Abstract
We previously reported that knock-down of tumor suppressor Pdcd4 (Programmed Cell Death 4) down-regulates E-cadherin expression and activates β-catenin/Tcf (T cell factor) dependent transcription in colon tumor cells. However, the underlying mechanism of these observations remains unknown. In this study, we demonstrated that knock-down of Pdcd4 down-regulates E-cadherin expression through elevated protein level of Snail. Over-expression of Pdcd4 up-regulates E-cadherin expression and inhibits β-catenin/Tcf dependent transcription. We then showed that knock-down of E-cadherin activates β-catenin/Tcf dependent transcription. Conversely, over-expression of E-cadherin in Pdcd4 knock-down cells inhibits β-catenin/Tcf dependent transcription. In addition, Pdcd4 knock-down stimulates u-PAR and c-Myc expression, while u-PAR and c-Myc expression can be reversed by over-expressing E-cadherin in Pdcd4 knock-down cells. Using chromatin immunoprecipitation, we demonstrated that β-catenin/Tcf4 directly binds to the promoters of u-PAR and c-myc in Pdcd4 knock-down cells. Futhermore, knock-down of u-PAR or c-Myc inhibits invasion in Pdcd4 knock-down cells, suggesting that both u-PAR and c-Myc contribute to invasion induced by Pdcd4 knock-down. Taken together, our data demonstrated that elevated Snail expression by Pdcd4 knock-down leads to down-regulation of E-cadherin resulting in activating β-catenin/Tcf dependent transcription and stimulating the expression of c-Myc and u-PAR, thus providing molecular explanation of how Pdcd4 suppresses tumor invasion.
Keywords: Pdcd4, β-catenin/Tcf dependent transcription, E-cadherin, u-PAR, c-Myc, stably knock-down
Introduction
Programmed Cell Death 4 (Pdcd4) is a novel tumor suppressor that frequently exhibits down-regulated expression in several types of cancer. In colon cancer tissues, expression level of Pdcd4 is continuously down-regulated in the order of normal-adenoma-carcinoma (Mudduluru et al., 2007). Over-expression of Pdcd4 has been demonstrated to inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced transformation (Yang et al., 2001) and tumor phenotype (Yang et al., 2003b). Knock-down of Pdcd4 inhibits retinoic acid induced terminal differentiation in NB4 cells (Ozpolat et al., 2007) and UV radiation-induced apoptosis in HeLa cells (Bitomsky et al., 2008). In addition to cultured cells, Pdcd4 transgenic mice that over-express Pdcd4 in the epidermis show significant reductions in 7,12-dimethylbenz(a)anthracene (DMBA)/TPA induced skin papilloma formation and carcinoma incidence (Jansen et al., 2005). Knock-out of Pdcd4 expression in mice resulted in induction of lymphomas with frequent metastases to the liver and kidneys (Hilliard et al., 2006), and an increase in DMBA/TPA induced skin papilloma formation and carcinoma incidence (Schmid et al., 2008). These findings suggest that Pdcd4 suppresses carcinogenesis not only at an early stage (tumor promotion), but also at a late stage (tumor progression) in both cultured cells and transgenic mice.
Ectopic expression of pdcd4 cDNA in invasive colon RKO cells inhibited invasive capacity and AP-1 dependent transcription (Yang et al., 2006), and tumor cell intravasation (Leupold et al., 2007). Inhibition of AP-1 dependent transcription by Pdcd4 is believed to contribute to invasion inhibition since many AP-1-regulated genes are involved in tumor invasion and metastasis (Ozanne et al., 2000). Recently, we found that Pdcd4 knock-down in colon tumor HT29 cells led to a fibroblast-like morphological change and promoted invasion (Wang et al., 2008). Concurrently, Pdcd4 knock-down in HT29 cells resulted in down-regulation of E-cadherin expression, accumulation of β-catenin in the nucleus, and activation of β-catenin/Tcf (T cell factor) and AP-1 dependent transcription (Wang et al., 2008). In addition, transient knock-down of Pdcd4 increased expression of endogenous urokinase-type plasminogen activator receptor (u-PAR) (Leupold et al., 2007), a glycosylated membrane protein that mediates degradation of extracellular matrix components and promotes invasion and metastasis (Laufs et al., 2006). However, the mechanism by which Pdcd4 knock-down activates β-catenin/Tcf dependent transcription and promotes invasion remains unclear.
E-cadherin is a transmembrane glycoprotein whose cytoplasmic domain interacts with actin, α-catenin, p120, and β-catenin (Kemler, 1992). It has been proposed that loss of E-cadherin-mediated cell-cell adhesion is an essential event for the invasion of epithelial tumor cells (Cavallaro and Christofori, 2004). Upon loss of binding with E-cadherin, free β-catenin is rapidly phosphorylated by glycogen synthase kinase-3β (GSK-3β) in the complex of adenomatous polyposis coli (APC)-axin-GSK-3β-casein kinase I (CKI), and degraded by the proteasome pathway. If the APC is mutated or GSK-3β activity is blocked by Wnt signaling, β-catenin will not be phosphorylated, and will translocate into the nucleus (Gregorieff and Clevers, 2005). Subsequent to nuclear translocation, β-catenin binds to a member of Tcf family to form the β-catenin/Tcf complex, which activates β-catenin/Tcf dependent transcription. Numerous target genes of β-catenin/Tcf dependent transcription have been shown to be involved in colon tumor invasion (Fuchs et al., 2005).
In the present study, we investigated the mechanism by which Pdcd4 knock-down activates β-catenin/Tcf dependent transcription and identified its downstream target genes. We demonstrated that E-cadherin is a mediator for activating β-catenin/Tcf dependent transcription to stimulate u-PAR and c-Myc expression, which promotes invasion in Pdcd4 knock-down cells.
Results
Pdcd4 knock-down in GEO cells activates β-catenin/Tcf dependent transcription despite of the decreased β-catenin level
Previously, we have demonstrated that Pdcd4 knock-down in HT29 cells resulted in down-regulation of E-cadherin and a slight decrease in β-catenin protein level (Wang et al., 2008). Although Pdcd4 knock-down in GEO cells also down-regulated E-cadherin expression, the protein level of β-catenin decreased dramatically with more than 70% relative to GEO-shLacZ cells (Figure 1a). The GEO-shLacZ and parental GEO cells had similar levels of β-catenin protein (Figure 1a). This finding thus raises a question as to whether Pdcd4 knock-down is able to activate β-catenin/Tcf dependent transcription in GEO cells. To address this question, we transfected TOPflash and FOPflash luciferase reporter constructs into GEO-shLacZ and GEO-shPdcd4 cells. The TOPflash and FOPflash contain the wild type and mutated β-catenin/Tcf binding site, respectively. As shown in Figure 1b, β-catenin/Tcf dependent transcription was approximately 3-fold-higher in GEO-shPdcd4 cells relative to GEO-shLacZ cells (filled bars). To confirm the specificity of this induction, the FOPflash plasmid was transfected into GEO-shLacZ and GEO-shPdcd4 cells. There was no significant difference in luciferase activity between the GEO-shLacZ and GEO-shPdcd4 cells (Figure 1b, open bars). In addition, we found that knock-down of Pdcd4 in GEO cells also led to translocation of β-catenin into nucleus (Supplementary Figure S1) and promotion of invasion (Supplementary Figure S2). These data clearly show that knock-down of Pdcd4 expression activates β-catenin/Tcf dependent transcription and promotes invasion in GEO cells. The extent of activation of β-catenin/Tcf dependent transcription in GEO-shPdcd4 cells is lower compared to that of the HT29-shPdcd4 cells (Wang et al., 2008); this might be due to reduced accumulation of β-catenin in the nuclei of GEO-shPdcd4 cells as a consequence of decreased β-catenin protein level.
