Abstract
Plants acquire essential trace elements from the rhizosphere and must adapt to conditions that can range from deficiency to excess. Knowledge of how trace elements move from root to shoot to seed is critical for agriculture and human nutrition.
Introduction and context
Plants require an assortment of trace elements for growth, yet the properties that make these minerals essential also make them toxic at increasing concentrations. As plants primarily acquire their micronutrients from the rhizosphere, an ability to adapt to soil conditions that can range from deficiency to excess is critical for optimal growth. Ultimately, human nutrition is dependent on how plants respond to trace element availability in terms of both nutrient accumulation and yield. Deciphering trace element transport and tolerance allows the breeding of crops that are more nutrient rich and more tolerant of extreme soil conditions. Currently, many aspects of metal homeostasis in plants remain unknown. These include how plants sense metal deficiencies and respond and whether chaperones exist for all redox-active metals. Although these core questions remain unanswered, our understanding of metal tolerance and detoxification has increased greatly in the past two years with the publication of several insightful papers.
Major recent advances
Advances in understanding metal tolerance
It is well known that metal hyperaccumulating species increase their tolerance to metals by dispersing them from the root to aerial tissues. Arabidopsis halleri is closely related to Arabidopsis thaliana, yet A. halleri grows readily in soils polluted with zinc and cadmium, accumulating high concentrations of these metals in its leaves [1]. A series of microarray experiments had previously found significant increases in the expression of zinc transporters in A. halleri relative to A. thaliana [2-4], but the regulatory and evolutionary basis of these changes remained unknown.
Hanikenne et al. [5] shed light on this question by showing that the ability of A. halleri to move large amounts of zinc and cadmium from root to shoot (Figure 1) is mediated by a triplication of the gene encoding the vascular-loading transporter HMA4, as well as by changes to cis-regulatory sequences controlling the expression of HMA4. The authors used RNA interference to knockdown HMA4 levels, resulting in zinc accumulation in the roots, reduced expression of other zinc transporters, and elimination of zinc tolerance. This showed that HMA4 is not only essential for zinc tolerance, but also serves as a ‘physiological master switch’ to control expression of other zinc transporters. They also found that the AhHMA4 promoter expressed in A. thaliana drove much higher levels of expression than the endogenous AtHMA4 promoter in the leaves and root vasculature. Interestingly, when AhHMA4 was overexpressed in A. thaliana, the plants became more sensitive to zinc and cadmium, indicating that additional genes in A. halleri are also required for hyperaccumulation. Quantitative trait locus (QTL) analyses now underway in A. halleri may provide new candidates, in addition to those suggested from microarray studies, that can be tested [1].
Figure 1. Examples of metal tolerance mechanisms in plants.
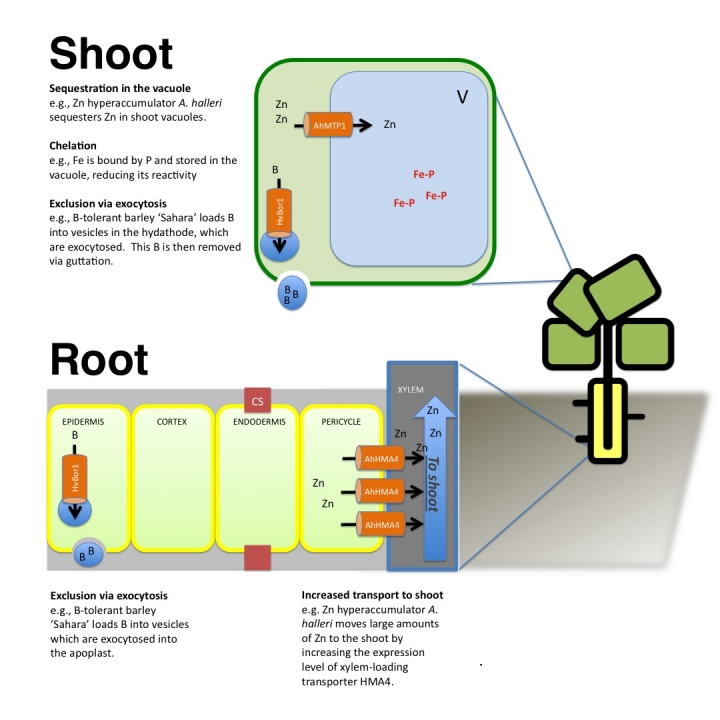
In the past two years, several genes required for metal tolerance in plants have been identified. In roots, excess metal can be loaded into vesicles and is likely exocytosed into the apoplast (e.g. HvBOR1 and boron). Tolerance is also increased by dispersing metal from the roots to the shoot. This is achieved by increasing the expression of xylem-loading metal transporters, like zinc-transporter AhHMA4. Once in the shoot, the metal can be sequestered (e.g. AhMTP1 pumps zinc into the vacuole), or pumped into vesicles and exocytosed into the hydathode (e.g. HvBOR1 and boron). CS, Casparian strip; V, vacuole.
