Abstract
The tectorial membrane (TM) is widely believed to play an important role in determining the ear's ability to detect and resolve incoming acoustic information. While it is still unclear precisely what that role is, the TM has been hypothesized to help overcome viscous forces and thereby sharpen mechanical tuning of the sensory cells. Lizards present a unique opportunity to further study the role of the TM given the diverse inner-ear morphological differences across species. Furthermore, stimulus-frequency otoacoustic emissions (SFOAEs), sounds emitted by the ear in response to a tone, noninvasively probe the frequency selectivity of the ear. We report estimates of auditory tuning derived from SFOAEs for 12 different species of lizards with widely varying TM morphology. Despite gross anatomical differences across the species examined herein, low-level SFOAEs were readily measurable in all ears tested, even in non-TM species whose basilar papilla contained as few as 50–60 hair cells. Our measurements generally support theoretical predictions: longer delays/sharper tuning features are found in species with a TM relative to those without. However, SFOAEs from at least one non-TM species (Anolis) with long delays suggest there are likely additional micromechanical factors at play that can directly affect tuning. Additionally, in the one species examined with a continuous TM (Aspidoscelis) where cell-to-cell coupling is presumably relatively stronger, delays were intermediate. This observation appears consistent with recent reports that suggest the TM may play a more complex macromechanical role in the mammalian cochlea via longitudinal energy distribution (and thereby affect tuning). Although significant differences exist between reptilian and mammalian auditory biophysics, understanding lizard OAE generation mechanisms yields significant insight into fundamental principles at work in all vertebrate ears.
Introduction
The tectorial membrane (TM), a gelatinous ribbon sitting atop the sensory cells of the inner ear, is widely believed to play a critical role in producing the ear's remarkable sensitivity and selectivity (1). This latter feature, also referred to as sharpness of tuning, determines the ear's ability to resolve different frequency components of incoming sound. However, the precise functional role of the TM is still not well known due to the fragility and complexity of the inner ear. A traditional point of view posits that the mammalian TM provides an additional mechanical resonance in the cochlea and thereby a means for sharpened tuning (2,3), a prediction supported by direct empirical observation (4). More-recent reports, both theoretical and experimental, have indicated additional possible roles the TM plays. Several theoretical studies have suggested the TM contributes toward sharper tuning at the level of the stereovillar bundle by counteracting viscous forces (5,6). Other recent studies have examined the role the TM plays in coupling responses across groups of hair cells (7–9) and longitudinally along the mammalian cochlea (10), indicating that such coupling is likely important. Observations in a genetic knockout mouse model have suggested that mutations to the underlying fibrillar network of the mammalian TM can have large consequences for longitudinal coupling in the cochlea and thereby lead to changes in sharpness of tuning (11).
Remarkably, the ear not only responds to sound, but somehow in the process of forward transduction (i.e., converting mechanical sound stimuli to electrical signals at the level of the auditory nerve), the inner ear generates and subsequently emits sound. These sounds, known as otoacoustic emissions (OAEs), are emitted from the ear either spontaneously or via an evoking stimulus, and provide a noninvasive window into the function of the peripheral auditory system. Emissions have been demonstrated to be present in a wide variety of species (12,13), including nonvertebrates (14). Furthermore, the development of extensive clinical OAE applications have been of great value to audiologists (15), motivating further study of the underlying generation mechanisms. Spontaneous otoacoustic emissions (SOAEs) present compelling (but not definitive) evidence for active mechanisms at work inside the inner ear that lead to an amplification of low-level stimuli (e.g., (16)). Stimulus frequency emissions (SFOAEs), evoked using a single tone and occurring at that same stimulus frequency, have been suggested as a means to noninvasively probe the sharpness of tuning (or bandwidths) of the underlying auditory filters inside the ear (17,18). Specifically, the SFOAE phase-gradient delays, expressed nondimensionally as NSF (see Methods), are hypothesized to reflect build-up time toward the steady-state response of the underlying filters. Theoretical models for the mammalian (19) and lizard (20) ear provide a foundation for such a correlation, indicating a proportionality between phase-gradient delays associated with low-level SFOAEs and filter bandwidths. Specifically, the larger the emission delay, the sharper the tuning.
This study utilizes the morphological diversity of the inner ear across the Lacertilia (lizards) to noninvasively probe functional consequences of TM structure (or lack thereof). Lizards exhibit robust emissions (13,21–24), in addition to wide variations in inner ear structure (25). As put forth by Manley, the lizard inner ear represents “a playground of evolution” (26) and differs significantly from mammals in that lizard ears lack a traveling wave that propagates along the basilar membrane (27,28). Lizards have been described as having two populations of different hair cell types (29):
Type I—Cells whose stereovillar bundles are unidirectionally oriented, sensitive to frequencies <∼1 kHz (30,31), and have a continuous overlying TM.
Type II—Cells whose bundles are bidirectionally oriented, responsive to frequencies >1 kHz, and have a diverse TM morphology (differing across species).
The latter cell group typically comprise the majority of the basilar papilla (e.g., ∼70–90% for iguanids) and are the focus of this study. We diverge somewhat from the convention of Wever (25) and Miller (29) with regard to defining the TM. In their framework, a TM refers to a structure that is attached at another anchoring point (i.e., the limbic lip). Here, we refer to any structure composed of a gelatinous/fibrillar matrix that resides directly atop the sensory cells as a TM, regardless of whether there is a secondary attachment point.
Lizard TM morphology comes in many different forms. A continuous TM attached to the limbic lip is present in some families over the entirety of the papilla (e.g., teiids), superficially similar to that of mammals. Several lizard families have, essentially, what amounts to a discretized TM (sallets), such as in skinks, geckos, and gerrhosaurids. While unconnected to the limbic lip, there are interconnecting processes coupling adjacent sallets (25), but the functional significance of these connections is unknown. One possibility is that the connections introduce a small degree of longitudinal coupling that might improve sensitivity (28) at the sacrifice of selectivity (32). Other families lack a TM altogether over the bulk of the papilla, such as iguanids and anguids. Much of this diversity in TM morphology has been proposed to stem from various selection pressures (26,32). It is worth noting that a previous study has taken a similar approach to that here: utilizing OAEs and the diversity of TM morphology in nonmammals to infer filtering properties of the TM with respect to certain types of emissions (33). However, that work differs significantly from ours in that it was focused upon how the TM might act as a bandpass filter in producing distortion product emissions (DPOAEs), OAEs evoked by the simultaneous presentation of two tones. Another study (34) also examined the effects of TM detachment upon DPOAEs in the mammalian cochlea via a genetically modified mouse model.
