL versus the CON group. Matrix uptake of Ca2+ continued in the CON group. Adding RR after 180 s stopped this slow uptake, resulting in a smaller increase in [Ca2+]m after adding 10 μM (114 nM [Ca2+]e). However, extending the time of Ca2+ uptake before adding RR in the 10-μM group resulted in an increase in [Ca2+]m beyond 180 s similar to that of the CON group. Adding ADP after RR then increased [Ca2+]m as much as in the CON group (data not shown).
[Ca2+]m increased as a function of [Ca2+]e, and this was attenuated during state 3 respiration (Fig. 4). These data show that [Ca2+]m reached a value twofold higher than that of [Ca2+]e at t = 180 s (state 2 respiration). [Ca2+]m was 80 ± 5, 212 ± 10, and 518 ± 44 nM, whereas [Ca2+]e was 26 ± 3, 112 ± 12, and 266 ± 12 nM after adding 0, 10, and 25 μM CaCl2. This plot also shows that [Ca2+]m increased 1.75- and twofold during state 3 versus state 2 respiration (from 518 ± 44 nM to 1039 ± 83 nM), whereas [Ca2+]e decreased slightly. These data support the proposal that as more Ca2+ becomes buffered in the matrix after CaCl2 addition, some is released during ADP/ATP antiport and ADP phosphorylation, possibly due to a decrease in matrix Ca2+ buffering sites (e.g., ADP, proteins).
Figure 4.
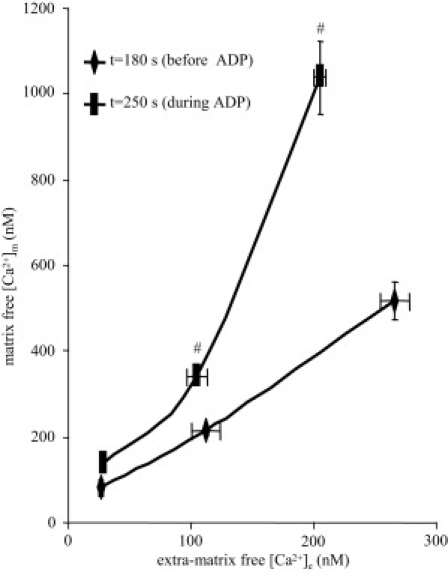
[Ca2+]m as a function of [Ca2+]e. [Ca2+]e and [Ca2+]m were measured at t = 100 s (before added CaCl2), at t =180 s (after added CaCl2), and at t = 250 s (in the presence of ADP). ADP caused a more than additive increase in [Ca2+]m as a function of [Ca2+]e, and the increase was dependent on the existing [Ca2+]m level. Data were taken from Figs. 2 and 3A. For P < 0.05, # indicates state 3 versus states 2 and 4 respiration.
Effect of buffer Pi and Mg2+ on matrix free [Ca2+]
The effect on [Ca2+]m of altering buffer [Pi] was evaluated at different [CaCl2]e and during ADP addition (Fig. 5). Buffer [KCl] was adjusted against [K2HPO4] to maintain the same osmolarity. With no added CaCl2, there were no detectable changes in [Ca2+]m among the three [Pi] groups. A change in buffer [Pi] from 5 to 1, and from 5 to 10 mM, did not significantly affect [Ca2+]m after adding CaCl2; however, at 25 μM CaCl2 and 1 mM [Pi] (LP), the initial (fast) uptake of Ca2+ was followed by a slow decrease in [Ca2+]m. The increase in [Ca2+]m after adding ADP did not differ among the Pi groups.
Figure 5.
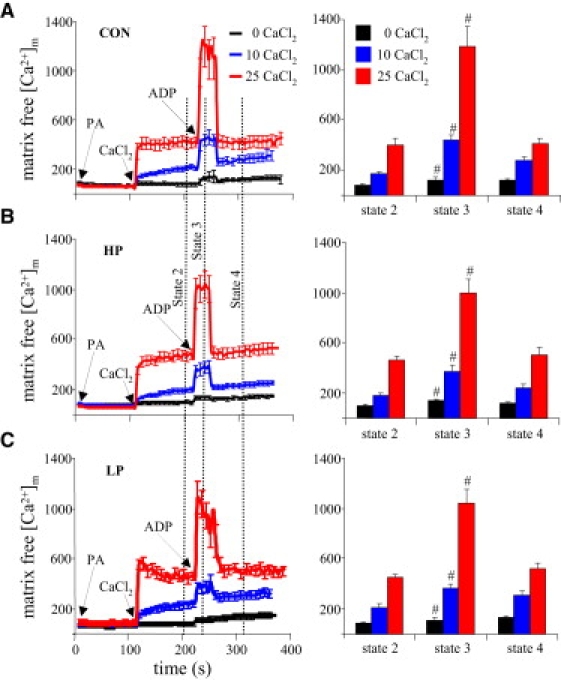
Effects of ADP and altered buffer Pi on [Ca2+]m. (A) Same as Fig. 3A, where [Pi] is 5 mM. (B) In both the 10-mM and 1-mM [Pi] groups, there were no significant differences in response to added CaCl2 or ADP versus the corresponding 5-mM [Pi] group. For P < 0.05, # indicates state 3 versus states 2 and 4 respiration; ∗ indicates the HP and LP versus CON groups at the same respiration state and CaCl2. See Fig. 3 legend for additional details.