Another means of metal tolerance, exocytosis, had been documented in humans and yeast. This mechanism has now been reported in plants, with the discovery of what is likely exocytosis of metal-loaded vesicles in both Arabidopsis and barley. Manganese (Mn) detoxification in Arabidopsis can be conferred in part by sequestration in the vacuole [6] and endoplasmic reticulum [7]. Further elucidating Mn homeostasis in Arabidopsis, Peiter et al. [8] showed that Mn detoxification also involves exclusion via exocytosis. The transporter MTP11 was localized to trans-Golgi vesicles, and rescued manganese sensitive yeast via microsomal loading; at the same time, overexpression of MTP11 in Arabidopsis improved manganese tolerance, while the loss-of-function mutant was hypersensitive to manganese. Additionally, Delhaize et al. [9] found that an intermediate expression level of MTP11-GFP resulted in intermediate manganese tolerance (although this construct appeared to localize to pre-vacuolar compartments). Peiter et al. [8] found MTP11 expressed in the hydathodes and root tips, suggesting it loads vesicles with excess manganese that are then exocytosed, with the manganese being removed via guttation in the shoots, and via efflux into the root apoplast. Interestingly, the vesicular MTP11 was expressed in different tissues than the vacuolar manganese transporter MTP1; this suggests tissue-specific, tissue-specialized roles in Mn homeostasis for different members of the MTP family.
Boron is needed for cross-linking of plant cell walls, yet too much boron can be toxic [10]. It is only recently that boron transporters have been identified. NIP5;1, a member of the aquaporin protein family, acts to take up boron [11] and BOR1 was identified as a boron effluxer required for efficient xylem loading of boron for transport to the shoot [12]. Boron efflux also plays an important role in boron tolerance. QTL analysis in barley between the highly tolerant Sahara cultivar and the susceptible Clipper cultivar revealed that the genomic region of interest contained a BOR1-like gene [13], which the authors designated Bot1. This is another great example of where basic research in Arabidopsis facilitated the identification of an agronomically significant gene in barley. They discovered that boron tolerance was mediated by elevated expression of the effluxer Bot1 (Figure 1); when compared to a boron-sensitive variety, Sahara had four times as many copies of Bot1, much like the triplication of HMA4 found in A. halleri. Sahara Bot1 also contained two amino acid changes that significantly increased its boron transport capability compared to Clipper Bot1 when expressed in yeast. And like AtMTP11, Sahara Bot1 was also highly expressed in the root tips and hydathodes, likely expelling boron and allowing the landrace Sahara to thrive in boron-rich soils. The authors note that boron toxicity causes a 17% decrease in barley yield in Australia, and the discovery of the BOR1-like genes will speed the breeding of boron-tolerant crops.
Sometimes the pathway for the transport of an important nutrient can also be used to transport toxic elements. Two silicon transporters have recently been implicated in arsenite transport in rice [14]. Human arsenic intake from rice consumption can be substantial because rice is particularly efficient in assimilating arsenic from paddy soils. The silicon/arsenite transporters belong to the nodulin-like intrinsic protein (NIP) subfamily.
Finally, a phenotype long attributed to phosphorous deficiency was found to be caused by iron toxicity. Root elongation in Arabidopsis ecotype Col-0 is inhibited during phosphorous deficiency, and was believed to be caused by a phosphorous-sensing regulatory pathway. Instead, Ward et al. [15] found they could restore root growth during phosphorous deficiency by simply removing iron from the growth medium - the inhibition was actually caused by the toxic effects of iron that was likely no longer in complex with phosphate, greatly increasing its bioavailability.
Future directions
In the past year, several interesting large-scale experiments have shed light on previously unknown aspects of metal homeostasis in plants, and may serve as a preview of the style and scale of experiments to come.
Dinneny et al. [16] undertook the task of isolating individual root cell types and measuring the transcriptional changes in response to iron deficiency and salt stress. The authors found that the majority of the transcriptome was altered in response to environmental conditions, especially in the epidermis. At the same time, they identified a small core set of stress-resistant genes that likely define the essential features of each cell layer, and appear to regulate the environment-specific expression of the rest of the transcriptome. Their dataset is a valuable resource for both deducing the role of individual iron-regulated genes, and understanding the broad spatial and temporal changes that make up the iron-deficiency response. Previous studies with salt stress in particular seem to have only identified semi-ubiquitous responses, showing the power of using a genome scale, high-resolution map of gene expression changes.