The specific goal of this study is to make a broad comparison of OAE-derived estimates of tuning across lizards with known differences in TM morphology. Theoretical studies have hypothesized that the presence of a TM, which presumably plays a role in how the hair cells are coupled, can provide sharpened tuning to the underlying auditory filters (5,6,8). However, to our knowledge, there is little direct empirical verification of such, aside from comparisons of auditory nerve fiber (ANF) responses across various different studies (e.g., (35,26)). Assuming SFOAEs provide an objective measure of peripheral auditory tuning (17,20), we hypothesize that non-TM species would exhibit shorter SFOAE delays relative to those species with a TM. We systematically test such a prediction by examining 12 different lizard species, spanning across eight different families and four infraorders. Salient morphological features for all species are summarized in Table 1. Additionally, Figs. 1–3 contain highly simplified schematics to illustrate the TM structure over the bidirectional portion of the papilla (type II hair cells) for the indicated species. For the sake of clarity, the results initially focus on three species (Anolis carolinensis, Gerrhosaurus flavigularis, and Aspidoscelis tigris) in order to demonstrate results from non-TM, salletal, and continuous TM papillae, respectively. Data from several other species can be found in the Supporting Material.
Table 1.
Species examined in this study
| Anatomical parameters |
||||
|---|---|---|---|---|
| Species (common name) | Family | TM type (≥1 kHz) | Papilla length [mm] | No. of hair cells |
| Anolis carolinensis (green anole) | Po | None | 0.45 (0.5) | 160 (182) |
| Aspidoscelis tigris† (whiptail lizard) | Te | Continuous | 0.65 | 370 (465) |
| Callisaurus draconoides† (zebra-tail lizard) | Ph | None | (0.2) | 65 (73) |
| Elgaria multicarinata† (Southern alligator lizard) | An | None | 0.4 | 160 |
| Eublepharis macularius∗ (leopard gecko) | Gk | Sallets and continuous | 1.25 | 970 |
| Eumeces schneideri (Schneider's skink) | Sk | Sallets | ? | 500? |
| Gekko gecko∗ (Tokay gecko) | Gk | Sallets and continuous | 1.8 | 1620 (2100) |
| Gerrhosaurus flavigularis (yellow-throated plated lizard) | Gr | Sallets | 0.8? | 530 |
| Pogona vitticeps (bearded dragon) | Ag | ? | ? | ? |
| Sceloporus magister† (desert spiny lizard) | Ph | None | 0.35 (0.35) | 80 (90) |
| Urosaurus ornatus† (ornate tree lizard) | Ph | None | 0.29? | 55 |
| Uta stansburiana† (common side-blotched lizard) | Ph | None | 0.22? (0.2) | 52 (55) |
Cited values are from Wever (25) and Miller (64) (the latter in parentheses). Where unknown, inferences based upon similar species are included (designated via the notation “?”). Family abbreviations as follows: Ag, Agamidae; Po, Polychrotidae; Ph, Phrynosomatidae; An, Anguidae; Sk, Scincidae; Gr, Gerrhosauridae; Gk, Gekkonidae; and Te, Teiidae. Families Ag, Po, and Ph all fall within infraorder Iguania. The designations non-TM, salletal, and continuous TM are meant simply to indicate the morphology of the TM over the majority of the papilla (i.e., for the bidirectional hair cells). All species except Eumeces have a continuous TM attached to the limbic lip overlying the portion of the papilla sensitive to frequencies <1 kHz (see (26,32)). For Eumeces, the TM over the low frequency portion of the papilla is unconnected to the limbic lip (46). Note that for clarity, the TM morphologies listed here are a simplification; see the literature (25,29) for more detailed descriptions. Total hair cell counts in the last column are per ear.
Species data from a previous study (13).
Species data from animals that were locally native/wild-caught.
Figure 1.
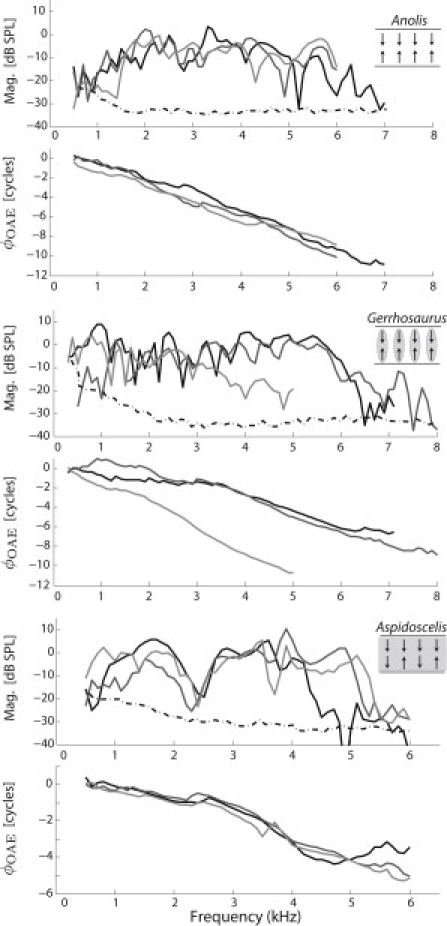
Low-level SFOAE magnitude and phase (φOAE) for three representative individuals from each of three species chosen to highlight differences in TM morphology. (Top) Non-TM (Anolis); (middle) sallets (Gerrhosaurus); (bottom) continuous TM (Aspidoscelis). A guiding schematic is included. (Shading) Presence of the TM. (Arrows) Bundle polarization (direction from shortest to tallest villi) for radially clustered groups of hair cells (note the variability in orientation for Aspidoscelis). Different individuals are illustrated by different line shadings. SFOAE magnitude and phase (φOAE) were evoked using a 20 dB SPL tone. Error bars have been excluded for clarity (see Supporting Material). Stimulus conditions and steady-state body temperatures (∼32–33°C) were identical for all curves. (Dashed-dotted line) Approximate noise floor.
Figure 2.