In a few studies, 1 mM MgCl2 was added to the buffer (Fig. S1 A in the Supporting Material). Calculated matrix [Mg2+] (see Supporting Material, Methods) was 0.51 ± 0.03 mM without added MgCl2 and 0.85 ± 0.02 mM after adding 1 mM MgCl2 to the buffer. In the presence of MgCl2 the same amount of added CaCl2 resulted in a lesser rise in [Ca2+]m; however, adding ADP caused a proportional increase in [Ca2+]m when more CaCl2 was added to the buffer to counter the Mg2+-inhibited transport of Ca2+ through the CU (Fig. S1 A).
Effect of ADP versus ATP on mitochondrial matrix volume versus [Ca2+]
Added ADP resulted in a transient volume decrease of 7% (photon count) with a corresponding 51% increase in [Ca2+]m in the 25 μM CaCl2 group, whereas the maximal volume increase after valinomycin (VAL) was 52% with a corresponding decrease in [Ca2+]m after VAL of only 18% (Fig. S1 B). The ratio of volume to [Ca2+]m during responses to VAL over ADP—(52/18) divided by (7/51) = 0.05—indicated that this small decrease in volume caused by addition of ADP could account for only ∼5% of the increase in [Ca2+]m.
Effect of blocking ADP and ATP transport on matrix free [Ca2+]
Mitochondria were treated with OMN or CATR, which inhibit the F1F0-ATPase and the AAC respectively, to evaluate the effect of the organic phosphates ADP and ATP on [Ca2+]m. In the presence of OMN, [ADP]m and [ATP]m change when ADP is added, because ATP is exchanged for ADP at the AAC. With blocked phosphorylation of ADP by OMN, matrix [Pi] is expected to remain unchanged. In the presence of CATR, however, there can be no changes in [ADP]m or [ATP]m during added buffer ADP, because the AAC is blocked.
OMN and CATR had no effect on [Ca2+]m when 0, 10, or 25 μM CaCl2 was added; thus, there was no effect of these drugs on matrix Ca2+ uptake (Fig. 6), but the response to ADP after OMN was a small but significant increase in [Ca2+]m. Moreover, this increase in [Ca2+]m was sustained beyond the period when ADP would have been completely phosphorylated. This increase in [Ca2+]m may indicate limited ADP entry into the matrix in exchange for ATP efflux. The [Ca2+]m response to ADP was abolished after CATR as both ATP efflux and ADP influx were blocked.
Figure 6.
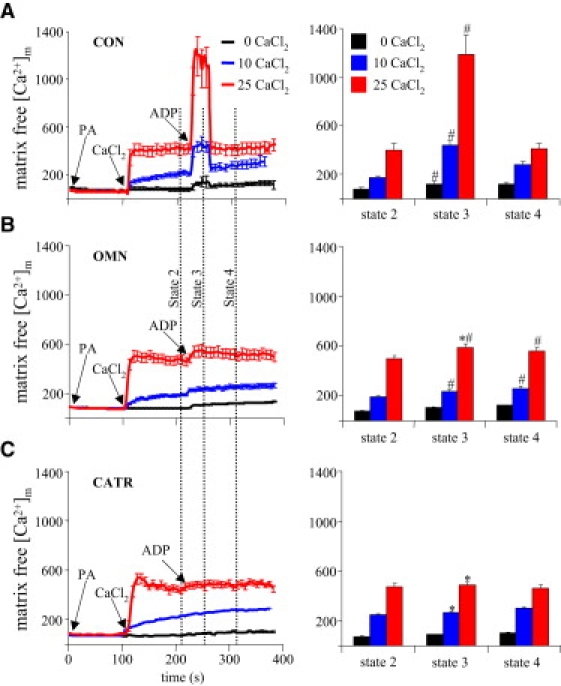
Effect of ADP on [Ca2+]m during inhibited phosphorylation and blocked ADP/ATP transport. (A) Same as Fig. 3A. (B) Adding ADP after CaCl2 and blocking F1F0-ATPase with OMN caused a significant increase in [Ca2+]m after addition of both 10 and 25 μM CaCl2. (C) Adding ADP after CaCl2 when the ADP/ATP carrier was blocked with CATR inhibited the ADP-induced increase in [Ca2+]m. For P < 0.05, # indicates state 3 versus states 2 and 4 respiration; ∗ indicates the OMN and CATR versus CON groups at the same respiration state and CaCl2. See Fig. 3 for additional details.
Effect of increasing [Ca2+]m on the NADH redox state
PA increased NADH in all groups. Adding either 10 or 25 μM CaCl2 did not change NADH compared to vehicle (CON, Fig. 7 A). Adding ADP caused a transient and reversible oxidation of NADH (i.e., a decrease in signal) in all groups, except in the OMN and CATR groups (data not shown), because ADP transport and phosphorylation were blocked in these groups. NADH returned to pre-ADP levels as ADP was phosphorylated to ATP. The presence of RR or a different [Pi] did not affect redox state (data not shown).