Figure 1.
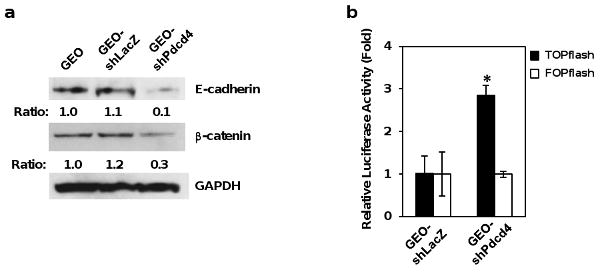
Knock-down of Pdcd4 down-regulates E-cadherin and activates β-catenin/Tcf dependent transcription in GEO cells. (a) Knock-down of Pdcd4 down-regulates E-cadherin. Western blot was performed using antibodies against E-cadherin, β-catenin, and GAPDH. The ratios of E-cadherin/GAPDH and β-catenin/GAPDH in GEO cells are designated as 1.0. The number indicates the relative level of E-cadherin or β-catenin protein. (b) Knock-down of Pdcd4 activates β-catenin/Tcf dependent transcription. GEO-shLacZ and GEO-shPdcd4 cells were transfected with either 0.2 μg of TOPflash or FOPflash along with 10 ng of pRL-SV40. TOPflash and FOPflash constructs are widely used to evaluate β-catenin/Tcf dependent signaling events that regulated by TCF/LEF family. TOPflash construct driven by thymidine kinase minimal promoter contains 6 wild-type Tcf binding sites upstream of a luciferase reporter gene. FOPflash is similar as TOPflash except that it contains 6 mutated Tcf binding sites. FOPflash is used as a specificity control for TOPflash activity. The luciferase activity was normalized against Renilla activity, and the activity of GEO-shLacZ cells transfected with either TOPflash or FOPflash plasmid is designated as 1. These experiments were repeated three times, each with five independent transfections; representative data are shown. Results are expressed as mean ± standard deviation (SD). The asterisk denotes a significant difference compared with GEO-shLacZ cells transfected with TOPflash, as determined by one-way ANOVA (p < 0.0001).
Elevated Snail expression contributes to the down-regulation of E-cadherin in Pdcd4 knock-down cells
To understand the mechanism by which Pdcd4 knock-down leads to down-regulation of E-cadherin expression, the E-cadherin promoter-luciferase plasmid (E7) was transfected into GEO-shLacZ and GEO-shPdcd4 cells. The E7 construct comprises E-cadherin promoter region from −38 to +135 bp (containing two E-box sites) fused with luciferase reporter (Liu et al., 2005). The luciferase activity in GEO-shPdcd4 cells was approximately 35% of that in GEO-shLacZ cells (Figure 2a), indicating that E-cadherin promoter activity is inhibited by Pdcd4 knock-down. To test whether the E-boxes mediate the inhibition of E-cadherin promoter activity in Pdcd4 knock-down cells, we mutated both E-box sites and transfected the mutated construct (E7-m) into GEO-shLacZ and GEO-shPdcd4 cells. The E7-m exhibited approximately 70% of the basal level of E-cadherin promoter activity (Figure 2a, filled bars). However, the luciferase activity transfected with E7-m construct was similar in GEO-shLacZ and GEO-shPdcd4 cells (Figure 2a, E7-m filled vs open bar), indicating that mutation of E-boxes abolished the inhibition of E-cadherin promoter activity in Pdcd4 knock-down cells. It has been known that transcription factor Snail can binds to E-boxes on the E-cadherin promoter and inhibits E-cadherin expression (Batlle et al., 2000; Cano et al., 2000). To determine whether Pdcd4 knock-down leads to the up-regulation of Snail expression, the protein level of Snail was examined. As shown in Figure 2b, the Snail protein level in GEO-shPdcd4 cells was dramatically induced while the Snail protein was undetectable in GEO-shLacZ cells. We then knocked down Snail expression and tested whether Snail knock-down can restore the E-cadherin promoter activity in GEO-shPdcd4 cells. The level of Snail protein in Snail knock-down cells (si-snail) was approximately 50% of that in GEO-shPdcd4 cells expressing control siRNA (control) (Figure 2c) whereas the E-cadherin promoter activity in Snail knock-down cells was approximately 180% of that in control cells (Figure 2d). These results indicate that Snail contributes to the inhibition of E-cadherin expression in GEO-shPdcd4 cells. Our findings indicate that Pdcd4 knock-down leads to up-regulation of Snail resulting in inhibition of E-cadherin expression.
Figure 2.
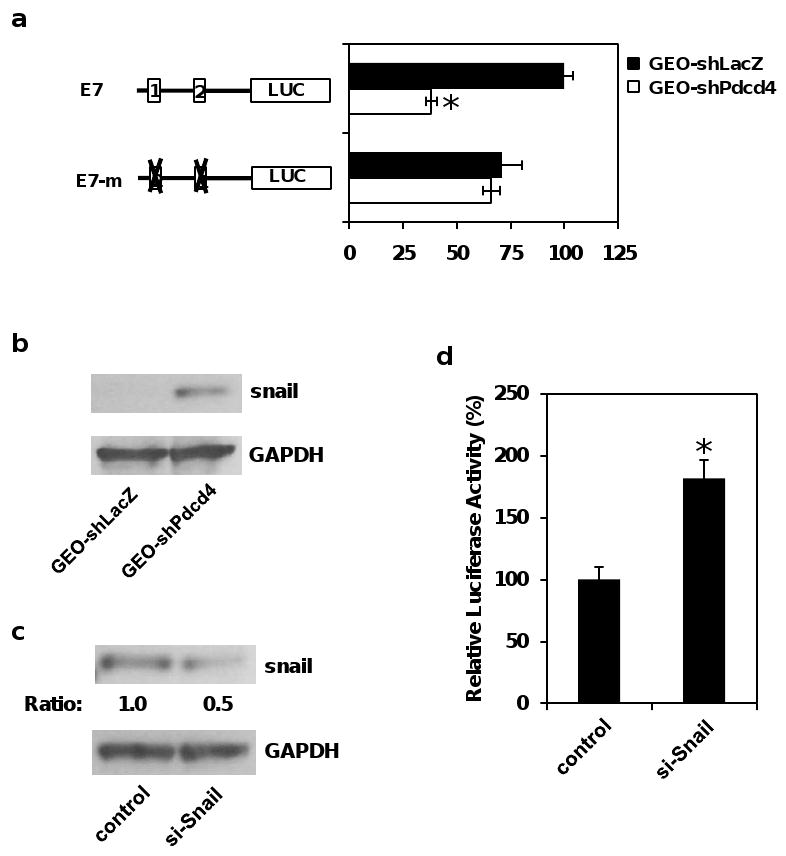
Knock-down of Pdcd4 inhibits E-cadherin expression through elevated Snail expression. (a) Pdcd4 knock-down inhibits E-cadherin promoter activity. The E-cadherin promoter reporter constructs (0.2 μg), wild-type (E7) and E-box mutant (E7-m) were transfected into GEO-shLacZ and GEO-shPdcd4 cells along with 10 ng of pRL-SV40. The luciferase activity was normalized against Renilla activity. The activity of GEO-shLacZ cells transfected with E7 is designated as 100%. The asterisk denotes a significant difference as determined by one-way ANOVA (p< 0.005). (b) Snail expression is up-regulated in GEO-shPdcd4 cells. Western blot was performed using antibodies against Snail and GAPDH as described in Materials and Methods. (c) Knock-down of Snail expression in GEO-shPdcd4 cells. The snail siRNA (si-snail) and control siRNA (control) was transfected into GEO-shPdcd4 cells. Western blot was performed using antibodies against Snail and GAPDH. The ratios of Snail/GAPDH in control cells are designated as 1.0. The number indicates the relative level of Snail protein. (d) Knock-down of Snail in GEO-shPdcd4 cells activates E-cadherin promoter activity. The wild-type E7 promoter reporter construct (0.2 μg) along with 10 ng of pRL-SV40 were transfected into control and si-snail cells. The luciferase activity was normalized against Renilla activity. The activity of control cells transfected with E7 is designated as 100%. The asterisk denotes a significant difference compared with GEO-shLacZ cells transfected with E7 as determined by one-way ANOVA (p < 0.05).