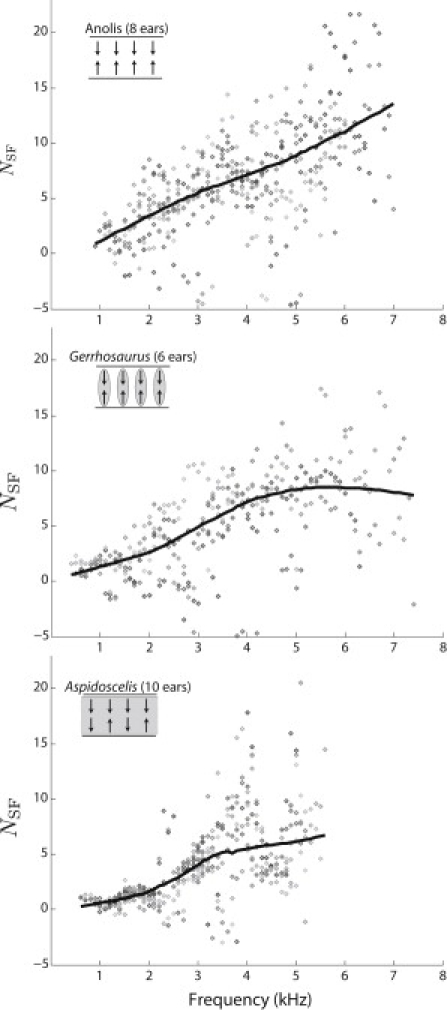
Low-level SFOAE phase-gradient delays, expressed in dimensionless form (NSF). SFOAEs were evoked using a 20-dB SPL stimulus level with body temperature stable at ∼32–33°C. Only points whose magnitudes were at least 10 dB above the acoustic noise floor were included. Similar to Fig. 1, different shadings represent different individuals. While significant spread is present, trends within a given species are apparent. (Solid lines) Locally weighted regression (loess) trend.
Figure 3.
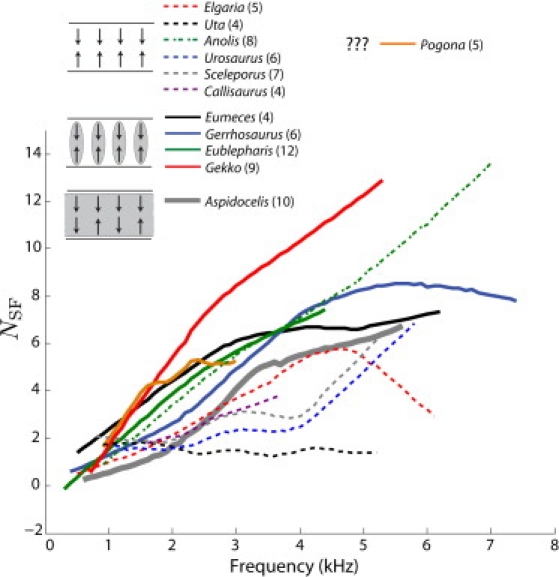
Comparison of NSF across 12 different species. Species lacking a TM over the majority of their papilla are denoted by dashed lines whereas TM-species are denoted by a solid line. Non-TM exhibit smaller NSF values than those with a TM for frequencies >∼1–2 kHz. All data were obtained using a 20-dB SPL stimulus level with body temperature stable at ∼32–33°C. For a given species, the illustrated curve was obtained via a locally weighted regression (see Fig. 2). The number of individual ears included is specified in parentheses. Only points whose magnitudes were at least 10 dB above the acoustic noise floor were included. Note that the inner ear morphology for Pogona is presently unknown.
Methods
All measurements reported in this study were obtained using the same stimulus paradigms, acquisition codes, and OAE probe for all species/individuals (13). A desktop computer housed a 24-bit soundcard (Lynx TWO-A; Lynx Studio Technology, Costa Mesa, CA), whose synchronous root mean-square input/output was controlled using a custom data-acquisition system. A sample rate of 44.1 kHz was used to transduce signals to/from an ER-10C probe containing a microphone and two earphones (Etymotic, Elk Grove Village, IL). The microphone response was amplified by 40 dB and high-pass-filtered with a cutoff frequency of 0.41 kHz to minimize the effects of noise. The OAE probe was coupled to the external ear using a short tube attached to the foam tip and sealed to the head using vaseline or silicone grease. This ensured a tight (closed) acoustic coupling and minimized low-frequency losses. The probe tip was ∼0.5–1.25 cm from the tympanic membrane. The probe earphones were calibrated in situ by presenting flat-spectrum, random-phase noise. By computing the ratio of response to that of the output signal, the frequency response and associated delays could be determined. Calibrations were verified repeatedly throughout the experiment. All stimulus frequencies were quantized such that an integral number of cycles were contained within the sampling window.
To evoke the SFOAEs, a low probe level (Lp = 20 dB SPL) was chosen for several reasons:
First and foremost, this level was large enough to evoke a detectable emission with suitable signal/noise ratio in all species examined and was low enough such that the SOAE activity was not suppressed by the stimulus tone (see Supporting Material).
Second, SFOAE phase-gradient delays (defined below) are relatively insensitive to stimulus level at these lower intensities, whereas moderate and higher level stimuli exhibit significantly smaller NSF values (e.g., (36,13)), consistent with the general observation of broadened tuning in ANF responses at higher stimulus levels.
Third, as previously reported (22), lizard OAEs evoked using a relatively low stimulus level are highly dependent upon the physiological state of the animal (e.g., hypoxia) while those evoked at higher levels are less sensitive to such. Thus, emissions generated via low-level stimuli are presumably more critically tied to active mechanisms at work in the inner ear.
Fourth, given that there is evidence for multiple OAE generation sources in at least certain types of lizard ears (13), a low stimulus level helps minimize the potentially confounding factor of (nonlinear) source-mixing from different generation mechanisms (37).
Fifth and lastly, for low stimulus levels the acoustic noise floor completely masks any system distortion that can create artifactual emissions.
The range of stimulus frequencies (fp) employed was typically 0.4–8 kHz. The stimulus and emission frequency are one and the same for SFOAEs. A two-tone suppression paradigm was employed to extract the SFOAE (13,37). The suppressor parameters were where fs and Ls are the suppressor frequency and level, respectively. A total of 35 waveforms (8192 sample window) were averaged, excluding any flagged by an artifact-rejection paradigm (37). A period of 20 ms was allotted before the start of the sample window to allow for the associated response to reach steady state. Frequency step-size during sweeps was small enough to avoid ambiguity during the phase unwrapping. Delays associated with the measurement system were determined and subtracted out. The noise floor was defined as the average sound-pressure level centered about (but excluding) the frequency of interest. It was quantified via averaging the magnitudes of the ±3 bins in the fast-Fourier transform of the response.
The phase-gradient delay (NSF) expressed in number of periods is the product of the derivative of the phase function with respect to frequency (τOAE) and the emission frequency (fp). For linear systems, this is directly related to the group delay. By quantifying the frequency dependence of the steady-state response of a system, the phase-gradient delay provides a useful means to identify delays inherent in the dynamics of the system. It is given by
| (1) |
where φOAE is the emission phase (in radians) and fp is in hertz.
