Abstract
Agents that inhibit histone deacetylases (HDAC inhibitors) have been shown to enhance radiation response. The aim of this study was to evaluate the effects of low, minimally cytotoxic concentrations of the HDAC inhibitor, valproic acid (VPA), on radiation response of colorectal cancer cells. Cell lines LS174T and an isogenic pair of HCT116, which differed only for the presence of wild-type p53, were exposed to ionizing radiation (IR) alone, VPA alone, or the combination. Clonogenic survival, γ-H2AX induction, apoptosis, changes in mitochondrial membrane potential, and mitochondrial levels of p53 and Bcl-2 family proteins were assessed. In vivo studies monitored tumor growth suppression after therapy in mice bearing HCT116/p53+/+ and HCT116/p53−/− tumor xenografts. VPA led to radiosensitization, which was dependent on p53 status. A decrease in clonogenic survival, an increase in apoptosis, and an increase in levels of γ-H2AX were observed after VPA+IR, compared to IR alone, in wild-type p53 cells (LS174T and HCT116/p53+/+), as opposed to p53 null cells (HCT116/p53−/−). Exposure to VPA resulted in enhancement of IR-induced mitochondrial localizations of Bax and Bcl-xL, mitochondrial membrane potential, and cytochrome c release only in wild-type p53 cell lines. VPA also enhanced tumor growth suppression after IR only in wild-type p53 xenografts. These data suggest that VPA may have an important role in enhancing radiotherapy response in colorectal cancer, particularly in tumors with the wild-type p53 genotype.
Key words: histone deacetylase inhibitors, apoptosis, colorectal cancer, p53, radiation therapy
Introduction
Although the molecular basis of radiation response is complex and multifactorial,1,2 the predominant mechanism by which therapeutic irradiation kills most tumor cells is through clonogenic death. DNA double-stranded breaks (DSBs) are regarded as the specific lesions that initiate this lethal response,3,4 and the repair of DNA DSBs is critical in determining radiosensitivity.5 Therefore, agents such as histone deacetylase inhibitors (HDIs), targeting DNA and abrogating the responses of the DNA/protein complex to ionizing radiation (IR)-induced DSBs, may serve as a potential radiosensitizing strategy.
Reversible, post-translational acetyl modifications of proteins are increasingly being recognized as critical modulators of protein-protein and protein-nucleic acid interactions in eukaryotic cells. Acetylation has been shown to mediate certain intracellular signaling events by governing such macromolecular interactions, much like the perhaps better-known phosphorylation modifications.6 Histone acetyltransferases (HATs) were among the first activities capable of mediating protein acetylation to be described, and they were originally defined as enzymes that can acetylate core histones, with resulting regulatory effects on chromatin structure and gene expression.6 The counterpart-regulated removal of acetyl modifications is mediated by histone deacetylase activity (HDAC).7 HDACs play important roles in many diverse and essential biologic processes, including intracellular signaling.7 These activities have been categorized into four groups termed classes I through IV.7 The enzymes in classes I, II, and IV contain a structurally conserved catalytic domain, and for this reason, they are quite broadly and commonly susceptible to inhibitory drugs (“HDAC inhibitors”; here, “HDI”) that chelate an essential active-site zinc cation.8,9 Commonly used agents of this kind include valproic acid (VPA), trichostatin A (TSA), and suberoylanilide hydroxamic acid (SAHA).8,9 The mammalian class III enzymes (homologs of the yeast Sir2 protein; hence, the names “Sirtuins” or “SIRTs”) are structurally distinct and have an NAD-dependent mechanism; for this reason, they are insensitive to conventional HDI (but they are inhibited by distinct drug classes).10
HDIs are currently showing considerable promise as therapeutic agents for human malignant diseases.8,9 The use of these drugs is largely empirical at this point in time, and there is little information regarding the structure-function relationship of HDIs, their targeting specificity (if any) for individual HDACs, or the specific biologic processes they impact in mediating anticancer actions.8,9,11
HDIs also show promise in laboratory studies as candidate radiosensitizers in many types of tumors. The initial idea for HDI as a radiosensitizer is that the blockage of HDAC activity by HDI drugs promotes hyperacetylation in core histones and leads to a more relaxed chromatin structure12,13; DNA in decondensed chromatin is relatively sensitive to DNA damage induced by IR.14,15 This model is supported by recent studies that have demonstrated that HDI, such as trichostatin A (TSA), sodium butyrate (NaB), FK228, and MS-275, enhance radiation-induced cell death and increase IR-induced DNA DSBs in a variety of cancer cells.16–19 However, details of molecular mechanisms mediating radiosensitization by HDI are, at present, not clear. Therefore, more information is needed to be able to identify cancer patients for whom HDI treatment with radiation might be helpful.