Over-expression of Pdcd4 up-regulates E-cadherin and inhibits β-catenin/Tcf dependent transcription
If E-cadherin expression is regulated by Pdcd4, over-expression of Pdcd4 should increase E-cadherin expression. To test this, we stably expressed Pdcd4 in human colon tumor LoVo cells (LoVo-Pdcd4) using the lentivirus-mediated expression system. As shown in Figure 3a, the protein levels of Pdcd4 and E-cadherin in LoVo-Pdcd4 cells were approximately 8-fold and 2-fold higher than those of cells transduced with lentivirus-empty vector (LoVo-control), respectively. These results suggest that over-expression of Pdcd4 induces E-cadherin expression. To test whether over-expression of Pdcd4 inhibits β-catenin/Tcf dependent transcription, LoVo-control and LoVo-Pdcd4 cells were transfected with TOPflash and FOPflash plasmids. As shown in Figure 3b, the β-catenin/Tcf dependent transcription reduced approximately 50% in LoVo-Pdcd4 cells relative to LoVo-control cells (filled bars). In contrast, a similar luciferase activity was observed when FOPflash was transfected into LoVo-control and LoVo-Pdcd4 cells (open bars). In addition, we found that Pdcd4 inhibited β-catenin/Tcf dependent transcription in a concentration dependent manner when Pdcd4 expression plasmid (pcDNA-Pdcd4) and TOPflash were transiently transfected into LoVo cells (Figure 3c, filled bars). The β-catenin/Tcf dependent transcription was inhibited approximately 60% when 1.5 μg of pcDNA-Pdcd4 was transfected while Pdcd4 does not affect the luciferase activity when FOPflash was co-transfected with either pcDNA-Pdcd4 or control plasmid (Figure 3c). Our findings indicate that not only knock-down of Pdcd4 activates β-catenin/Tcf dependent transcription (Figure 1), but also over-expression of Pdcd4 inhibits β-catenin/Tcf dependent transcription (Figure 2). Thus, Pdcd4 regulates the expression of E-cadherin and activation of β-catenin/Tcf dependent transcription.
Figure 3.
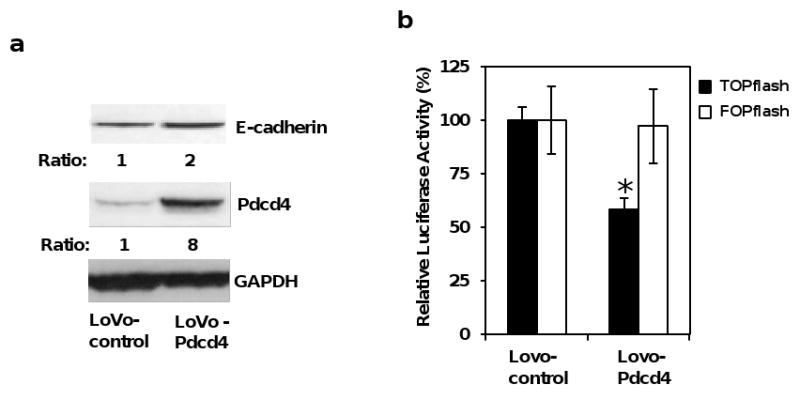
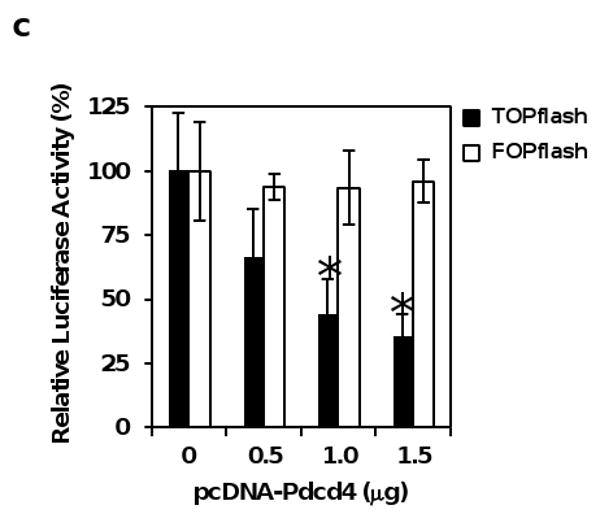
Over-expression of Pdcd4 up-regulates E-cadherin and inhibits β-catenin/Tcf dependent transcription. (a) Over-expression of Pdcd4 in LoVo cells up-regulates E-cadherin expression. Western blot was performed using antibodies against E-cadherin, Pdcd4, and GAPDH. The ratios of E-cadherin/GAPDH and Pdcd4/GAPDH in LoVo-control cells are designated as 1. The number indicates the relative level of either E-cadherin or Pdcd4 protein. (b) Pdcd4 inhibits β-catenin/Tcf dependent transcription. TOPflash (0.2 μg, filled bar) and FOPflash (0.2 μg, open bar) along with 10 ng of pRL-SV40 were transfected into LoVo-control and LoVo-Pdcd4 cells. The luciferase activity was normalized against Renilla activity. The activity of LoVo-control cells transfected with TOPflash and FOPflash is designated as 100%. The asterisk denotes a significant difference compared with cells transfected with TOPflash as determined by one-way ANOVA (P < 0.005). (c) Pdcd4 inhibits β-catenin/Tcf dependent transcription in a dose-dependent manner. Increasing amount (0-1.5 μg) of pcDNA-Pdcd4 plasmid was transfected along with 0.2 μg of TOPflash (filled bar) or FOPflash (open bar) into LoVo cells. The luciferase activity was normalized against Renilla activity. The total DNA was maintained at 1.7 μg by adding empty vector pcDNA3.1 DNA. The activity of cells transfected with TOPflash or FOPflash plasmid but without pcDNA-Pdcd4 plasmid is designated as 100%. The asterisk denotes a significant difference compared with cells transfected with TOPflash but without pcDNA-Pdcd4 plasmid, as determined by one-way ANOVA (P < 0.005). The experiments in (b) and (c) were repeated three times each with five independent transfections and representative data are shown. Results are expressed as mean ± SD.