Expressing the delay in dimensionless form as NSF is useful when making comparisons to other dimensionless quantities such as the filter bandwidths (Q). Delays (i.e., τOAE) were computed from individual (unwrapped) phase responses using centered differences (38). As shown in Fig. 2, delay trends were computed across individuals of a given species via a locally weighted regression (loess) (39) (weighting factor α ≈ 0.1–0.2, polynomial of degree one, robust fit). To further reduce the effects of outliers at the end points, only NSF values whose corresponding magnitude (as well as the magnitude of its neighbors) was at least 10 dB above the noise floor were included in the fits. The data in general do not appear well-fit by a simple power-law (38) across all frequencies tested, and it is desirable to make comparisons without ad hoc statistical modeling assumptions. While loess fits are a first-order approximation (e.g., note deviation in Fig. 2 between 4 and 5 kHz for Aspidoscelis), they do provide a useful starting point for quantitatively comparing delay trends across species (38).
All experiments were performed at the University of Arizona with approval from the Institutional Animal Care and Use Committee. Experiments were performed during the months of March–August. For all species in this study, OAE data were collected from both adult males and females and from both ears in a given individual; the results as presented here do not distinguish between sex nor between data collected from left-versus-right ears. Species native to southern Arizona/California were wild-caught while nonnative species were obtained via local vendors. All lizards were housed in glass terraria with a 9 h light cycle and fed meal worms and crickets (occasionally dusted with calcium powder) 2–3 times a week. All lizards were healthy and active. Before each experiment, an animal was anesthetized via a 25–36 mg/kg Nembutal intraperitoneal injection to prevent movement. Anguids and iguanids required higher doses while the teiids and scincomorphs were given lower doses to obtain similar anesthetic states such that they do not move. These doses were effective for ∼2–5 h. The animal recovered completely within a few hours after the experiment. During the experiment, lizards were placed in a noise-attenuating chamber. Body temperature was kept constant by the use of a regulated heating blanket (Harvard Apparatus, Holliston, MA) and monitored using a calibrated thermocouple placed in the mouth (propping it open) or in the leg pit for cases where the lizard spit out the thermometer. Body temperature was kept in the range of ∼32–33°C (verified via a quick-reading cloacal thermometer). Preliminary data indicate SFOAE phase-gradient delays appear relatively insensitive to temperature (40) or depth of anesthetic state.
Results
Of the 49 different ears examined during this study, in addition to the 21 gecko ears from a previous study using the same system/paradigms (13), low-level SFOAEs were readily detectable in all ears (magnitude traces in Fig. 1). Significant phase accumulation was also apparent as the stimulus tone was swept in frequency (φOAE in Fig. 1), indicative of delays on the order of milliseconds. Spontaneous activity, as identified via temperature dependence and suppressibility due to external tones (41,42), was apparent in the vast majority of ears examined (see Supporting Material). In two instances of accidental overdose, both SOAEs and low-level SFOAEs were found to rapidly disappear upon death. These observations indicate these emissions are dependent upon a healthy physiological state for the animal, consistent with previous studies of lizard emissions (21–23).
For each species, three different individuals (chosen at random) are shown in Fig. 1 to demonstrate SFOAE similarities and differences across ears. For all species examined, SFOAE magnitudes fall off into the noise floor by fp ≈ 7–8 kHz, though typically at lower frequencies depending upon species (Fig. 1 and Supporting Material). Low-level SFOAE magnitudes extend beyond the highest frequency of SOAE activity (Supporting Material), though how far beyond varies from animal to animal. While SFOAE magnitude and phase trends are broadly apparent for a given species (as fp was varied), significant variations across individuals are observed (Fig. 1). Each ear exhibits a distinct set of peaks and valleys in magnitude and these spectral characteristics were highly reproducible within a given recording session, provided body temperature was kept constant. In some instances, individual responses appear qualitatively different from the others within a species. For example, one individual Gerrhosaurus shown in Fig. 1 shows reduced magnitudes at >∼3 kHz, although the rate of phase accumulation is similar to the others. Other magnitude features, such as the notch at 2–2.5 kHz for Aspidoscelis, are consistent across individuals within a species.
As shown in Figs. 2 and 3, the delay (NSF, expressed in number of stimulus cycles) was computed by numerically differentiating the phase responses shown in Fig. 1. While a steady rate of phase accumulation is apparent for a given individual (Fig. 1), sudden magnitude variations such as notches can lead to phase discontinuities and thus ambiguities in the phase unwrapping (e.g., negative delays). This ambiguity can make it difficult to precisely quantify the phase-gradient delay for a given ear, despite the clear trend. However, given a sufficiently large population for a particular species (≥4 ears), a suitable trend in NSF can be determined via a locally weighted regression (see Methods) (38,36), as shown in Fig. 2. Only delays whose corresponding magnitude (as well as that of its neighbors) was at least 10 dB above the noise-floor were included in further analysis.
Comparing SFOAE phase-gradient delays across species, Fig. 3 shows that delays are typically larger in TM-species for fp at >∼1–2 kHz. One notable exception is Anolis, whose emissions extend out to higher frequencies relative to other non-TM species and exhibit relatively large NSF values. Of all species examined, Gekko exhibits the largest delays above ∼1.5 kHz. The one species with a continuous TM, Aspidoscelis, has intermediate NSF values. In all species, with the exception of Elgaria and Uta, NSF generally increases with frequency.
Discussion
While it is commonly accepted that the tectorial membrane plays an important role in the ear's ability to transduce sound into neural signals, there is still presently much debate as to precisely what that role is. Given the robust OAEs and diverse TM morphology across the lizard taxa, this study systematically explores SFOAE properties across a broad array of lizard species. Specifically, we make use of the notion that SFOAEs (emissions evoked in response to a single stimulus tone) can be used to objectively determine auditory filter bandwidths (17,18). Thus, an underlying goal here is to examine the functional role the TM potentially plays in determining the ear's ability to discriminate different frequencies.
SFOAE delays and tuning
A theoretical model inspired by the gecko ear (20) predicts a proportionality between SFOAE phase-gradient delay and the reciprocal of the auditory filter bandwidth (for example, as derived from ANF responses). In a nutshell, the model is a collection of coupled, tuned oscillators (manifesting a small degree of irregularity) that act as the underlying filters (43,44). There is no direct TM coupling: longitudinal coupling comes entirely via the rigid papilla. The model assumes that the underlying auditory filters are second-order, although the model predictions do not appear constrained by this assumption. More complicated filter assumptions can still lead to a prediction of proportionality between NSF (phase-gradient delay) and Q (tuning bandwidth) (20). For second-order filters, the model predicts that
| (2) |
where Q is the quality factor of the resonance of the filter and Q10dB is derived from the bandwidth 10 dB from the peak response, as is commonly reported in physiological measurements (see (1)). Although the model SFOAEs receive contributions from all the oscillators, the response (and subsequent rate of phase accumulation) is dominated by those tuned about the stimulus frequency. The basic intuition is that the more sharply tuned the filter is, the longer it takes to build up to steady state and hence a sluggish response, or longer emission delay apparent via the phase-versus-frequency relationship.