Figure 7.
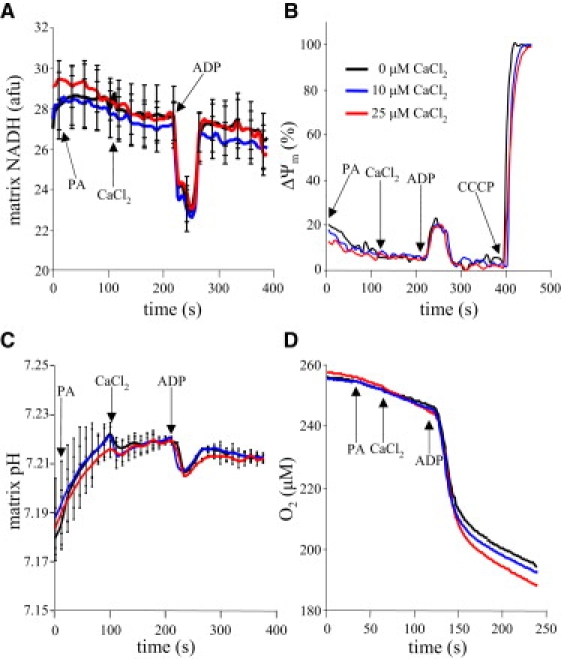
Effects of adding CaCl2 and ADP on mitochondrial bioenergetics. All data correspond to [Ca2+]m data displayed in Fig. 3A (CON). (A) PA increased NADH as the TCA cycle was activated. Adding CaCl2 did not significantly alter NADH values. (B) ADP transiently oxidized mitochondria (i.e., reduced NADH (A)) as energy contained in ΔΨm was consumed, as shown by the transiently lowered ΔΨm. (C) PA induced a mild alkalinization; adding CaCl2 did not further alter the matrix pH. ADP transiently reduced matrix pH as matrix proton influx temporarily exceeded proton pumping. (D) O2 consumption (respiration) increased on addition of PA, and more so on addition of 25 μM CaCl2. Adding ADP markedly enhanced O2 consumption, but adding CaCl2 did not produce any additional effect on respiration. See Table 1 for summary data and statistics on respiration.
Effect of increasing [Ca2+]m on ΔΨm
Energizing mitochondria with PA increased ΔΨm in all groups. Adding CaCl2 had no significant effect on ΔΨm (CON, Fig. 7 B). Adding ADP caused a transient and reversible partial depolarization of ΔΨm in all groups, as ADP was transported into the matrix and phosphorylated to ATP, which was also not affected by added CaCl2. ΔΨm was not affected by RR or at different buffer [Pi] or [Mg2+] (data not shown). The ADP-induced depolarization did not occur when ADP and ATP transport was prevented by CATR or when phosphorylation of ADP to ATP was blocked by OMN (data not shown). CCCP maximally depolarized the IMM.
Effect of increasing [Ca2+]m on matrix pH
Matrix pH increased on energizing mitochondria with PA. Adding CaCl2 had no significant effect on matrix pH compared to vehicle (CON, Fig. 7 C). Adding ADP acidified the matrix as protons enter the matrix through the F1F0ATP-ase to generate ATP. This alteration in pH by ADP was not affected by adding RR or by altering buffer [Pi], but was inhibited by CATR or OMN (data not shown). Adding CCCP caused matrix acidification, as this protonophore facilitates transport of protons across the IMM (data not shown).
Effect of increasing [Ca2+]m on respiration
Respiration rates in states 2 and 4 were significantly increased only at 518 nM [Ca2+]m (Fig. 7 D and Table 1); state 3 respiration was higher but unaltered at any [Ca2+]m. At 183 nM [Ca2+]m, respiration rates in states 2 and 4 were not significantly altered. Therefore, the respiratory control index (RCI) remained unchanged at 183 nM [Ca2+]m, and slightly decreased at 518 nM [Ca2+]m, due to the change in state 4 respiration. Adding MgCl2 had no added effect on state 3 or state 4 respiration (Fig. S1 C). Respiratory effects of [Ca2+]m were also not altered at any [Pi] (data not shown).
Table 1.
O2 consumption in different respiratory states
| State 2 | State 3 | State 4 | RCI | |
|---|---|---|---|---|
| 0 CaCl2 | 0.91 ± 0.04 | 14.07 ± 0.58 | 1.01 ± 0.05 | 13.6 ± 0.41 |
| 10 CaCl2 | 0.87 ± 0.04 | 14.15 ± 0.58 | 1.08 ± 0.02 | 13.0 ± 0.36 |
| 25 CaCl2 | 1.04 ± 0.03∗ | 14.44 ± 0.48 | 1.19 ± 0.02∗ | 12.1 ± 0.28∗ |
Oxygen consumption was measured in μmol O2/h/mg protein. Adding CaCl2 (485 nM [Ca2+]e) significantly increased O2 uptake in states 2 and 4 and lowered RCI (state 3/state 4 ratio).