Baxter et al. [17] analyzed the shoot metal accumulation data of over 880 Arabidopsis mutants and ecotypes from the Purdue Ionomics Information Management System (PiiMS) database to identify a metal accumulation signature for iron-deficiency. Because iron is highly reactive, its concentration in shoots is tightly regulated and does not fluctuate, even in chlorotic plants. The authors found that iron-deficient plants do exhibit signature changes in other metals in the shoot: increased manganese, zinc, cobalt, and cadmium, and decreased molybdenum. The increases of all but molybdenum are explained by the transport of these metals by the iron-regulated root transporter IRT1 [18]; although they enter the root with iron, their localization is not as highly regulated, and they are able to accumulate in the shoot. The decrease in molybdenum is likely due to the acidification of the rhizosphere by the iron-regulated H+ATPases, which increases access to iron, but reduces molybdenum solubility.
Finally, the use of association mapping is quickly proving an invaluable tool in identifying novel genes. Arabidopsis is well suited for association mapping because of its rich pool of variation between natural populations. Baxter et al. [19] examined 98 ecotypes for variation in shoot molybdenum, and mapped a polymorphism in seven low molybdenum ecotypes. The source of the phenotype was a 54 base-pair deletion in the promoter region of what is now known as MOT1 - a mitochondrial molybdenum (Mo) transporter. Again, like HMA4 in A. halleri, metal accumulation was greatly affected by changes in the promoter. MOT1 is likely just one of many genes that will soon be identified using association mapping.
Acknowledgments
We would like to thank the Guerinot lab members for many constructive discussions about metals. Work in the Guerinot lab is supported by grants from the National Science Foundation (IOB 0419695; DBI-070119), the Physical Biosciences Program of the Department of Energy (DE-FG02-06ER15809), the National Institutes of Health (R01 GM 078536) and the National Institute of Environmental Health Sciences (5 P42 ES007373).
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://F1000.com/Reports/Biology/content/1/14
References
- 1.Roosens NH, Willems G, Saumitou-Laprade P. Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends Plant Sci. 2008;13:208–15. doi: 10.1016/j.tplants.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Becher M, Talke IN, Krall L, Krämer U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 2004;37:251–68. doi: 10.1046/j.1365-313x.2003.01959.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Ramon Serrano 8 Jun 2004
- 3.Weber M, Harada E, Vess C, Roepenack-Lahaye E, Clemens S. Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J. 2004;37:269–81. doi: 10.1046/j.1365-313x.2003.01960.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Ramon Serrano 8 Jun 2004
- 4.Talke IN, Hanikenne M, Krämer U. Zinc-dependent global transcriptional control, transcriptional deregulation and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2006;142:148–67. doi: 10.1104/pp.105.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–5. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.5 Must ReadEvaluated by Enrico Martinoia 6 May 2008, Nicolaus Von Wiren 15 May 2008, Michael Gjedde Palmgren 28 May 2008
- 6.Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 2000;124:125–33. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, Liang F, Hong B, Young JC, Sussman MR, Harper JF, Sze H. An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1 supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol. 2002;130:128–37. doi: 10.1104/pp.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiter E, Montanini B, Gobert A, Pedas P, Husted S, Maathuis FJ, Blaudez D, Chalot M, Sanders D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc Natl Acad Sci U S A. 2007;104:8532–7. doi: 10.1073/pnas.0609507104. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Nico Von Wiren 20 Jun 2007
- 9.Delhaize E, Gruber BD, Pittman JK, White G, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J. 2007;51:198–210. doi: 10.1111/j.1365-313X.2007.03138.x. [DOI] [PubMed] [Google Scholar]
- 10.Takano J, Miwa K, Fujiwara T. Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 2008;13:451–7. doi: 10.1016/j.tplants.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. Arabidopsis boron transporter for xylem loading. Nature. 2002;420:337–40. doi: 10.1038/nature01139. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.6 Must ReadEvaluated by Enrico Martinoia 10 Dec 2002, Wolfgang Junge 20 Dec 2002, Mary Lou Guerinot 2 Jan 2003, Ramon Serrano 21 Mar 2003
- 13.Sutton T, Baumann U, Hayes J, Collins NC, Shi BJ, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, Langridge P. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science. 2008;318:1446–9. doi: 10.1126/science.1146853. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Nico Von Wiren 18 Dec 2007
- 14.Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008;105:9931–5. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008;147:1181–91. doi: 10.1104/pp.108.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Brian Forde 16 May 2008
- 16.Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–5. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Mary Lou Guerinot 4 Jun 2008
- 17.Baxter IR, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, Guerinot ML, Salt DE. The leaf ionome as a multivariable system to detect a plant's physiological status. Proc Natl Acad Sci U S A. 2008;105:12081–6. doi: 10.1073/pnas.0804175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and plant growth. Plant Cell. 2002;14:1223–33. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter I, Muthukumar B, Park HC, Buchner P, Lahner B, Danku J, Zhao K, Lee J, Hawkesford MJ, Guerinot ML, Salt DE. Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1) PLoS Genet. 2008;4:e1000004. doi: 10.1371/journal.pgen.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Brian Forde 13 May 2008