As predicted by the model, bandwidth estimates derived from both ANF responses and SFOAEs have been shown to correlate well for Gekko gecko (20). Additionally, while the model does not directly distinguish the role of the TM, the model's prediction (i.e., NSF ∝ Q) holds well in the non-TM species Elgaria multicarinata (20), indicating the model is applicable across a variety of species. Furthermore, the results shown in Fig. 3 are strikingly similar to comparisons of tuning estimated directly from the auditory nerve for a variety of lizard species (see Fig. 4.17 in (35)). Lastly, estimates of tuning derived from SOAE suppression tuning curves (24,42) also correlate well to low-level SFOAE estimates for geckos (20) and anoles (see Supporting Material).
The larger SFOAE phase-gradient delays observed in the majority of TM-species at above 1.5–2 kHz (Fig. 3) are generally consistent with the hypothesis that those species have sharper tuning. Several possible explanations could account for how the TM leads to sharper tuning. The increased mass due to loading by TM could lead to a reduction in the effective damping working against the hair cell bundle (5,6). Longitudinal and radial coupling could also play a significant role in the mechanical response of groups of hair cells by concentrating the efforts of active hair cell bundles (e.g., (7,8,45)). Thus, the role of the TM in sharpening mechanical tuning could stem from both passive and active force considerations. Note that there are apparent exceptions to this rule, such as Anolis (non-TM, large delays) and Aspidoscelis (continuous TM, intermediate delays). These observations indicate that increased coupling does not always result in sharpened tuning, as discussed further in subsequent sections.
As shown in Fig. 3, it is difficult to distinguish differences in NSF across species <1–1.5 kHz where there is significant overlap. All species included in this study (except for Eumeces) have a TM connected back to the limbic lip that covers the low frequency portion of the papilla (25). Without further knowledge of how OAE generation mechanisms differ in the lower frequency portion of the papilla (where SOAE activity is not readily observable), one might reasonably expect NSF to be similar across species at these lower frequencies. It is possible that there is source mixing (37) between generators in the low-frequency TM region (present in most species tested here) and the higher frequency region as outlined in Table 1. At sufficiently high frequencies, such mixing becomes negligible and a more apparent distinction can be made. It is worthwhile to note that the TM in the Scincidae family (including Eumeces) is unconnected to the limbic lip (25,46). As such, for the only skink species examined here, NSF sits above that for all other species at the lowest frequencies where SFOAEs were detectable.
The majority (∼80%) of ears examined in this study also produced detectable SOAEs, consistent with previous studies (e.g., (24); see Supporting Material). However, low-level SFOAE activity was more readily produced than SOAE activity in most ears (i.e., when little or no SOAE activity was detected, SFOAEs were clearly present). This observation suggests that SFOAEs might provide a more robust probe into OAE generation mechanisms than SOAEs alone. For example, suppression tuning curves derived from SOAE peaks have been shown to correlate well to tuning curves derived from ANF responses (e.g., (42)), but SOAE peaks only manifest at certain frequencies, limiting their practicality. Tuning measures derived from SFOAEs, which appear to correlate well to those derived from SOAE suppression (see Supporting Material), can provide a more rapid measure with finer frequency resolution.
Within a given species, significant magnitude differences exist across individuals (Fig. 1). This observation supports the hypothesis that individualized irregularity, such as manifest in bundle stiffness or variations in active force contributions, has functional consequences as observed in the OAEs (e.g., (7,20,47)). Such individualized differences presumably relate to the unique SOAE spectra measured in different individuals (see Supporting Material).
Mammalian/nonmammalian differences
The functional role of the TM could be quite different between mammals and nonmammals. The demonstration that the mammalian TM is capable of propagating energy longitudinally along the length of the cochlea (10), coupled with the lack of a basilar membrane (BM) traveling wave in the lizard ear (27,28), could point toward significant differences in the underlying mechanics between the two types of ears. For example, one proposed cochlear model (48) posits what essentially amounts to two coupled parallel transmission lines that trade energy back and forth to produce sharp and spatially localized tuning as observed in mammalian BM responses. If such a model holds, the TM could potentially play a critical role in one of those paths (e.g., (11)). Given the absence of lizard BM traveling waves, such a model may be implausible for their ears. Thus, the TM's role toward creating a “second resonance” (49) could be a feature specific to the mammalian cochlea.
The only species examined with a continuous TM, Aspidoscelis, exhibited relatively moderate delays (Fig. 3). This observation, barring additional unknown morphological considerations at the micromechanical scale (e.g., differences in how the TM specifically attaches to the stereovillar bundles, nonuniform bundle bidirectionality (29)), suggests that stronger TM coupling does not always lead to sharper tuning. One possibility is that in the lizard, too strong of a coupling may be disadvantageous (32). Potential reasons could stem from both passive (e.g., strong coupling on papillae with steep tonotopic gradients would smear out responses) and active considerations (e.g., phase cancellations from active force contributions), similar in essence to differences between lizards and mammals discussed in the previous paragraph. Such a limiting effect could have led to the discretized nature of the TM in the salletal species, such as geckos who indeed appear to be auditory specialists among the lizards (32). Put another way, there may be an optimal amount of TM coupling that works to effectively balance active force contributions: too much or too little can ultimately lead to broader tuning. Such a hypothesis would be consistent with recent observations in the mammalian ear that indicated weaker longitudinal coupling via the TM leads to sharper tuning at the level of the BM (11).
Additionally, the morphological and mechanical properties of the TM itself might be very different in nonmammals. For example, the collagen-based fibrillar network in the mammalian TM (50,51) that appears functionally important (11,52) could take on a very different composition in the lizard TM. As noted by Miller, the skink TM (an individual sallet specifically) is “thrown into complicated folds and twisted structures that are interconnected by both thick and very fine strands of material” (53).
One possible means to gain further insight into differences between mammalian and nonmammalian OAE generation mechanisms (and the subsequent role of the TM) could be a comparative study of SFOAEs across bird species. There is evidence to suggest that there is some degree of similarity in OAE generation mechanisms between chickens and humans (13), despite large morphological differences of the inner ear: chickens lack hair-cell somatic motility (54), (55) and have a massive TM more firmly coupled to the apical surface of the basilar papilla (56).