Discussion
Changes in matrix free [Ca2+]m during respiration
This study demonstrates that in energized heart mitochondria, a transient ADP influx coupled to instantaneous ATP efflux markedly increases [Ca2+]m. Moreover, this study demonstrates that acute physiological changes in [Ca2+]m have little effect on mitochondrial bioenergetics other than to slightly increase resting-state respiration. The ADP-induced rise in [Ca2+]m may be in large part due either to the lower Ca2+ buffering capacity of ADP versus ATP or to an ADP-induced protein release of stored matrix Ca2+. This transient increase in [Ca2+]m is not measurably altered by Mg2+ or Pi,, and is little affected by an ADP-induced matrix contraction, or by ADP-induced uptake of matrix Ca2+ via the CU.
The uptake of [Ca2+]m at a given [Ca2+]e (Fig. 4) supports earlier studies (34,35) showing that Ca2+ influx through the CU causes Ca2+ to accumulate in the matrix, as predicted by the Nernst equation. However, the twofold gradient between [Ca2+]m and [Ca2+]e observed in this study after adding CaCl2 (114 or 485 nM [Ca2+]m) is smaller than that reported in earlier studies (34,35). Differences in species, isolation techniques, energy state, buffering capacities, equilibration rate, and measurement and calibration techniques for ionized [Ca2+] could underlie some of the differences.
The small, steady decline in [Ca2+]e after addition of ADP (Fig. 2) could be due to increased sequestration of matrix free Ca2+ by matrix proteins. The continuous decrease in [Ca2+]e without any further change in [Ca2+]m after a rapid rise in [Ca2+]m upon addition of 25 μM CaCl2 to the buffer (485 nM [Ca2+]e) is an interesting phenomenon that might also be explained by accumulating matrix Ca2+ storage in the form of Ca-PO4 complexes (26), such as Ca3(PO4)2, as Ca2+ enters the matrix through the CU. Formation of these complexes could result in a steady-state [Ca2+]m during continued matrix Ca2+ uptake, as additional Ca2+ entering the matrix will precipitate with PO43−. Although there is no direct evidence for matrix Ca-PO4 complex formation, there are indications that it occurs (36–38). However, we could not observe a clear difference in [Ca2+]m at different buffer [Pi], perhaps because of the low, but physiologic, [Ca2+]m.
Adding ADP at any [Ca2+]m caused a further 1.6- to 2-fold increase in [Ca2+]m during state 3 compared to state 2 respiration (Fig. 4). This effect of ADP on [Ca2+]m was more than additive at higher [Ca2+]m and is unlikely to be due to activation of the CU by ADP because the ADP-induced increases in [Ca2+]m were not appreciably reduced by RR (Fig. 3). Although ADP transiently reduced matrix volume, this was not in itself sufficient to account for the much larger increases observed in [Ca2+]m. Further, it is possible that a change in light scattering during state 3 respiration may also arise from a change in mitochondrial shape. Overall, these data suggest that matrix contraction by ADP is not an important factor.
There was no clear correlation among the different buffer [Pi]s and increases in [Ca2+]m on ADP addition. The relationship between [Ca2+]m and [Pi] has been shown previously (26), although at a relatively high [Ca2+]m, and that study did not examine for any difference in [Ca2+]m during state 3 respiration. It was not feasible to use Pi carrier blockers in our experiments to examine the importance of matrix [Pi] on [Ca2+]m during state 3 respiration, because these blockers affect ADP/ATP transport as well (39).
In contrast to [Pi], the differential binding of ADP and ATP to Ca2+ apparently plays an important role in the ADP-induced increase in [Ca2+]m, as shown by the slight increase of [Ca2+]m in the OMN versus the CATR group. The lack of an increase in [Ca2+]m on ADP addition after blocking the AAC shows that the ADP-induced increase in [Ca2+]m is not due to contamination of ADP with divalent cations that can bind to indo-1. The small but significant increase in [Ca2+]m in the OMN group shows that a change in [ADP]m, without altering matrix [Pi], can change [Ca2+]m. As discussed below, ADP binds to Ca2+ with a 10-fold lower affinity than does ATP; therefore, a decrease in matrix ATP due to ADP/ATP exchange alone may explain some of the increase in [Ca2+]m. Another possible explanation for the ADP effect is release of ionized Ca2+ from proteins similar to calsequestrin, a release mechanism that is observed in the sarcoplasmic reticulum (40).
To better understand how ATP, ADP, and Pi affect [Ca2+]m, it is first necessary to understand how these molecules are taken up into mitochondria and how they differentially bind to Ca2+. ADP3− enters mitochondria via the AAC in exchange for ATP4−. Since this transport is electrogenic, and constitutes a net influx of one positive charge, it is driven by, and uses the energy of, the ΔΨm (41–43). In the matrix, ADP is phosphorylated to ATP by the F1F0ATP-ase, a process driven by proton flux into the matrix. The Pi carrier is responsible for electroneutral cotransport of Pi−/H+ (or Pi−/OH− antiport), which is driven by the proton gradient, ΔpH (44–46).