Knock-down of E-cadherin activates β-catenin/Tcf dependent transcription
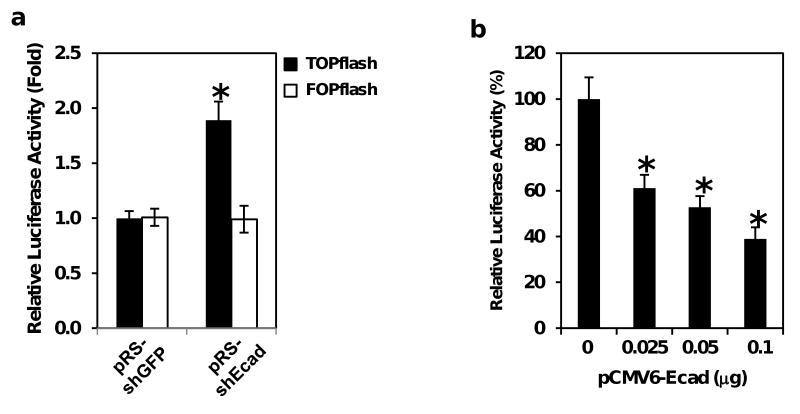
We demonstrated herein that Pdcd4 knock-down led to E-cadherin down-regulation and activation of β-catenin/Tcf dependent transcription (Figure 1). However, it is unclear whether down-regulation of E-cadherin is necessary for activating β-catenin/Tcf dependent transcription when Pdcd4 is knocked down. To address this issue, E-cadherin shRNA expression plasmid (pRS-shEcad) was transiently transfected into HT29 cells along with either TOPflash or FOPflash construct. Knock-down of Pdcd4 in GEO and HT29 cells results in similar changes in cell morphology as well as the cellular functions including down-regulation of E-cadherin (Figures 1 and Supplementary Figure S2) (Wang et al., 2008). Thus, these two cell lines are interchangeable for the experiment of knocking-down E-cadherin. Since the transfection efficiency is very low for GEO cells, HT29 cells were used in the experiment of transiently knocking down E-cadherin. The luciferase activity in cells transfected with pRS-shEcad and TOPflash was approximately 2-fold higher than that in cells transfected with pRS-shGFP (a plasmid expressing GFP shRNA) and TOPflash (Figure 4a, filled bars). In contrast, co-transfection of pRS-shEcad or pRS-shGFP with FOPflash showed no significant difference in β-catenin/Tcf dependent transcription activity (Figure 4a, open bars), indicating that knocking down E-cadherin specifically activates β-catenin/Tcf dependent transcription rather than activates transcription in general.
Figure 4.
E-cadherin modulates β-catenin/Tcf dependent transcription in Pdcd4 knock-down cells. (a) Knock-down of E-cadherin activates β-catenin/Tcf dependent transcription in HT29 cells. One microgram of either E-cadherin shRNA (pRS-shEcad) or control shRNA (pRS-shGFP) was transfected into HT29 cells along with either 0.2 μg of TOPflash (filled bar) or FOPflash (open bar) plus 10 ng of pRL-SV40. The luciferase activity was normalized against Renilla activity, and the activity of cells transfected with pRS-shGFP plasmid is designated as 1.0. The asterisk denotes a significant difference compared with cells transfected with pRS-shGFP, as determined by one-way ANOVA (p < 0.001). (b) and (c) Over-expression of E-cadherin inhibits β-catenin/Tcf dependent transcription in Pdcd4 knock-down cells. Increasing amounts (0-0.1 μg) of pCMV6-Ecad plasmid were transfected into GEO-shPdcd4 cells with either 0.2 μg of TOPflash (b) or FOPflash (c) reporter as described in (a). The total DNA for each transfection was maintained at 0.3 μg by adding empty vector pcDNA3.1 DNA. The activity of cells transfected with either TOPflash or FOPflash but without pCMV6-Ecad plasmid is designated as 100%. The asterisk denotes a significant difference compared with cells transfected 0.1 μg of pCMV6-Ecad plasmid, as determined by one-way ANOVA (p < 0.005). (d) β-Galactosidase does not inhibit β-catenin/Tcf dependent transcription. One tenth microgram of pcDNA3.1, pCMV6-Ecad or pSV-β-gal plasmid was transfected into GEO-shPdcd4 cells with 0.2 μg of TOPflash plus 10 ng of pRL-SV40. The luciferase activity was normalized against Renilla activity, and the activity of the cells transfected with pcDNA3.1 is designated as 100%. The asterisk denotes a significant difference compared with cells transfected with pcDNA3.1, as determined by one-way ANOVA (p < 0.001). These experiments were repeated three times each with five independent transfections, and representative data are shown. Results are expressed as mean ± SD.
To further confirm the essential role of E-cadherin in inhibiting β-catenin/Tcf dependent transcription in Pdcd4 knock-down cells, an E-cadherin expression plasmid (pCMV6-Ecad) was co-transfected with TOPflash into GEO-shPdcd4 cells. Transient co-transfection of pCMV6-Ecad and TOPflash plasmids inhibited β-catenin/Tcf dependent transcription in a concentration dependent manner in Pdcd4 knock-down cells (Figure 4b). β-catenin/Tcf dependent transcription was inhibited approximately 60% when 0.1 μg of pCMV6-Ecad plasmid was transfected. On the contrary, no significant difference in β-catenin/Tcf dependent transcription was observed when pCMV6-Ecad was co-transfected with FOPflash (Figure 4c). Inhibition of β-catenin/Tcf dependent transcription by E-cadherin is specific since transient expression of β-galactosidase did not inhibit β-catenin/Tcf dependent transcription (Figure 4d). Therefore, activation of β-catenin/Tcf dependent transcription by Pdcd4 knock-down can be reversed by over-expressing E-cadherin. These results collectively indicate that down-regulation of E-cadherin is essential in activating β-catenin/Tcf dependent transcription in Pdcd4 knock-down cells.
Pdcd4 knock-down stimulates the expression of u-PAR and c-Myc
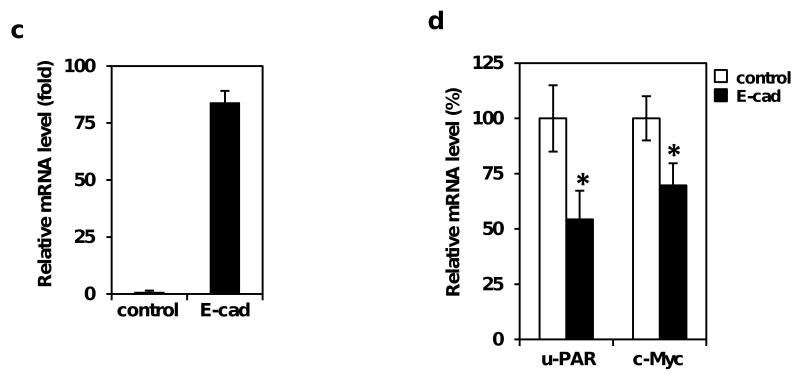
In order to understand how Pdcd4 knock-down promotes invasion, we evaluated some target genes of β-catenin/Tcf dependent transcription that are involved in colon tumor cell invasion. It has been reported that u-PAR and c-Myc are the target genes of β-catenin/Tcf dependent transcription (He et al., 1998; Mann et al., 1999). u-PAR is frequently over-expressed in the invasion front of colon cancer (Laufs et al., 2006), whereas c-Myc expression is elevated at both early and late stages of colon carcinogenesis (Smith et al., 1993). To test whether Pdcd4 knock-down elevates u-PAR and c-myc mRNAs, we performed quantitative real-time PCR (qPCR) using total RNAs isolated from GEO-shLacZ and GEO-shPdcd4 cells. As shown in Figure 5a, the u-PAR mRNA level in GEO-shPdcd4 cells was approximately 2.3-fold higher comparing to that in the GEO-shLacZ cells; this is in agreement with a previous report wherein Pdcd4 was transiently knocked-down (Leupold et al., 2007). In addition, the level of c-myc mRNA in GEO-shPdcd4 cells was about 1.7-fold higher comparing to that in GEO-shLacZ cells (Figure 5a). The protein levels of u-PAR and c-Myc in GEO-shPdcd4 cells were approximately 5- and 2.5-fold higher than that in GEO-shLacZ cells, respectively (Figure 5b). These results indicate that Pdcd4 knock-down stimulates the expression of u-PAR and c-Myc.