Species specifics
As shown in Fig. 3, NSF for the Tokay gecko (Gekko) sits well above the other species for frequencies ≥1–2 kHz. This species is the only one examined here known to extensively vocalize for territorial and mating purposes, save for Eublepharis, whose vocalizations are much more limited. In fact, Gekkonidae appears unique among lizard families in that it is the only one to possess elasticized vocal cords (57,58), allowing them to make more spectrally rich vocalizations than the “hissing” observed in other lizard groups. Sharper peripheral tuning, as indicated by the larger SFOAE delays, could potentially provide significant benefits to Gekko for the perception of these vocalizations. Furthermore, the spectral content apparent in the SFOAE responses of Tokay geckos ((13), Supporting Material) correlates well with that of their vocalizations (57), where responses remain relatively flat up to ∼4–5 kHz and fall off sharply above. It has been proposed that geckos are similar to mammals (and birds) in that evolution has produced a dichotomy of hair cell types in the high frequency portion of their papilla (i.e., within the type II hair cell region): those that act as detectors (to send information to the brain) and those that act as amplifiers (to boost low-level stimuli) (59). It is presently unclear how such a distinction might extend across the Lacertilia, but further study of OAE properties should help determine whether such a dichotomy is present in other lizard groups.
Of all species examined, emission magnitudes were smallest in Callisaurus. This observation is not surprising given their close relation to the Holbrookia and Cophosaurus genera, also known as the “earless lizards” due to their apparent lack of an external ear. While Callisaurus has a more pronounced external tympanum than Holbrookia, it nonetheless has reduced function relative to the external/middle ears of other species examined including those in the Phrynosomatidae family. Thus, the smaller magnitudes in Callisaurus presumably stem from poorer forward and reverse transmission via the middle and external ear (see (60)).
Relative to other non-TM species, anole (Anolis carolinensis) OAEs appear unique: robust SOAEs and relatively large SFOAE delays with emission magnitudes extending well out to higher frequencies. It is not presently clear why their delays are relatively larger: as Table 1 indicates, their papilla is roughly the same length and contains the same number of hair cells as the alligator lizard (Elgaria) whose delays are significantly shorter. Furthermore, there does not presently appear to be any outwardly obvious differences in the hair cells themselves across these two species (e.g., number of stereovilli in a given bundle (61,62)), though Anolis carolinensis does have an enclosed external auditory meatus. It seems unlikely that the longer SFOAE delays in anoles are due to some other factor not associated with tuning, given that tuning estimates derived from SOAE suppression in a similar Anolis species are relatively large (24) and appear to correlate well to tuning estimates derived from SFOAEs (see Supporting Material). One possibility is that anoles (and possibly other non-TM species) have evolved such that the underlying amplification mechanisms (at the micromechanical level) could be enhanced relative to other species. Indeed, the Anolis genus is highly diversified and has served as an prime example of adaptive radiation in evolutionary biology (63). These observations suggest that anoles could serve as excellent models for future auditory research, given the relative simplicity of their papilla and robust OAEs, as well as potential applications stemming from the sequencing of the Anolis carolinensis genome (see (63)). Lastly, the anole OAE data presented here further reinforce the observation that reptile hearing, even in those with a relatively simple papilla, need not be confined to lower frequencies (i.e., <5 kHz) (24).
Lastly, SFOAE magnitudes in Pogona fell off at lower frequencies (typically by ∼3 kHz) but exhibited relatively large NSF values (Fig. 3). While the inner ear morphology of Pogona is presently unknown, it may likely be similar to that of iguanids (i.e., minimal TM) (32). If this is the case, perhaps agamids are similar to Anolis in that they have evolved mechanisms to sharpen mechanical tuning despite the lack of a TM. Other possibilities: Pogona has a more complex TM morphology, as is apparent in some agamids such as Leiolepis belliana (25) or that there is some additional source of delay present in their ears that does not contribute to tuning, as appears to be the case for the frog (13).
Conclusions
This study demonstrates that features of SFOAEs, a noninvasive measure of auditory function, appear critically tied to local mechanical and morphological properties of the inner ear. Specifically, our results imply that the structure of the TM, in a region where the emissions are presumably being generated, likely plays an important role in determining the observed OAE properties in lizards. A lack of a TM in general leads to shorter emission delays, while species with a TM (either continuous or discretized) typically exhibit longer delays. However, at least one non-TM species exhibits relatively large delays, suggesting that additional factors could be at work across species that can affect tuning (e.g., differences in an underlying active process). In light of theoretical considerations that hypothesize that TM-coupling can lead to sharper tuning and that SFOAE delays are inversely proportional to auditory filter bandwidths, our results generally support these predictions, but with the added caveat that there might be an optimal amount of TM coupling: too little or too much can lead to broadened tuning.
Given the significant differences that exist between the lizard inner ear and the mammalian cochlea, the functional role of the TM could very well be different between these two groups. Whereas in lizards the TM's primary role might be to help overcome viscous forces and thereby sharpen mechanical tuning, the TM in mammals could have the added role of coupling energy longitudinally along the length of the cochlea. Regardless, further understanding of emission generation mechanisms in nonmammals will inevitably lead to deeper insights into the function of the mammalian ear.
Acknowledgments
J. Jarchow and N. McMullen provided veterinary assistance. G. Manley and C. Shera provided encouragement and technical assistance. Comments from A. J. Aranyosi, A. J. Hudspeth, L. Jones, J. Rosowski, C. Shera, and two anonymous reviewers improved upon an earlier draft. The Green Diamond Resource Company, E. Bjorkstedt, K. M. Bonine, and the Arizona Game and Fish Department facilitated lizard procurement.
Financial support came from the Howard Hughes Medical Institute (grant No. 52003749), the National Science Foundation Division of Mathematical Sciences (grant No. 0602173), and the National Institutes of Health (grant No. R01 DC3687).