In the present study, we examined the effects of the HDAC inhibitor, VPA, on radiosensitivity in human colorectal cancer cells lines in vitro and in vivo. The results of this study demonstrate that exposure to VPA leads to a decrease in clonogenic survival and an increase in radiation-induced apoptosis in wild-type p53 cell lines (LS174T and HCT116/p53+/+), but not in the p53 knock-out cell line (HCT116/p53−/−). VPA also results in modulation of the radiation induced by p53-dependent mitochondrial localization of Bax and Bcl-xL, as well as mitochondrial membrane potential and cytochrome c release. Taken together, these data indicate an important role of p53 in HDAC inhibitor-mediated radiosensitization.
Materials and Methods
Cell lines and external beam radiotherapy
The human colorectal cancer cell line, LS174T, was obtained from the American Type Culture Collection (Manassas, VA). Isogenic HCT116 cell lines (p53+/+ and p53−/−) were kindly provided by Doctor Bert Vogelstein (John Hopkins, Baltimore, MD). LS174T cells were maintained in minimum essential medium with 2 mM of l-glutamine, 0.1 mM of non-essential amino acids, 1.0 mM of sodium pyruvate, 1.5 g/L of sodium bicarbonate, and 10% fetal bovine serum (FBS). HCT-116 cells were maintained in McCoy`s 5A medium containing 10% heat-inactivated FBS. Cells were irradiated by using a Mark I Cs-137 Irradiator (J.L. Shepherd Association, San Fernando, CA) at a dose rate of 1.49 Gy/min.
Reagents and antibodies
VPA was obtained from Sigma Aldrich Co., Ltd. (St. Louis, MO). Monoclonal antibody (mAb) with specificity for phospho-histone H2A.X and DNA-PK, and polyclonal antibodies with specificity for acetyl-H4 and Rad51, were purchased from Upstate (Charlottesville, VA). Polyclonal antibodies against cyclin B1, Bcl-xL (H-62), Bax (N-20), Ku-86 (H-300), and a mAb against p53 (DO-1), p21 (F-5), and Ku-70 (E-5) were from Santa Cruz Biotech, Inc. (Santa Cruz, CA). The mAb against mitochondria Hsp-70 (MA3-028) was from Affinity Bioreagents (Golden, CO). The mAb against cytochrome c (SA-226) was obtained from BIOMOL (Plymouth Meeting, PA). The mAb against beta-actin was obtained from Abcam, Inc. (Cambridge, MA).
Clonogenic assay
To evaluate radiosensitivity, cells in the log phase were plated into individual wells of six-well plates or 60-mm dishes. Six (6) hours later, cells were treated with VPA, or phosphate-buffered saline (PBS) as a control, for 16 hours, followed by irradiation to various doses. Colonies were allowed to form over the next 14–20 days in complete medium with or without VPA. Colonies greater than 50 cells were counted and the number of colonies normalized to that observed in unirradiated controls. Mean inactivation doses were determined by the method of Fertil et al.,20 and the sensitizer enhancement ratio for VPA was calculated as the ratio of mean inactivation dose control/mean inactivation dose of VPA.
Flow cytometry assay and cell-cycle phase analysis
Cells in log phase were treated with or without VPA for 16 hours, followed by exposure to 4 Gy IR. Cells were then fixed at indicated time intervals with 80% ethanol, stained with propidium iodide (50 μg/mL), and analyzed by flow cytometry with 5 × 104 events counting per run, as described previously.21 The percentage of cells in the sub-G1, G0/G1, S, and G2/M phases of the cell cycle were determined by using ModFitLT software (Verity Software, Topsham, ME).