Phosphorylation of ADP and dephosphorylation of ATP are dependent on the mitochondrial bioenergetic state (e.g., ΔpH) and ATP utilization by the cell, respectively. ADP levels rise in the matrix when extramatrix ADP is added and the ATP that is formed is ejected rapidly from the matrix by the AAC; matrix ADP levels remain elevated until all ADP is phosphorylated to ATP (4,47). It is important to note that the dissociation constants (Kds) for ATP and ADP (Table S1) indicate a 10-fold greater binding affinity of Ca2+ and Mg2+ with ATP4− over ADP3−, and a twofold greater binding affinity of Mg2+ versus Ca2+ to these phosphates. The binding affinities of these phosphate entities are uniformly high for H+ and very low for K+. Our premise is that these differences in binding affinities for ATP and ADP largely account for the ADP-induced increase in [Ca2+]m.
The results of these experiments imply a small role for Pi and a larger role for the ADP/ATP ratio in buffering of Ca2+ by mitochondria. Nevertheless, the ADP-induced increase in [Ca2+]m may be ascribed in part to other mechanisms that we explored. Our experiments performed with RR indicate that the ADP-induced Ca2+ flux through the CU cannot explain the phenomenon. The NCE was not active in the Na+-free buffer as verified by the NCE blocker CGP 37157. However, as there are currently no known blockers for Na+-independent Ca2+ exchange (NICE) such as Ca2+/H+ (CHE), it is not possible to exclude a decrease in Ca2+ efflux through NICE during state 3 respiration as an alternative explanation for the net increase of Ca2+ flux into the matrix (Jnet = JCU − JNICE) that causes the increase in [Ca2+]m. Because Ca2+ efflux through the NICE is dependent on both ΔpH ([H+]e − [H+]m) and Δ[Ca2+] ([Ca2+]m − [Ca2+]e) (20,48), we would not expect a major change in Ca2+ efflux through the NICE during state 3 respiration, since Δ[Ca2+] increases (Fig. 4) and ΔpH decreases (Fig. 7 C).
Another possible factor for the ADP-induced increase in [Ca2+]m is the change in pH upon ADP addition. During state 3 respiration, pHm decreases, albeit slightly (Fig. 7 C), due to influx of protons through the F1F0-ATPase (49). This transient acidification might affect the Kd between indo-1 and Ca2+ (50). However, the measured pH change was minimal (0.03 pH unit) and the reported changes in Kd were measured with a ΔpH of 1 pH unit. We expected the effect of ΔpH on [Ca2+]m to be minimal in this study because of 1), the small change in pHm, 2), the difference in [Ca2+]m after ADP addition in the presence of low buffer Pi (no significant change in pH compared to control; data not shown), and 3), the increase in [Ca2+]m after ADP addition in the presence of OMN (no significant change in pH on ADP addition; data not shown). Finally, the ADP-induced increase in [Ca2+]m could be due to an alteration in Ca2+ buffering capacity by TCA cycle intermediates, which can bind to Ca2+ as well (51).
Changes in [Ca2+]m and mitochondrial bioenergetics
These experiments did not show significant changes in mitochondrial bioenergetics when CaCl2 was added to the buffer except for small increases in respiration in states 2 and 4, and only after 25 μM CaCl2. Possible explanations for the increased resting-state respiration are opening of Ca2+-dependent K+ channels (31,52) or proton cycling through the putative NICE (48). In a previous study (53), in which respiration was measured in permeabilized cardiac cells, it was reported that adding CaCl2 increased state 2 respiration, but decreased state 3 respiration. However, other studies report that an increase in [Ca2+]m is associated with an increase in state 3 as well as state 2 respiration (5,54,55). In many of these studies, though, higher concentrations of CaCl2 might have induced a large increase in respiration due to other factors, such as mPTP opening. In this study, irreversible mPTP opening did not occur, as there was no decrease in ΔΨm at the highest [Ca2+]m observed with ADP. The small increase in O2 consumption on CaCl2 addition was not accompanied by other changes in bioenergetics, indicating that any slight uncoupling effect of Ca2+ could easily be corrected by increasing TCA cycle activity.
Many studies have shown a correlation between mitochondrial NADH and [Ca2+]m (1–3,6). A possible explanation for the lack of an increase in NADH with increased [Ca2+]m is that the highest [Ca2+]m only just reached the K0.5 for activation of TCA cycle dehydrogenases of ∼1 μM (55,56) during state 3 respiration, a period in which there is much fluctuation in bioenergetics. Other studies also question the role of Ca2+ in activation of NADH-producing dehydrogenases (57,58). Although these studies did not disprove the hypothesis of Ca2+ activation of TCA dehydrogenases, they did ascribe the changes in bioenergetics at least in part to other mechanisms (e.g., Mg2+ and ADP/ATP ratio), indirectly altered by Ca2+.