Figure 5.

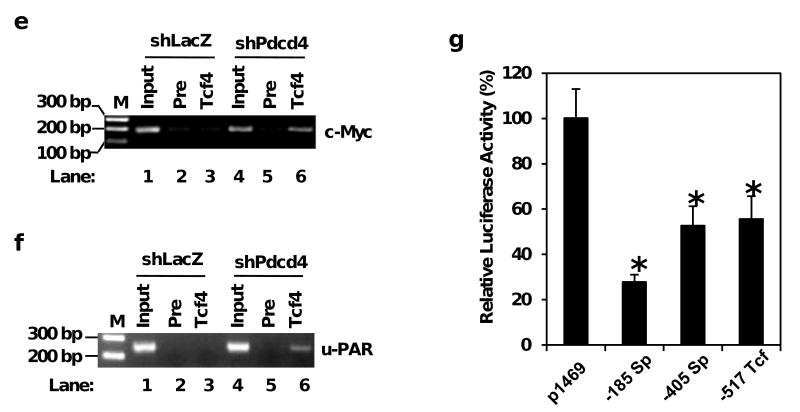
Pdcd4 knock-down stimulates the expression of u-PAR and c-Myc. (a) The mRNA levels of u-PAR, c-myc, and GAPDH were determined by qPCR using total RNA isolated from GEO-shLacZ and GEO-shPdcd4 cells. The ratio of u-PAR/GAPDH or c-myc/GAPDH in GEO-shLacZ cells is designated as 1.0. The asterisk indicates a significant difference, as determined by one-way ANOVA (p < 0.01). (b) The protein levels of u-PAR, c-myc, and GAPDH were determined by Western blotting analysis using cell lysates from GEO-shLacZ and GEO-shPdcd4 cells. The ratio of u-PAR/GAPDH and c-Myc/GAPDH in GEO-shLacZ cells is designated as 1. The number indicates the relative level of u-PAR or c-Myc protein. (c) and (d) Over-expression of E-cadherin inhibits u-PAR and c-Myc expression. Either pCMV6-Ecad (E-cad) or pcDNA3.1 (control) was transfected into GEO-shPdcd4 cells. After 72h, the total RNA was isolated and the mRNA levels of E-cadherin, u-PAR and c-myc were determined by qPCR. The ratio of E-cadherin/GAPDH in cells transfected with pcDNA3.1 is designated as 1 (c). The ratio of u-PAR/GAPDH or c-myc/GAPDH in cells transfected with pcDNA3.1 is designated as 100% (d). The asterisk denotes a significant difference, as determined by one-way ANOVA (p < 0.01). (e) and (f) The β-catenin/Tcf4 complex directly binds to the promoters of u-PAR and c-myc in Pdcd4 knock-down cells. ChIP assays were performed with cell lysates from either GEO-shLacZ or GEO-shPdcd4 cells using anti-Tcf4 antibody (Tcf4) or control IgG (Pre). ChIP enriched DNA and input samples (Input) analyzed with PCR using primers for amplifying β-catenin/Tcf4 binding site on promoters of c-myc (e) and u-PAR (f). PCR products were resolved onto 2% agarose gels. M indicates the DNA markers. (g) β-catenin/Tcf4 binding site mediates Pdcd4-regulated u-PAR promoter activity. Wild-type and mutated u-PAR promoter reporter constructs were transiently transfected into GEO-shPdcd4 cells. Luciferase activity was assayed 48 h post-transfection. The luciferase activity was normalized against Renilla activity, and the activity of cells transfected with pu-PAR-1469 plasmid is designated as 100%. These experiments were repeated three times each with five independent transfections, and representative data are shown. Results are expressed as mean ± SD. The asterisk denotes a significant difference compared with cells transfected with pRS-shGFP, as determined by one-way ANOVA (p < 0.001).
As over-expression of E-cadherin in Pdcd4 knock-down cells inhibited β-catenin/Tcf dependent transcription (Figure 4), over-expression of E-cadherin should repress the expression of u-PAR and c-Myc. To test this, we performed qPCR to determine the mRNA levels of u-PAR and c-myc in E-cadherin expression plasmid (E-cad) or empty vector (control) expressing GEO-shPdcd4 cells. As shown in Figure 5c, the mRNA level of E-cadherin was approximately 84-fold higher in the E-cad cells than that in the control cells. However, the mRNA levels of u-PAR and c-myc in E-cad cells decreased approximately 50% and 30%, respectively, compared with that in control cells (Figure 5d). These findings demonstrate that E-cadherin plays a key role in regulating u-PAR and c-Myc expression in Pdcd4 knock-down cells.
To determine the functional significance of β-catenin/Tcf complex in the elevated expression of u-PAR and c-Myc in Pdcd4 knock-down cells, we performed chromatin immunoprecipitation (ChIP) assays to examine the binding of the β-catenin/Tcf complex to the u-PAR and c-myc promoters. We have demonstrated that β-catenin binds with Tcf4 in the nucleus of Pdcd4 knock-down cells (Wang et al., 2008). We therefore used Tcf4 antibody in the ChIP assay and the primers specifically amplify the β-catenin/Tcf4 binding site on the u-PAR and c-myc promoters at position -517 bp and -1001 bp, respectively, upstream of translation initiation codon ATG. The input chromatin before immunoprecipitation was used as a control in the PCR reaction. A high level of PCR products (185 bp) was observed using Tcf4 antibody precipitated chromatin from lysates of GEO-shPdcd4 cells (Figure 5e, lane 6). However, using Tcf4 antibody precipitated chromatin from GEO-shLacZ cell lysates, the same primers only generated a low level of PCR products (Figure 5e, lane 3). A similar background level of PCR products was also observed using the pre-immune serum precipitated chromatin (Figure 5e, lanes 2 and 5), thus this background might be due to the non-specific binding of the magnetic beads used in the immunoprecipitation. Similarly, when u-PAR specific primers were used, a 247 bp DNA fragment was generated from lysates of GEO-shPdcd4 cells (Figure 5f, lane 6), but only a background level of PCR product was generated from the lysates of GEO-shLacZ cells (Figure 5f, lane 3). These results indicate that Pdcd4 knock-down leads to the binding of β-catenin/Tcf4 complex to the promoters of u-PAR and c-myc to stimulate their expression.