Assessment of apoptosis
Cells were treated with or without VPA for 16 hours and then irradiated. After incubation in drug-containing medium for additional 48 hours, 5 × 105 cells were collected and analyzed by Annexin V–FITC (fluorescein isocyonate) staining, as per the manufacturer's instructions (MBL Co. LTD., Woburn, MA). The extent of apoptosis was quantified by using a Becton-Dickinson (Franklin Lakes, NJ) flow cytometer.
Assay of mitochondrial membrane potential
Changes in mitochondrial membrane potential (MMP) were evaluated by staining cells with the fluorescent cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzamidazolocarbocyanin iodide (JC-1), as per the manufacturer's instructions (MitoProbe™ JC-1 Assay Kit for Flow Cytometry; Molecular Probes, Eugene, OR). Briefly, 1 × 106 cells were collected and suspended in fresh medium and stained with 2 μM of JC-1 for 15 minutes. Fluorescence was monitored by using flow cytometry, measuring both the monomer (527-nm emission; green) and J-aggregate (590-nm emission; red) forms of JC-1 following 488-nm excitation. The percentage of monomeric form or fluorescence green was then quantified as the MMP.
Preparation of mitochondrial and cytosolic protein fractions and whole-cell lysates for immunoblotting
After treatment, approximately 2 × 107 cells were collected. The mitochondria and cytosolic protein fractions were prepared from cell pellets and separated by utilizing a reagent-based method per the manufacturer's instructions (Mitochondria Isolation Kit; Pierce, Rockford, IL). Mitochondria were lysed with RIPA buffer [150 mM NaCl, 50 mM Tris-HCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 2 mM ethylene diamine tetraacetic acid (EDTA), 2 mM phenylmethylsulfonyl fluorade (PMSF), 10 μg/mL aprotinin, 10 μg/mL leupeptin, 50 mM NaF, 1 mM Na2VO4, 50 μg/mL soybean trypsin inhibitor, and 20 mM iodoacetamide]. Then, 10 μg of mitochondrial protein or 20 μg of the cytosolic fraction were directly loaded onto SDS-PAGE polyarylamide gel electrophoresil gels for immunoblotting analysis. Anti-PCNA (Santa Cruz, CA), anti-mtHSP70, and anti-cytochrome c antibodies were utilized to test the purity of the preparation. For whole-cell lysates, cells were washed with cold PBS twice and lysed in RIPA buffer with mild sonication. To determine the acetylation status of histone H4, cells were washed twice with cold PBS and resuspended in lysis buffer containing Tris (0.02 M, pH 7.4), 1% Triton X-100, 0.02% 2-mercaptoethanol, and 2 ng/mL of aprotinin.
Tumor growth assay
HCT116/p53+/+ and HCT116/p53−/− cells (3 × 106/0.2 mL HBSS 1x + 1% HSA) were inoculated subcutaneously (s.c.) into the right leg of 4–6-week-old female athymic nude mice (Charles River/NCI, Frederick, MD). When tumor volumes reached a size of 50–100 mm3 (approximation day 7 after inoculation), mice were randomly grouped into four groups, each with 5–7 mice that received the following: (1) saline (0.2 mL); (2) VPA (300 mg/kg); (3) IR (10 Gy) and (4) VPA (300 mg/kg)+IR (10 Gy). Mice were treated with intraperitoneal (i.p.) injections of VPA (300 mg/kg) every 12 hours for 3 days. Localized irradiation of 10 Gy was delivered after the third VPA injection. Tumors were measured biweekly and tumor volumes were determined from caliper measurements of tumor length (L) and width (W), according to the following formula: (L × W2)/2.
