Abstract
The Rho-family GTPases are proving to have a variety of biological functions apart from their well known effects on the cytoskeleton. Recent work indicates their involvement in signaling between the adhesion receptors integrin and syndecan, effects on the recruitment of beta-catenin to the nucleus, and potential roles in the nucleus as well as the cytoplasm.
Introduction and context
The ability of cells to move and to adhere to neighboring cells and the extracellular matrix is the foundation of multicellularity, and provides tissues with the ability to remodel and recover from injury, yet invasive motility is the basis for deadly cancer metastasis. Thus, new insights into the molecular mechanisms that regulate cell motility and adhesion are likely to broaden our understanding of how embryos develop, wounds heal, immune cells hit their targets and tumors metastasize.
In many types of cultured cells, motility depends upon the ability of the cell to generate protrusions at the leading edge (lamellipodia) and contraction in the body of the cell. Protrusion and contraction require different types of actin filament arrays: protrusion of the lamellipodial edge requires rapidly growing, branched filament arrays, whereas contractility requires myosin-rich, parallel bundles of filaments called stress fibers, and also firm adhesion to a substrate. Thus, it seems that motility presents a major challenge for the cell - it must construct functionally distinct actin-based structures in different sub-cellular sites simultaneously, and then constantly remodel those arrays as the cell moves forward. In 1992, a pair of landmark papers from Anne Ridley, Alan Hall and co-workers appeared in Cell [1,2] and established roles for the small guanine nucleoside triphosphatases (GTPases) RhoA and Rac1 in the generation of stress fibers and lamellipodia, respectively, suggesting that RhoA and Rac1 might have important roles in regulating the sequential stepwise process by which cells migrate. These key discoveries have spun off an entire sub-field of investigation into the complex pathways that regulate a large family of 22 different Rho-family GTPases, with the goal of understanding how their activities and localizations are controlled within cells. In addition, new research has uncovered functions for the Rho GTPases in unexpected subcellular sites. Also, major advances have been made by researchers venturing beyond the original model system used by Ridley and Hall (cultured Swiss 3T3 fibroblasts) to identify novel functions for Rho and Rac in a variety of cell types and model organisms. One important example comes from the field of developmental neuroscience, where Rho GTPases have been shown to have a key role in neurite outgrowth and axonal guidance, and thus in the correct wiring of the nervous system. As Rho GTPases are highly conserved among eukaryotic species, this has facilitated the use of both vertebrate and invertebrate model systems for exploring the roles of Rho family members in embryonic development and adult physiology.
Major recent advances
Rho-family GTPases cycle between an active and an inactive state, and cells have evolved elaborate mechanisms to regulate the timing of their activation, and to ensure that the active molecules are targeted to appropriate subcellular destinations. While tremendous progress has been made in this area overall, one of the most interesting recent observations comes from a paper by Palamidessi et al. [3] that links Rac1 activation to the process of membrane trafficking. This carefully designed study shows that Rac1 is activated on a specific class of membrane-bound vesicles, the early endosomes, and then recycled back to the plasma membrane, where it controls actin assembly and membrane dynamics. By linking Rac1 activation to membrane trafficking, the cell efficiently achieves a high degree of control over its spatial targeting, restricting active Rac1 to the sites where it is needed. The authors test their hypothesis both in cultured cells grown on a thick matrix, to mimic the basement membranes that define tissue boundaries in the body, and in migrating germ cells in zebrafish, thus providing a powerful combination of approaches to support a novel idea about the targeting of active Rac1 and its role in cell motility.
Important advances have also been made in understanding how signals initiated from outside of the cell can be transmitted through the plasma membrane to result in activation or inhibition of small GTPases (Figure 1). Almost ten years ago, it was shown that engagement of transmembrane integrin receptors results in a transient inhibition of RhoA [4]. More recently, an interesting study by Bass et al. [5] explored a mechanism that permits cross-talk between two different classes of adhesion receptors: integrins and syndecans (Figure 2a). This paper showed that the Rho inhibitor p190RhoGAP is phosphorylated as a consequence of integrin engagement (specifically, the integrin α5β 1), and then targeted to the membrane downstream of syndecan engagement. This suggests that cells have evolved both primary and secondary systems for regulating the small GTPases, an interesting idea that will require further investigation in motile cells.
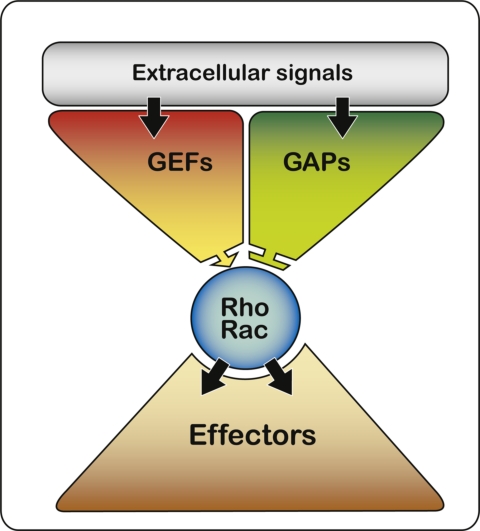
Figure 1.