Sp and Tcf4 binding sites regulate u-PAR promoter activity
We have shown that the Sp binding sites located at -185 bp and -405 bp relative to the translational initiation codon of the u-PAR promoter mediate Pdcd4-regulated u-PAR expression (Leupold et al., 2007). To access the relative strength of Sp binding motifs and Tcf4 binding site in mediating Pdcd4 regulation of u-PAR promoter activity, we mutated these motifs individually and used in the reporter assays. Using pu-PAR-1469 (wild-type) as the template, Sp binding sites at -185 bp (CGCGGCC) and -405 bp (AGGAGGG), and the Tcf4 binding site at -517 bp (CTTTGCT) were mutated to AGAGGAA, ATTATTT, and CTGTCGC, respectively. The mutated and wild-type promoter constructs were then transfected into GEO-shPdcd4 cells. As shown in Figure 5g, the mutation of Sp site at -185 bp (-185 Sp) decreased the u-PAR promoter activity to 30% of wild-type promoter while mutation of Sp site at -405 (-405 Sp) and Tcf4 site at -517 (-517 Tcf) reduced the u-PAR promoter activity about 50%. These results suggest that Tcf4 binding site along with two Sp motifs are mediators for Pdcd4-regulated u-PAR promoter activity.
Pdcd4 knock-down induced invasion promotion can be reversed by knocking down u-PAR or c-Myc
To test whether elevated u-PAR and c-Myc expression contributes to the promotion of invasion in Pdcd4 knock-down cells, we knocked down u-PAR or c-Myc by transient transfection of u-PAR or c-myc siRNA into GEO-shPdcd4 cells. After 7 days, the u-PAR or c-Myc knock-down cells were then assayed for the capacity to pass through a Matrigel barrier in a modified Boyden chamber assay using FBS (0.5%) and EGF (20 ng/ml) as attractants. As shown in Figure 6a, u-PAR knock-down (si-u-PAR) and c-Myc knock-down (si-Myc) cells displayed about 50% and 80% reduction of u-PAR and c-Myc expression, respectively. The ability of si-u-PAR and si-Myc cells to invade through Matrigel was reduced to 50% and 30% of that in control cells, respectively (Figure 6b). These results indicate that both u-PAR and c-Myc contribute to the invasion induced by Pdcd4 knock-down, providing molecular explanation of how Pdcd4 suppresses tumor invasion.
Figure 6.
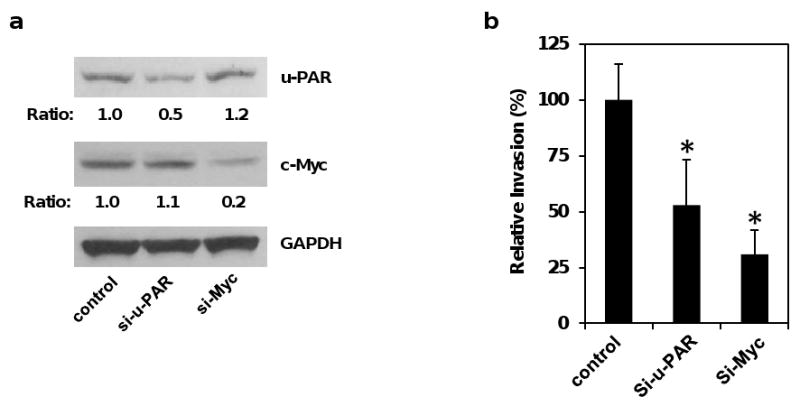
Knock-down of u-PAR or c-Myc inhibits invasion in Pdcd4 knock-down cells. (a) Knock-down of u-PAR and c-Myc in GEO-shPdcd4 cells. The u-PAR and c-Myc expression was knocked down by transfection with u-PAR siRNA and c-myc siRNA, respectively. The u-PAR and c-Myc protein levels from control siRNA (control), u-PAR siRNA (si-u-PAR), and c-myc siRNA (si-Myc) expressing cells were determined by Western blot using antibodies against u-PAR, c-Myc, and GAPDH. The ratio of u-PAR/GAPDH and c-Myc/GAPDH in control cells is designated as 1. The number indicates the relative protein level of u-PAR or c-Myc. (b) Knock-down of u-PAR and c-Myc inhibits invasion. Matrigel invasion assays were performed with the indicated cells for 48 h using FBS (0.5%) and EGF (20 ng/ml) as attractants. Each value is the mean ± SD of three independent experiments. The asterisk denotes a significant difference compared with control cells as determined by one-way ANOVA (P < 0.05).
Discussion
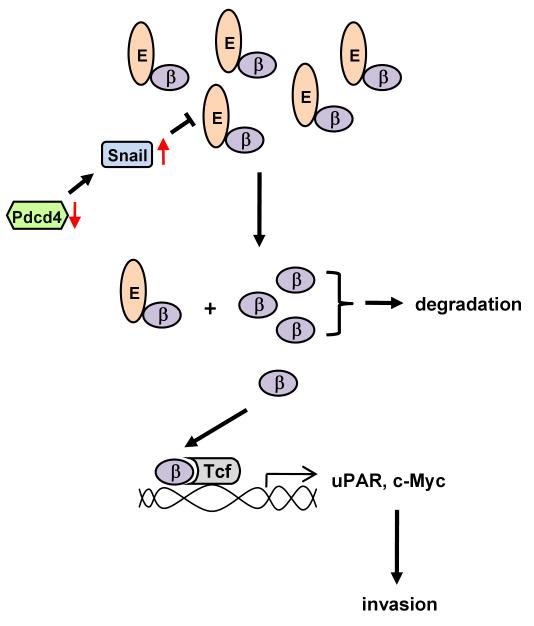
Our previous study showed that Pdcd4 knock-down activates β-catenin/Tcf dependent transcription and promotes invasions in colon tumor cells (Wang et al., 2008). In this study, we demonstrated that Pdcd4 knock-down up-regulates Snail resulting in down-regulation of E-cadherin expression. In addition, we showed that down-regulation of E-cadherin is a key event for activating β-catenin/Tcf dependent transcription. Activation of β-catenin/Tcf dependent transcription subsequently leads to the expression of invasion promoting genes, u-PAR and c-myc. Thus, our previous (Wang et al., 2008) and present studies collectively reveal the mechanism by which Pdcd4 knock-down promotes invasion in colon tumor cells. Our model shows that (1) Pdcd4 knock-down leads to down-regulation of E-cadherin through up-regulating Snail expression, (2) decreased E-cadherin expression changes cell morphology and releases β-catenin, and (3) the free β-catenin then translocates into the nucleus, binds to Tcf4 transcription factors, and activates β-catenin/Tcf dependent transcription to stimulate the expression of u-PAR and c-Myc, which promote tumor cell invasion (Figure 7).
Figure 7.
A model depicting the pathway involved in invasion promotion caused by Pdcd4 knock-down. See text for details. E: E-cadherin; β: β-catenin.