Supporting Material
References
- 1.Dallos P., Popper A., Fay R. Springer Handbook of Auditory Research. Springer; New York: 1996. The cochlea. [Google Scholar]
- 2.Zwislocki J., Kletsky E. Tectorial membrane: a possible effect on frequency analysis in the cochlea. Science. 1979;204:639–641. doi: 10.1126/science.432671. [DOI] [PubMed] [Google Scholar]
- 3.Allen J. Cochlear micromechanics—a physical model of transduction. J. Acoust. Soc. Am. 1980;68:1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- 4.Gummer A., Hemmert W., Zenner H. Resonant tectorial membrane motion in the inner ear: its crucial role in frequency tuning. Proc. Natl. Acad. Sci. USA. 1996;93:8727–8732. doi: 10.1073/pnas.93.16.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Authier S., Manley G. A model of frequency tuning in the basilar papilla of the Tokay gecko, Gekko gecko. Hear. Res. 1995;82:1–13. doi: 10.1016/0378-5955(94)00138-g. [DOI] [PubMed] [Google Scholar]
- 6.Nam J., Fettiplace R. Theoretical conditions for high-frequency hair bundle oscillations in auditory hair cells. Biophys. J. 2008;95:4948–4962. doi: 10.1529/biophysj.108.138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilfan A., Duke T. Frequency clustering in spontaneous otoacoustic emissions from a lizard's ear. Biophys. J. 2008;95:4622–4630. doi: 10.1529/biophysj.108.130286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dierkes K., Linder B., Jülicher F. Enhancement of sensitivity gain and frequency tuning by coupling of active hair bundles. Proc. Natl. Acad. Sci. USA. 2008;105:18651–18674. doi: 10.1073/pnas.0805752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J., Hemmert W., Aranyosi A. Frequency-dependent shear impedance of the tectorial membrane. Biophys. J. 2008;95:2529–2538. doi: 10.1529/biophysj.107.124727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaffari R., Aranyosi A., Freeman D. Longitudinally propagating traveling waves of the mammalian tectorial membrane. Proc. Natl. Acad. Sci. USA. 2007;104:16510–16515. doi: 10.1073/pnas.0703665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell I., Legan P., Richardson G. Sharpened cochlear tuning in a mouse with a genetically modified tectorial membrane. Nat. Neurosci. 2007;10:215–223. doi: 10.1038/nn1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köppl, C. 1995. Advances in Hearing Research: Proceedings of the 10th International Symposium on Hearing. World Scientific, Singapore.
- 13.Bergevin C., Freeman D., Shera C. Otoacoustic emissions in humans, birds, lizards, and frogs: evidence for multiple generation mechanisms. J. Comp. Physiol. A. 2008;194:665–683. doi: 10.1007/s00359-008-0338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coro F., Kössl M. Distortion-product otoacoustic emissions from the tympanic organ in two noctuoid moths. J. Comp. Physiol. A. 1998;183:525–531. [Google Scholar]
- 15.Probst R., Lonsbury-Martin B., Martin G. A review of otoacoustic emissions. J. Acoust. Soc. Am. 1991;89:2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- 16.Neely S., Kim D. An active cochlear model showing sharp tuning and high sensitivity. Hear. Res. 1983;9:123–130. doi: 10.1016/0378-5955(83)90022-9. [DOI] [PubMed] [Google Scholar]
- 17.Shera C., Guinan J., Oxenham A. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc. Natl. Acad. Sci. USA. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moleti A., Sisto R. Objective estimates of cochlear tuning by otoacoustic emission analysis. J. Acoust. Soc. Am. 2003;113:423–429. doi: 10.1121/1.1523389. [DOI] [PubMed] [Google Scholar]
- 19.Sisto R., Moleti A., Shera C.A. Cochlear reflectivity in transmission-line models and otoacoustic emission characteristic time delays. J. Acoust. Soc. Am. 2007;122:3554–3561. doi: 10.1121/1.2799498. [DOI] [PubMed] [Google Scholar]
- 20.Bergevin C., Shera C.A. Coherent reflection without traveling waves: on the origin of long-latency otoacoustic emissions in the gecko. J. Acoust. Soc. Am. 2010;127:2398–2409. doi: 10.1121/1.3303977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosowski J., Peake W., White J. Cochlear nonlinearities inferred from two-tone distortion products in the ear canal of the alligator lizard. Hear. Res. 1984;13:141–158. doi: 10.1016/0378-5955(84)90105-9. [DOI] [PubMed] [Google Scholar]
- 22.Manley G., Köppl C., Johnstone B. Distortion-product otoacoustic emissions in the bobtail lizard. I. General characteristics. J. Acoust. Soc. Am. 1993;93:2820–2833. [Google Scholar]
- 23.Stewart C., Hudspeth A. Effects of salicylates and aminoglycosides on spontaneous otoacoustic emissions in the Tokay gecko. Proc. Natl. Acad. Sci. USA. 2000;97:454–459. doi: 10.1073/pnas.97.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manley G. Spontaneous otoacoustic emissions from free-standing stereovillar bundles of ten species of lizard with small papillae. Hear. Res. 2006;212:33–47. doi: 10.1016/j.heares.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Wever E. Princeton University Press; Princeton, NJ: 1978. The Reptile Ear. [Google Scholar]
- 26.Manley G. Cochlear mechanisms from a phylogenetic viewpoint. Proc. Natl. Acad. Sci. USA. 2000;97:11736–11743. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peake W., Ling A. Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J. Acoust. Soc. Am. 1980;67:1736–1745. doi: 10.1121/1.384300. [DOI] [PubMed] [Google Scholar]
- 28.Manley G., Yates G., Köppl C. Auditory peripheral tuning: evidence for a simple resonance phenomenon in the lizard tiliqua. Hear. Res. 1988;33:181–190. doi: 10.1016/0378-5955(88)90031-7. [DOI] [PubMed] [Google Scholar]
- 29.Miller, M. 1992. The Evolutionary Biology of Hearing, Chapt. 23. Springer-Verlag, Berlin, Germany.
- 30.Weiss T., Mulroy M., Pike C. Tuning of single fibers in the cochlear nerve of the alligator lizard: relation to receptor morphology. Brain Res. 1976;115:71–90. doi: 10.1016/0006-8993(76)90823-4. [DOI] [PubMed] [Google Scholar]
- 31.Turner R. Neural tuning in the granite spiny lizard. Hear. Res. 1987;26:287–299. doi: 10.1016/0378-5955(87)90064-5. [DOI] [PubMed] [Google Scholar]
- 32.Manley G. Evolution of structure and function of the hearing organ of lizards. J. Neurobiol. 2002;53:202–211. doi: 10.1002/neu.10115. [DOI] [PubMed] [Google Scholar]
- 33.Taschenberger G., Gallo L., Manley G. Filtering of distortion-product otoacoustic emissions in the inner ear of birds and lizards. Hear. Res. 1995;91:87–92. doi: 10.1016/0378-5955(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 34.Lukashkin A., Lukashkina V., Russell I. Role of the tectorial membrane revealed by otoacoustic emissions recorded from wild-type and transgenic tecta mice. J. Neurophysiol. 2004;91:163–171. doi: 10.1152/jn.00680.2003. [DOI] [PubMed] [Google Scholar]
- 35.Manley, G. 2000. Comparative hearing: birds and reptiles. In Springer Handbook of Auditory Research, Vol. 13, Chapt. 4. Springer, New York.