That an increase in [Ca2+]m can activate TCA dehydrogenases in the matrix to enhance respiration was proposed long ago (55,56). Our study provides an alternative hypothesis to the correlation between work load (changes in NADH/NAD+) and [Ca2+]m. We clearly observed an increase in [Ca2+]m due to activation of oxidative phosphorylation with addition of ADP, and we believe this is due in part to a decrease in matrix Ca2+ buffering capacity of ADP versus ATP. It is conceivable that an increase in [Ca2+]m is a result of enhanced phosphorylation-induced respiration, rather than enhanced respiration being a result of increased [Ca2+]m. Our study was not designed to disprove or prove the hypothesis of Ca2+ and its role in the regulation of mitochondrial bioenergetics; however, this alternative hypothesis should be further explored.
[Ca2+]m and mitochondrial pH
Matrix pH was not significantly affected by increased [Ca2+]m. It was anticipated that [Ca2+]m would compete with protons for binding with ATP, ADP, and Pi, and result in matrix acidification, just as acidification results in an increase in Ca2+ through buffering pathways (59). The amount of protons that dissociate from these buffering sites, when these sites bind to Ca2+, apparently can be sufficiently buffered in the matrix to maintain pH. We observed that [H+]m varies between 10 and 100 nM (pH between 7 and 8), which is about one order of magnitude lower than the changes in [Ca2+]m in this study. This indicates a higher matrix buffering capacity for protons than for Ca2+. Apparently the increase in [Ca2+]m is insufficient to produce a significant decrease in the H+ buffering capacity of the matrix. Otherwise, it is likely that an increase in proton expulsion coupled to electron transfer corrected for this increase in [H+]m, as respiration was slightly increased by CaCl2 addition. We have shown in preliminary experiments (unblocked NCE) that pH decreases with the addition of higher [CaCl2] (50 or 100 μM) (J. Haumann, A. Camara, and D. Stowe, unpublished observations). This decrease in pH was not blocked by cyclosporin A, an mPTP inhibitor, indicating that mPTP opening did not cause the acidification.
Summary and Conclusions
To our knowledge, the up to twofold increase in [Ca2+]m during oxidative phosphorylation under conditions of blocked CU and inactive NCE has not been reported previously. This study demonstrates the importance of changes in ADP phosphorylation on matrix free [Ca2+] and suggests a change in matrix buffering of Ca2+ or release of matrix stores of Ca2+ as possible mechanisms for the ADP-induced increase in [Ca2+]m. This observed increase may be dependent on buffer conditions and the different binding constants of Ca2+ to ATP, ADP, Pi, and matrix proteins. Changes in redox state, matrix pH, buffer [Pi], and [Mg2+]m, did not alter [Ca2+]m. Activation of the CU, and contraction of the matrix volume by ADP did not appear to account substantially for this effect of ADP. The conventional postulation is that increased [Ca2+]m enhances respiration via NADH-linked substrates; we show, alternatively, that ADP-induced stimulation of respiration enhances [Ca2+]m. Computer modeling (23) of the dynamics of Ca2+ flux and mitochondrial buffering mechanisms will help to confirm our findings, or to iteratively delineate the relative importance of each of the several proposed mechanisms that may modulate [Ca2+]m between states 3 and 4.
The important and dependent relationship between [Ca2+]m and [ADP], [Pi], and [ATP] should lead to a reexamination of the relationship between [Ca2+]m and control of respiration. Although increased [Ca2+]m slightly enhanced respiration in states 2 and 4 (NCE blocked), the increase in [Ca2+]m via reduced binding in the matrix during state 3 respiration did not enhance respiration. We could not demonstrate any Ca2+-induced changes in ΔΨm or NADH, but we found a small decrease in RCI, indicating slight uncoupling. The evidence that ΔΨm and NADH were not different in the OMN and CATR groups suggests that the changes observed resulted from an altered buffering effect due to differences in the ADP and ATP concentrations or to release of Ca2+ from stores, and less from increased Ca2+ influx through stimulation of the CU, or decreased Ca2+ efflux through NCE.
Acknowledgments
We thank Runa Patel, Mohammed Aldakkak, Kalyan C. Vinnakota, and Matthew R. Vernon for providing assistance in some experiments and for participating in many useful discussions.
This research was supported by grants from the National Institutes of Health (HL059122 to R.K.D. and A.K.S.C., HL089514 to D.F.S., and HL094317 to D.A.B.), and the American Heart Association (0855940G to D.F.S. and 08564N to R.K.D.).