Results
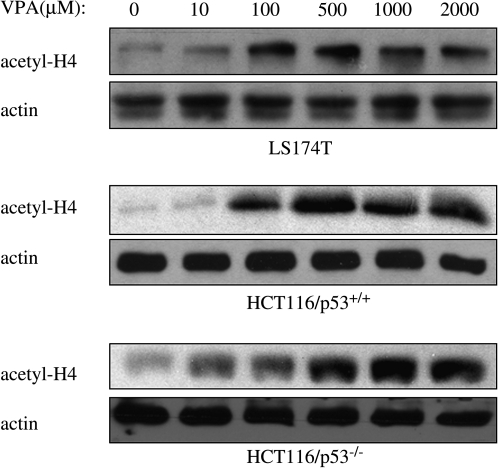
Effects of VPA on histone acetylation were first examined by exposing the human colorectal cancer cell lines, LS174T, HCT116/p53+/+, and HCT116/p53−/−, to different concentrations of VPA for 16 hours. As shown in Figure 1, acetylation of histone H4 increased in a dose-dependent manner. In all cell lines tested, an increase in the level of acetylated histone H4 was detected after the addition of VPA at concentrations ranging from 100 to 500 μM, with no further increase up to a maximum of 2 mM.
FIG. 1.
Changes in histone acetylation after valproic acid (VPA) exposure. LS174T and HCT116 cells were exposed to varying concentrations of VPA for 16 hours. Cellular protein extracts were prepared, as described in Materials and Methods, and analyzed by immunoblot assay with antibody against acetylated histone H4 (acetyl-H4). β-actin was included as a control to show equivalent protein loading.
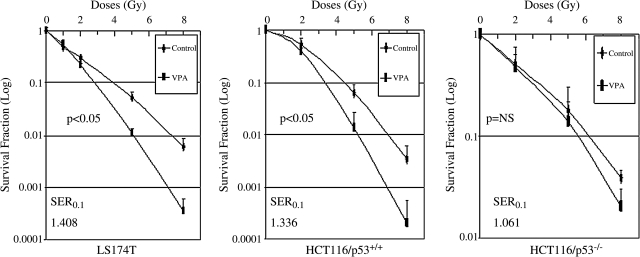
VPA differentially reduces clonogenic survival in irradiated colorectal cancer cells
We next determined the survival of colorectal cancer cells exposed to the combination of VPA and IR by clonogenic assay. Although exposure to VPA increased the levels of acetylated histone proteins in all cell lines, only LS174T and HCT/p53+/+ cells that express wild-type p53 showed significant reduction in IR-induced clonogenic survival when exposed to VPA (Fig. 2). VPA alone had no significant cytotoxic effects, compared to untreated controls, in all tested cell lines. The plating efficiencies in untreated control cells, compared to cells treated with VPA alone, were 28.67 ± 0.96 and 26.67 ± 1.41 in LS174T cells, 36.6 ± 0.73 and 32.7 ± 1.28 in HCT116/p53+/+ cells, and 36.93 ± 0.58 and 30.8 ± 1.05 in HCT116/p53−/− cells, respectively. The sensitizer enhancement ratio (SER) for VPA-treated cells at 0.1 and 0.01 isosurvival, compared to controls, were 1.408 and 1.480 for LS174T and 1.336 and 1.327 for HCT116/p53+/+, respectively. No obvious decrease (SER0.1:1.061) in clonogenic survival with the combination of VPA and IR, compared to IR alone, was observed in HCT116/p53−/− cells in which the p53 gene had been removed through genetic engineering.22 Therefore, our results suggest that p53 likely plays an important role in VPA-enhanced radiosensitization.
FIG. 2.
Clonogenic survival after valproic acid (VPA) and ionizing radiation (IR) exposure. Log-phase cells were trypsinized and plated as single cells. After 6 hours of incubation to allow for cell attachment, cells were pretreated with 500 μM of VPA for 16 hours and then exposed to different doses of IR. Colony survival was determined 14–20 days later. Values represent the mean from three to four independent experiments. Error bars indicate one standard deviation. There was a significant reduction in clonogenic survival with the addition of VPA for LS174T and HCT116/p53+/+, but not for HCT116/p53−/−.