- 36.Schairer K., Ellison J., Keefe D. Use of stimulus-frequency otoacoustic emission latency and level to investigate cochlear mechanics in human ears. J. Acoust. Soc. Am. 2006;120:901–914. doi: 10.1121/1.2214147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shera C.A., Guinan J.J. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J. Acoust. Soc. Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- 38.Shera C.A., Guinan J.J. Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J. Acoust. Soc. Am. 2003;113:2762–2772. doi: 10.1121/1.1557211. [DOI] [PubMed] [Google Scholar]
- 39.Cleveland W. Hobart Press; Summit, NJ: 1993. Visualizing Data. [Google Scholar]
- 40.Bergevin, C., D. Velenovsky, and K. Bonine. 2010. Otoacoustic emission temperature dependence across the lacertilia. In Abstracts of the Thirty-Third Midwinter Research Meeting. Association for Research in Otolaryngology, Anaheim, CA.
- 41.Köppl C., Manley G. Spontaneous otoacoustic emissions in the bobtail lizard. I. General characteristics. Hear. Res. 1993;71:157–169. doi: 10.1016/0378-5955(93)90031-u. [DOI] [PubMed] [Google Scholar]
- 42.Manley G., Gallo L., Köppl C. Spontaneous otoacoustic emissions in two gecko species, Gekko gecko and Eublepharis macularius. J. Acoust. Soc. Am. 1996;99:1588–1603. doi: 10.1121/1.414680. [DOI] [PubMed] [Google Scholar]
- 43.Frishkopf L., DeRosier D. Mechanical tuning of free-standing stereociliary bundles and frequency analysis in the alligator lizard cochlea. Hear. Res. 1983;12:393–404. doi: 10.1016/0378-5955(83)90008-4. [DOI] [PubMed] [Google Scholar]
- 44.Aranyosi A., Freeman D. Sound-induced motions of individual cochlear hair bundles. Biophys. J. 2004;87:3536–3546. doi: 10.1529/biophysj.104.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strimbu C., Ramunno-Johnson D., Bozovic D. Correlated movement of hair bundles coupled to the otolithic membrane in the bullfrog sacculus. Hear. Res. 2009;256:58–63. doi: 10.1016/j.heares.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Köppl C. Morphology of the basilar papilla of the bobtail lizard tiliqua rugosa. Hear. Res. 1988;35:209–228. doi: 10.1016/0378-5955(88)90119-0. [DOI] [PubMed] [Google Scholar]
- 47.Zweig G., Shera C.A. The origin of periodicity in the spectrum of evoked otoacoustic emissions. J. Acoust. Soc. Am. 1995;98:2018–2047. doi: 10.1121/1.413320. [DOI] [PubMed] [Google Scholar]
- 48.Hubbard A. A traveling-wave amplifier model of the cochlea. Science. 1993;259:68–71. doi: 10.1126/science.8418496. [DOI] [PubMed] [Google Scholar]
- 49.Lukashkin A., Russell I. A second, low-frequency mode of vibration in the intact mammalian cochlea. J. Acoust. Soc. Am. 2003;113:1544–1550. doi: 10.1121/1.1535941. [DOI] [PubMed] [Google Scholar]
- 50.Thalmann I., Thallinger G., Thalmann R. Composition and supramolecular organization of the tectorial membrane. Laryngoscope. 1987;97:357–367. [PubMed] [Google Scholar]
- 51.Hasko J., Richardson G. The ultrastructural organization and properties of the mouse tectorial membrane matrix. Hear. Res. 1988;35:21–38. doi: 10.1016/0378-5955(88)90037-8. [DOI] [PubMed] [Google Scholar]
- 52.Masaki K., Gu J., Aranyosi A. Col11a2 deletion reveals the molecular basis for tectorial membrane mechanical anisotropy. Biophys. J. 2009;96:4717–4724. doi: 10.1016/j.bpj.2009.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller M. Scanning electron microscope studies of some skink papillae basilares. Cell Tissue Res. 1974;150:125–141. doi: 10.1007/BF00220386. [DOI] [PubMed] [Google Scholar]
- 54.He D., Beisel K., Salvi R. Chick hair cells do not exhibit voltage-dependent somatic motility. J. Physiol. 2003;546:511–520. doi: 10.1113/jphysiol.2002.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koppl C., Forge A., Manley G. Low density of membrane particles in auditory hair cells of lizards and birds suggests an absence of somatic motility. J. Comp. Neurol. 2004;479:149–155. doi: 10.1002/cne.20311. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka K., Smith C. Structure of the avian tectorial membrane. Ann. Otol. Rhinol. Laryngol. 1975;84:287–296. doi: 10.1177/000348947508400302. [DOI] [PubMed] [Google Scholar]
- 57.Marcinelli D. Acoustic and visual display behavior in Gekkonid lizards. Am. Zool. 1977;17:251–260. [Google Scholar]
- 58.Russell A., Rittenhouse D., Bauer A. Laryngotracheal morphology of Afro-Madagascan geckos: a comparative survey. J. Morphol. 2000;245:241–268. doi: 10.1002/1097-4687(200009)245:3<241::AID-JMOR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 59.Chiappe M., Kozlov A., Hudspeth A. The structural and functional differentiation of hair cells in a lizard's basilar papilla suggests an operational principle of amniote cochleas. J. Neurosci. 2007;27:11978–11985. doi: 10.1523/JNEUROSCI.3679-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Dijk P., Mason M., Narins P. Distortion product otoacoustic emissions in frogs: correlation with middle and inner ear properties. Hear. Res. 2002;173:100–108. doi: 10.1016/s0378-5955(02)00605-6. [DOI] [PubMed] [Google Scholar]
- 61.Mulroy M., Williams R. Auditory stereocilia in the alligator lizard. Hear. Res. 1987;25:11–21. doi: 10.1016/0378-5955(87)90075-x. [DOI] [PubMed] [Google Scholar]
- 62.Manley G., Gallo L. Otoacoustic emissions, hair cells, and myosin motors. J. Acoust. Soc. Am. 1997;102:1049–1055. doi: 10.1121/1.419858. [DOI] [PubMed] [Google Scholar]
- 63.Losos J., Schneider C. Anolis lizards. Curr. Biol. 2009;19:R316–R318. doi: 10.1016/j.cub.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 64.Miller M. Quantitative studies of auditory hair cells and nerves in lizards. J. Comp. Neurol. 1985;232:1–24. doi: 10.1002/cne.902320102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