Supporting Material
References
- 1.Brandes R., Bers D.M. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ. Res. 1997;80:82–87. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 2.Brandes R., Bers D.M. Simultaneous measurements of mitochondrial NADH and Ca2+ during increased work in intact rat heart trabeculae. Biophys. J. 2002;83:587–604. doi: 10.1016/S0006-3495(02)75194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack J.G., Halestrap A.P., Denton R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen M.H., Dudycha S.J., Jafri M.S. Effect of Ca2+ on cardiac mitochondrial energy production is modulated by Na+ and H+ dynamics. Am. J. Physiol. Cell Physiol. 2007;292:C2004–C2020. doi: 10.1152/ajpcell.00271.2006. [DOI] [PubMed] [Google Scholar]
- 5.Rossi C.S., Lehninger A.L. Stoichiometry of respiratory stimulation, accumulation of Ca++ and phosphate, and oxidative phosphorylation in rat liver mitochondria. J. Biol. Chem. 1964;239:3971–3980. [PubMed] [Google Scholar]
- 6.Territo P.R., Mootha V.K., Balaban R.S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am. J. Physiol. Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 8.Brookes P.S., Yoon Y., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 9.Duchen M.R. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunter T.E., Gunter K.K., Gavin C.E. Mitochondrial calcium transport: physiological and pathological relevance. Am. J. Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 11.O'Rourke B., Cortassa S., Aon M.A. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argaud L., Gateau-Roesch O., Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 13.Bopassa J.C., Vandroux D., Ferrera R. Controlled reperfusion after hypothermic heart preservation inhibits mitochondrial permeability transition-pore opening and enhances functional recovery. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2265–H2271. doi: 10.1152/ajpheart.00209.2006. [DOI] [PubMed] [Google Scholar]
- 14.Feng J., Lucchinetti E., Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3β. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Halestrap A.P., Clarke S.J., Javadov S.A. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy D.J., Duchen M.R., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc. Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara M., Miura T., Shimamoto K. Modulation of the mitochondrial permeability transition pore complex in GSK-3β-mediated myocardial protection. J. Mol. Cell. Cardiol. 2007;43:564–570. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Gunter T.E., Pfeiffer D.R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 19.Brand M.D. Electroneutral efflux of Ca2+ from liver mitochondria. Biochem. J. 1985;225:413–419. doi: 10.1042/bj2250413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunter T.E., Chace J.H., Gunter K.K. Mechanism of sodium independent calcium efflux from rat liver mitochondria. Biochemistry. 1983;22:6341–6351. doi: 10.1021/bi00295a046. [DOI] [PubMed] [Google Scholar]
- 21.Rottenberg H., Marbach M. The Na+-independent Ca2+ efflux system in mitochondria is a Ca2+/2H+ exchange system. FEBS Lett. 1990;274:65–68. doi: 10.1016/0014-5793(90)81330-q. [DOI] [PubMed] [Google Scholar]
- 22.Wingrove D.E., Amatruda J.M., Gunter T.E. Glucagon effects on the membrane potential and calcium uptake rate of rat liver mitochondria. J. Biol. Chem. 1984;259:9390–9394. [PubMed] [Google Scholar]
- 23.Dash R.K., Qi F., Beard D.A. A biophysically based mathematical model for the kinetics of mitochondrial calcium uniporter. Biophys. J. 2009;96:1318–1332. doi: 10.1016/j.bpj.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dash R.K., Beard D.A. Analysis of cardiac mitochondrial Na+-Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J. Physiol. 2008;586:3267–3285. doi: 10.1113/jphysiol.2008.151977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litsky M.L., Pfeiffer D.R. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- 26.Chalmers S., Nicholls D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 27.Michailova A., McCulloch A. Model study of ATP and ADP buffering, transport of Ca2+ and Mg2+, and regulation of ion pumps in ventricular myocyte. Biophys. J. 2001;81:614–629. doi: 10.1016/S0006-3495(01)75727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 29.Chance B., Cohen P., Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 30.Aldakkak M., Stowe D.F., Camara A.K.S. Mitochondrial matrix K+ flux independent of large conductance Ca2+ activated K+ channel opening. Am. J. Physiol. Cell Physiol. 2010;298:C530–C541. doi: 10.1152/ajpcell.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinen A., Camara A.K., Stowe D.F. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am. J. Physiol. Cell Physiol. 2007;292:C148–C156. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 32.Riess M.L., Kevin L.G., Stowe D.F. Anesthetic preconditioning: the role of free radicals in sevoflurane-induced attenuation of mitochondrial electron transport in Guinea pig isolated hearts. Anesth. Analg. 2005;100:46–53. doi: 10.1213/01.ANE.0000139346.76784.72. [DOI] [PubMed] [Google Scholar]
- 33.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.McCormack J.G., Browne H.M., Dawes N.J. Studies on mitochondrial Ca2+-transport and matrix Ca2+ using fura-2-loaded rat heart mitochondria. Biochim. Biophys. Acta. 1989;973:420–427. doi: 10.1016/s0005-2728(89)80384-6. [DOI] [PubMed] [Google Scholar]