β-catenin is an important mediator for Wnt/β-catenin signaling pathway. Deregulation of the Wnt/β-catenin signaling pathway occurs in approximately 90% of colorectal cancer patients (Vogelstein and Kinzler, 2004). Given that E-cadherin is a binding partner of β-catenin, a decrease in E-cadherin expression results in an increase of free β-catenin. Non-sequestered free β-catenin is rapidly phosphorylated by GSK-3β in the APC-axin-GSK-3β-CKI complex, and subsequently degraded by the ubiquitin-proteasome pathway. Mutations in the proteins of APC-axin-GSK-3β-CKI complex prevent proteasomal degradation of β-catenin, thereby leading to translocation of β-catenin into the nucleus and activation of β-catenin/Tcf dependent transcription (Gregorieff and Clevers, 2005). Our data show that Pdcd4 knock-down led to an approximate 70% decrease in β-catenin protein levels in GEO cells (Figure 1a). Although β-catenin level is dramatically decreased, β-catenin is still able to translocate into the nucleus (Supplementary Figure S1), and activate β-catenin/Tcf dependent transcription (Figure 1b) in GEO cells with Pdcd4 knock-down. These results suggest that a small portion of non-sequestered β-catenin escapes proteasomal degradation and translocates into nuclei. Meanwhile, these results also indicate that the APC-axin-GSK-3β-CKI complex is, at least in part, functional in GEO cells. It is noteworthy that no mutation was found in exon 15 of the APC gene in GEO cells (Wang and Yang, unpublished data), since exon 15 of the APC gene is highly mutated in colon cancer patients (Miyaki et al., 1994). However, it is unknown whether other exons of APC in GEO cells contain mutations.
Clinical and experimental studies have shown that E-cadherin expression is frequently down-regulated during colon cancer progression (Birchmeier and Behrens, 1994). E-cadherin can be down-regulated through promoter methylation and/or transcriptional repression. Several transcriptional repressors have been identified to inhibit E-cadherin expression including zinc finger factors Snail and Slug, ZEB1/2, and basic helix-loop-helix transcription factors Twist (Peinado et al., 2007; Yang et al., 2004b) Snail and E-cadherin expression is inversely correlated in different carcinoma and melanoma cell lines (Cano et al., 2000; Poser et al., 2001). Over-expression of Snail in epithelial cells promotes invasion (Cano et al., 2000). Conversely, Knock-down of Snail up-regulates E-cadherin expression and inhibits invasion (Olmeda et al., 2007). These findings indicate that Snail is a key transcription factor for regulation of E-cadherin expression. Our findings showed that Pdcd4 knock-down up-regulates Snail expression and knock-down of Snail activates E-cadherin promoter activity (Figure 2) indicating that Snail mediates the down-regulation of E-cadherin by Pdcd4 knock-down. These findings provide a mechanism of how knock-down of Pdcd4 down-regulates E-cadherin expression. Pdcd4 is able to bind with translation initiation factor eIF4A and inhibits protein translation (Yang et al., 2004a; Yang et al., 2003a). Thus, it is possible that Pdcd4 translationally regulates Snail expression. Whether Pdcd4 translationally regulates Snail expression needs to further investigate.
Our results indicate that Pdcd4 regulates β-catenin/Tcf dependent transcription through modulating E-cadherin expression (Figures 3 and 4). How does activation of β-catenin/Tcf dependent transcription promote invasion in Pdcd4 knock-down cells? It is well known that many target genes of β-catenin/Tcf dependent transcription are involved in cell migration, invasion, and proliferation, including u-PAR and c-Myc (He et al., 1998; Mann et al., 1999). u-PAR, a 45-60 kD glycosylated membrane protein, exhibits elevated protein levels in advanced tumors that correlate with tumor progression (Memarzadeh et al., 2002; Nestl et al., 2001). Indeed, over-expression of u-PAR cDNA in human osteosarcoma cells enhances invasion (Kariko et al., 1993), whereas antisense-mediated down-regulation of u-PAR expression suppresses invasion (Ahmed et al., 2003). By binding to its ligand, uPA, u-PAR triggers the degradation of extracellular matrix components, thereby resulting in the promotion of invasion and metastasis. c-Myc is a transcription factor that activates genes involved in cell growth, protein synthesis, and mitochondrial function. In addition, c-Myc represses expression of genes that are involved in anti-proliferation and anti-metastasis (O'Connell et al., 2003). We demonstrated that both u-PAR and c-myc are up-regulated in the GEO-shPdcd4 cells (Figure 5). Over-expression of E-cadherin in Pdcd4 knock-down cells inhibits u-PAR and c-Myc expression (Figure 5), suggesting that down-regulation of E-cadherin contributes to the stimulation of u-PAR and c-Myc expression in Pdcd4 knock-down cells. In addition, up-regulation of u-PAR and c-Myc is achieved by direct binding of the β-catenin/Tcf4 complex to the u-PAR and c-myc promoters (Figure 5). Indeed, when knock-down of u-PAR or c-Myc expression in GEO-shPdcd4 cells inhibit invasive capacity (Figure 6). These observations suggest that Pdcd4 knock-down promotes invasion, at least in part, through stimulating u-PAR and c-Myc expression. In previous studies, it was shown that Pdcd4 regulates u-PAR gene expression via Sp1/Sp3 (Leupold et al., 2007). Our promoter analyses and ChIP assays (Figure 5) demonstrate for the first time that the β-catenin/Tcf4 complex directly binds to the u-PAR promoter and regulates u-PAR promoter activity in GEO-shPdcd4 cells. In addition, the β-catenin/Tcf4 binding site along with two Sp motifs mediates the Pdcd4-regulated u-PAR promoter activity. Whether or how Sp1/Sp3 and β-catenin/Tcf4 transcription factors cooperatively regulate u-PAR expression in colon tumor cells need to be further investigated.
In summary, our results reveal that down-regulation of E-cadherin is an important event for activating β-catenin/Tcf dependent transcription and expressing u-PAR and c-Myc in Pdcd4 knock-down cells, thus providing molecular explanation of how Pdcd4 suppresses tumor invasion in colon cancer cells.
Materials and Methods
Cell culture
GEO (kindly provided by Dr. Douglas Boyd, MD Anderson Cancer Center) and HT29 (ATCC, Manassas, VA, USA) cells were grown in McCoy's medium containing 10% FBS, 2 mM L-glutamine, and 500 U/ml penicillin-streptomycin. LoVo (ATCC) cells were grown in RPMI-1640 medium containing 10% FBS, 2 mM L-glutamine, and 500 U/ml penicillin-streptomycin. Cells were incubated at 37°C with 5% CO2 in a humidified incubator.
Matrigel invasion assay
Matrigel invasion assay was carried out as described previously (Yang et al., 2006). FBS (0.5%) and EGF (20 ng/ml) was used as attractant and added to the lower well. After 48 h, the number of cells that had traversed the filter into the lower compartment was determined.
Western blot analysis
Aliquots containing 20 or 40 μg of protein were separated on a SDS-PAGE, and transferred to nitrocellulose membranes as described previously (Yang et al., 2001). Subsequently, the membrane was incubated with antibodies against E-cadherin (Santa Cruz Biotechnology, Santa Cruz, USA), β-catenin (Chemicon, Temecula, CA, USA), Pdcd4 (Wang et al., 2008), c-Myc (Santa Cruz Biotechnology), u-PAR (Santa Cruz Biotechnology), GAPDH (Cell Signaling, Danvers, MA, USA), or Snail (Cell Signal) followed by horseradish peroxidase-linked secondary antibody. The target protein was visualized by chemiluminescence (Pierce, Rockford, IL, USA).