The activation of Rho-GTPases is mediated by specific guanine-nucleotide exchange factors (GEFs), which catalyze the exchange of GDP for GTP. In their active state, GTPases interact with one of several downstream effectors to modulate their activity and localization. The signal is terminated by hydrolysis of GTP to GDP, a reaction that is stimulated by GTPase-activating proteins (GAPs). Humans have more than 70 Rho-GEFs and approximately 70 Rho-GAPs, allowing the cell to regulate the activity of Rho-GTPases through multiple pathways.
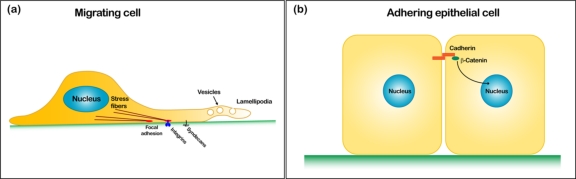
Figure 2.
Rac GTPases have a variety of roles. (a) Recent advances have shed new light on the upstream control of GTPases via adhesion receptors (integrins and syndecans), and on the pathways that localize active Rac to the membrane via vesicle trafficking. These are illustrated in a migrating fibroblast. (b) In adherent epithelial cells, signaling via Rac GTPases impacts on the localization of beta-catenin from the cell-cell junctions to the nucleus.
As GTPases clearly have important roles in organizing the cytoskeleton, many researchers have kept their focus on roles for RhoA and Rac1 in the cytoplasm. Currently, attention is also focused on identifying their roles as mediators of gene transcription in the nucleus. A recent study by Wu et al. [6] showed that Rac1 influences another well known pathway: canonical Wnt signaling, which results in the recruitment of beta-catenin to the nucleus, where it acts as a transcriptional co-activator. The authors show that phosphorylation of beta-catenin downstream of Rac1 activation controls the targeting of beta-catenin from the cytoplasm to the nucleus (Figure 2b). As signaling via beta-catenin is known to have an essential role in limb development in the mouse, the authors went on to use targeted Rac1 knockout mice to ask whether Rac1 activity is also critically required for this process. They found that knocking out Rac1 activity in the limb bud results in dramatic defects in limb outgrowth that resemble those observed in beta-catenin knockout mice. As beta-catenin signaling is involved in both embryonic development and in various cancers, particularly colon cancer, this research has important implications across multiple fields, and it opens up a new set of questions about the ways in which small GTPases may regulate cell behavior, independent of their effects on stress fibers and lamellipodia. Along these lines, Michaelson et al. [7] recently used subcellular fractionation to demonstrate that Rac1 cycles between the nucleus and the cytoplasm, and plays a part in controlling the cell cycle and the rate of mitotic division. This study introduces new questions about a possible role for Rho-family GTPases in the nucleoplasm in addition to the cytoplasm.
Future directions
A potential source of controversy in this field is an effect of a commonly used technique to probe the function of GTPases: the overexpression in cultured cells of mutant GTPases that are locked in the active or inactive state. Recent results suggest that this ‘dominant-negative approach’, in which inactive GTPases are transfected into cells, may be interfering nonspecifically with pathways involving distinct but closely related GTPases. This raises the question: do we need to reconsider the validity of earlier results obtained using dominant-negative approaches? A recent paper about Rac1 and another Rho-family member, RhoG, made this point by showing that results obtained with RhoG knockdown cells and cells that are genetically null for RhoG produce experimental results that are significantly different from those obtained using the dominant-negative approach [8]. This suggests the possibility that certain parts of the Rho and Rac story may undergo substantial rewriting as the field progresses.
Abbreviations
- GAP
GTPase-activating protein
- GEF
guanine-nucleotide exchange factor
- GTPase
guanine nucleoside triphosphatase
Competing interests
The authors declare that they have no competing interests
The electronic version of this article is the complete one and can be found at: http://F1000.com/Reports/Biology/content/1/4
References
- 1.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 3.Palamidessi A, Frittoli E, Garré M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–47. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.8 Must ReadEvaluated by Christopher Marshall 23 Jul 2008, Marie-France Carlier 31 Jul 2008, Harald Stenmark 18 Aug 2008, Gary Bokoch 27 Nov 2008
- 4.Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113:3673–8. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 5.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J Cell Biol. 2008;181:1013–26. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by John Couchman 19 Jun 2008
- 6.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–53. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 8.5 ExceptionalEvaluated by Chaya Kalcheim 22 Apr 2008, Filippo Giancotti 29 Apr 2008, Gregory Dressler 2 May 2008, John Wallingford 6 May 2008, Kathleen J Green with Cory L Simpson 17 Jul 2008
- 7.Michaelson D, Abidi W, Guardavaccaro D, Zhou M, Ahearn I, Pagano M, Philips MR. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J Cell Biol. 2008;181:485–96. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by Ed Manser 7 May 2008, Vania Braga 8 May 2008
- 8.Meller J, Vidali L, Schwartz MA. Endogenous RhoG is dispensable for integrin-mediated cell spreading but contributes to Rac-independent migration. J Cell Sci. 2008;121:1981–9. doi: 10.1242/jcs.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadSelected by Carol Otey 13 Jun 2008, Ed Manser 23 Jun 2008