- 35.Wan B., LaNoue K.F., Scaduto R.C., Jr. Regulation of citric acid cycle by calcium. J. Biol. Chem. 1989;264:13430–13439. [PubMed] [Google Scholar]
- 36.Nicholls D.G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem. J. 1978;176:463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls D.G., Scott I.D. The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membrane-potential-dependent calcium-ion efflux mechanisms. Biochem. J. 1980;186:833–839. doi: 10.1042/bj1860833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoccarato F., Nicholls D. The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria. Eur. J. Biochem. 1982;127:333–338. doi: 10.1111/j.1432-1033.1982.tb06875.x. [DOI] [PubMed] [Google Scholar]
- 39.McGivan J.D., Grebe K., Klingenberg M. On the coupling between the transport of phosphate and adenine nucleotides in rat liver mitochondria. Biochem. Biophys. Res. Commun. 1971;45:1533–1541. doi: 10.1016/0006-291x(71)90194-x. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen A.O., Shen A.C., Campbell K.P. The Ca2+-release channel/ryanodine receptor is localized in junctional and corbular sarcoplasmic reticulum in cardiac muscle. J. Cell Biol. 1993;120:969–980. doi: 10.1083/jcb.120.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan S.H., Barbour R.L. Adenine nucleotide transport in hepatoma mitochondria. Characterization of factors influencing the kinetics of ADP and ATP uptake. Biochim. Biophys. Acta. 1983;723:104–113. doi: 10.1016/0005-2728(83)90014-2. [DOI] [PubMed] [Google Scholar]
- 42.Klingenberg M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta. 2008;1778:1978–2021. doi: 10.1016/j.bbamem.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur. J. Biochem. 1968;6:66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 44.Coty W.A., Pedersen P.L. Phosphate transport in rat liver mitochondria. Kinetics and energy requirements. J. Biol. Chem. 1974;249:2593–2598. [PubMed] [Google Scholar]
- 45.Stappen R., Kramer R. Kinetic mechanism of phosphate/phosphate and phosphate/OH- antiports catalyzed by reconstituted phosphate carrier from beef heart mitochondria. J. Biol. Chem. 1994;269:11240–11246. [PubMed] [Google Scholar]
- 46.Wohlrab H. Molecular aspects of inorganic phosphate transport in mitochondria. Biochim. Biophys. Acta. 1986;853:115–134. doi: 10.1016/0304-4173(86)90007-8. [DOI] [PubMed] [Google Scholar]
- 47.Vignais P.V., Vignais P.M., Doussiere J. Functional relationship between the ADP/ATP-carrier and the F1-ATPase in mitochondria. Biochim. Biophys. Acta. 1975;376:219–230. doi: 10.1016/0005-2728(75)90013-4. [DOI] [PubMed] [Google Scholar]
- 48.Gunter K.K., Zuscik M.J., Gunter T.E. The Na+-independent Ca2+ efflux mechanism of liver mitochondria is not a passive Ca2+/2H+ exchanger. J. Biol. Chem. 1991;266:21640–21648. [PubMed] [Google Scholar]
- 49.Fillingame R.H. Coupling H+ transport and ATP synthesis in F1F0-ATP synthases: glimpses of interacting parts in a dynamic molecular machine. J. Exp. Biol. 1997;200:217–224. doi: 10.1242/jeb.200.2.217. [DOI] [PubMed] [Google Scholar]
- 50.Baker A.J., Brandes R., Weiner M.W. Protein and acidosis alter calcium-binding and fluorescence spectra of the calcium indicator indo-1. Biophys. J. 1994;67:1646–1654. doi: 10.1016/S0006-3495(94)80637-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranby M., Gojceta T., Lindahl T.L. Isocitrate as calcium ion activity buffer in coagulation assays. Clin. Chem. 1999;45:1176–1180. [PubMed] [Google Scholar]
- 52.Heinen A., Winning A., Weber N.C. The regulation of mitochondrial respiration by opening of mKCa channels is age-dependent. Eur. J. Pharmacol. 2008;578:108–113. doi: 10.1016/j.ejphar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Tsokos J., Bloom S. Effects of calcium on respiration and ATP content of isolated, leaky, heart muscle cells. J. Mol. Cell. Cardiol. 1977;9:823–836. doi: 10.1016/s0022-2828(77)80059-x. [DOI] [PubMed] [Google Scholar]
- 54.Roman I., Clark A., Swanson P.D. The interaction of calcium transport and ADP phosphorylation in brain mitochondria. Membr. Biochem. 1981;4:1–9. doi: 10.3109/09687688109065419. [DOI] [PubMed] [Google Scholar]
- 55.McCormack J.G., Denton R.M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem. J. 1980;190:95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denton R.M., McCormack J.G., Edgell N.J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem. J. 1980;190:107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi F., Chen X., Beard D.A. Detailed kinetics and regulation of mammalian NAD-linked isocitrate dehydrogenase. Biochim. Biophys. Acta. 2008;1784:1641–1651. doi: 10.1016/j.bbapap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hak J.B., Van Beek J.H., Westerhof N. Mitochondrial dehydrogenase activity affects adaptation of cardiac oxygen consumption to demand. Am. J. Physiol. 1993;264:H448–H453. doi: 10.1152/ajpheart.1993.264.2.H448. [DOI] [PubMed] [Google Scholar]
- 59.Gambassi G., Hansford R.G., Capogrossi M.C. Effects of acidosis on resting cytosolic and mitochondrial Ca2+ in mammalian myocardium. J. Gen. Physiol. 1993;102:575–597. doi: 10.1085/jgp.102.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