Transient transfection and luciferase activity assays
Cells (3 × 104 cells/well in 24-well plates) were transiently transfected with 0.2 μg of TOPflash or FOPflash (Upstate, Lake Placid, NY, USA) along with 10 ng of pRL-SV40 (Promega, Madison, WI, USA) by using TransIT-LT1 reagent (Mirus, Madison, WI, USA). After 48 h, the cells were lysed and the luciferase activity was determined as described previously (Wang et al., 2008). The luciferase activity of each sample is normalized against Renilla luciferase activity for monitoring transfection efficiency. Various amounts of E-cadherin expression plasmid (pCMV6-Ecad, Origene, Rockville, MD, USA), E-cadherin shRNA plasmid (pRS-shEcad, Origene), β-galactosidase expression plasmid (pSV-β-gal, Promega), and Pdcd4 expression plasmid (pcDNA-Pdcd4) were co-transfected with TOPflash or FOPflash reporter (as indicated in the corresponding figure legends).
For over-expression of E-cadherin, 10 μg of E-cadherin expression plasmid was transiently transfected into GEO-shPdcd4 cells (5 × 105) using Fugene 6 reagent (Roche Applied Science, Indianapolis, IN, USA). After 72 h, cells were collected for qPCR. For over-expressing Pdcd4, LoVo cells were infected with lentivirus particles containing Pdcd4 expression plasmid at MOI=5 as described previously (Yang et al., 2006)
qPCR
The total RNA isolation and qPCR were performed as described previously (Wang et al., 2008). The primers used for amplifying E-cadherin, u-PAR, c-myc, and GAPDH were purchased from SA Biosciences (Frederick, MD, USA).
ChIP assay
GEO-shLacZ and GEO-shPdcd4 cells were grown to 70-80% confluency and fixed with formaldehyde. ChIP assay was performed using ChIP-IT™ Express kit according to the manufacturer's instruction (Active Motif, Carlsbad, CA, USA). The Tcf4 antibody (Santa Cruz) was used to precipitate β-catenin/Tcf-DNA complex and the pre-immune rabbit serum was used as the negative control. The sequence of primers for amplifying u-PAR and c-myc promoters is available upon request. The input DNA before immunoprecipitation was used as a control in the PCR reaction. PCR products were analyzed on 2% agarose/Tris-borate EDTA gels.
Site-specific Mutagenesis
The Sp and Tcf4 binding sites on u-PAR promoter were mutated using pu-PAR-1469 as the template. The E-box sites on E-cadherin promoter were mutated using E7 construct as template. The site-specific mutagenesis was performed using QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to manufacturer's instruction.
Knock-down of Snail, u-PAR, and c-Myc
GEO-shPdcd4 cells (2 × 105 cells on a 60-mm plate) were transfected with 6 μl of 10 μM snail siRNA (Santa Cruz Biotechnology), u-PAR siRNA (Santa Cruz Biotechnology), or c-myc siRNA (Santa Cruz Biotechnology) using siRNA Transfection Reagent (Santa Cruz Biotechnology) according to manufacturer's protocol. After 7 days, cells were collected for Western blot, transfection, or Matrigel invasion assays.
Supplementary Material
Acknowledgments
We thank Dr. Ji Hshiung Chen (Tzu Chi University, Taiwan) for kindly providing E-cadherin promoter and Dr. Xiaoling Guo for technical support. This work was supported by NCI grant R01CA129015, ACS Institutional Research Grant IRG-85-001-19 and NCRR CORBE pilot grant P20RR020171.
References
- Ahmed N, Oliva K, Wang Y, Quinn M, Rice G. Downregulation of urokinase plasminogen activator receptor expression inhibits Erk signalling with concomitant suppression of invasiveness due to loss of uPAR-beta1 integrin complex in colon cancer cells. Br J Cancer. 2003;89:374–84. doi: 10.1038/sj.bjc.6601098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bitomsky N, Wethkamp N, Marikkannu R, Klempnauer KH. siRNA-mediated knockdown of Pdcd4 expression causes upregulation of p21(Waf1/Cip1) expression. Oncogene. 2008;27:4820–9. doi: 10.1038/onc.2008.115. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Ougolkov AV, Spiegelman VS, Minamoto T. Oncogenic beta-catenin signaling networks in colorectal cancer. Cell Cycle. 2005;4:1522–39. doi: 10.4161/cc.4.11.2129. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–90. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hilliard A, Hilliard B, Zheng SJ, Sun H, Miwa T, Song W, et al. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol. 2006;177:8095–102. doi: 10.4049/jimmunol.177.11.8095. [DOI] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–41. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- Kariko K, Kuo A, Boyd D, Okada SS, Cines DB, Barnathan ES. Overexpression of urokinase receptor increases matrix invasion without altering cell migration in a human osteosarcoma cell line. Cancer Res. 1993;53:3109–17. [PubMed] [Google Scholar]
- Kemler R. Classical cadherins. Semin Cell Biol. 1992;3:149–55. doi: 10.1016/s1043-4682(10)80011-x. [DOI] [PubMed] [Google Scholar]
- Laufs S, Schumacher J, Allgayer H. Urokinase-receptor (u-PAR): an essential player in multiple games of cancer: a review on its role in tumor progression, invasion, metastasis, proliferation/dormancy, clinical outcome and minimal residual disease. Cell Cycle. 2006;5:1760–71. doi: 10.4161/cc.5.16.2994. [DOI] [PubMed] [Google Scholar]
- Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007 doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277–90. doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–8. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarzadeh S, Kozak KR, Chang L, Natarajan S, Shintaku P, Reddy ST, et al. Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc Natl Acad Sci U S A. 2002;99:10647–52. doi: 10.1073/pnas.152127499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994;54:3011–20. [PubMed] [Google Scholar]
- Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- Nestl A, Von Stein OD, Zatloukal K, Thies WG, Herrlich P, Hofmann M, et al. Gene expression patterns associated with the metastatic phenotype in rodent and human tumors. Cancer Res. 2001;61:1569–77. [PubMed] [Google Scholar]
- O'Connell BC, Cheung AF, Simkevich CP, Tam W, Ren X, Mateyak MK, et al. A large scale genetic analysis of c-Myc-regulated gene expression patterns. J Biol Chem. 2003;278:12563–73. doi: 10.1074/jbc.M210462200. [DOI] [PubMed] [Google Scholar]
- Olmeda D, Jorda M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 2007;26:1862–74. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- Ozanne BW, McGarry L, Spence HJ, Johnston I, Winnie J, Meagher L, et al. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur J Cancer. 2000;36:1640–8. doi: 10.1016/s0959-8049(00)00175-1. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Akar U, Steiner M, Zorrilla-Calancha I, Tirado-Gomez M, Colburn N, et al. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid-induced terminal granulocytic differentiation of human myeloid leukemia cells. Mol Cancer Res. 2007;5:95–108. doi: 10.1158/1541-7786.MCR-06-0125. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem. 2001;276:24661–6. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68:1254–60. doi: 10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- Smith DR, Myint T, Goh HS. Over-expression of the c-myc proto-oncogene in colorectal carcinoma. Br J Cancer. 1993;68:407–13. doi: 10.1038/bjc.1993.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene. 2008;27:1527–35. doi: 10.1038/sj.onc.1210793. [DOI] [PubMed] [Google Scholar]
- Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004a;24:3894–906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003a;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–76. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003b;22:3712–20. doi: 10.1038/sj.onc.1206433. [DOI] [PubMed] [Google Scholar]
- Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, et al. Tumorigenesis suppressor Pdcd4 down-regulates MAP4K1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004b;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.