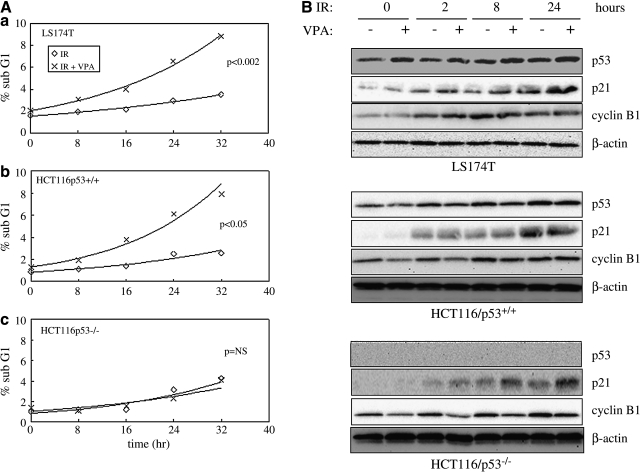
Effects of VPA on cell-cycle phase redistribution after IR
Cells were analyzed by flow cytometry after VPA and IR exposure to determine whether cell-cycle phase redistribution contributed to the observed VPA-induced radiosensitization. VPA pretreatment resulted in a significant increase in the sub-G1 population after irradiation in LS174T and HCT116/p53+/+, but not in HCT116/p53−/−, cells (Fig. 3A). Since the sub-G1 population represents cells undergoing apoptosis, these data suggest that the increase in radiosensitivity seen with VPA on colony formation assay may be due to VPA enhancement of IR-induced apoptosis in the p53-positive lines, LS174T and HCT116/p53+/+. All cell lines exhibited some degree of G2/M delay, after IR alone. Pretreatment with VPA led to a modest increase in G2/M delay, compared to IR alone, which was not significant (Table 1). There were no major differences seen for the other cell-cycle subpopulations with VPA and IR, compared to IR alone.
FIG. 3.
Effects of valproic acid (VPA) on ionizing radiation (IR)-induced sub-G1 cell-cycle distribution and expression of p53, p21, and cyclin B1. Cells were pretreated with or without 500 μM of VPA for 16 hours before γ-radiation (4 Gy). (A) Cells were then stained with propidium iodide and cell-cycle distribution analyzed by flow cytometry at 0, 8, 16, 24, and 32 hours after IR. The data from the two separate experiments were averaged and plotted. Because the percentage of cells in sub-G1 followed more closely an exponential curve than a linear one, a linear regression was performed on the logarithm of the percentages. The slopes of the curves for IR were compared to that of IR+VPA, using a t statistic, and the pooled variances for the pair-wise regressions. (B) Total protein extracts were also prepared at 0, 2, 8, and 24 hours after IR and analyzed by immunoblot assay with antibodies against p53, p21, and cyclin B1. β-actin was included as a control to show equivalent protein loading.
Table 1.
Effects of VPA on IR-Induced Cell-Cycle Distribution
| Cell lines | Cell cycles | Times after IR (hours) | 0 | 8 | 16 | 24 |
|---|---|---|---|---|---|---|
| LS174T | Sub-G1 | Control | 1.56 | 1.93 | 2.15 | 2.97 |
| VPA | 2.04 | 3.07 | 3.99 | 6.55 | ||
| G1/G0 | Control | 70.06 | 53.76 | 59.39 | 71.79 | |
| VPA | 70.08 | 53.90 | 53.63 | 64.96 | ||
| S | Control | 16.29 | 15.08 | 15.96 | 9.80 | |
| VPA | 15.42 | 15.98 | 11.37 | 8.33 | ||
| G2/M | Control | 12.04 | 29.05 | 23.23 | 15.20 | |
| VPA | 12.34 | 26.98 | 30.91 | 19.98 | ||
| HCT116-p53+/+ | Sub-G1 | Control | 0.85 | 1.13 | 1.14 | 2.51 |
| VPA | 1.21 | 1.95 | 3.80 | 6.12 | ||
| G1/G0 | Control | 45.63 | 40.58 | 19.71 | 41.47 | |
| VPA | 44.19 | 39.81 | 16.40 | 30.08 | ||
| S | Control | 29.91 | 19.32 | 11.77 | 17.06 | |
| VPA | 26.56 | 21.04 | 8.36 | 15.04 | ||
| G2/M | Control | 27.21 | 39.37 | 66.85 | 38.46 | |
| VPA | 28.68 | 37.93 | 70.86 | 48.71 | ||
| HCT116-p53−/− | Sub-G1 | Control | 1.03 | 1.22 | 1.28 | 3.14 |
| VPA | 1.43 | 1.11 | 1.60 | 2.31 | ||
| G1/G0 | Control | 41.26 | 9.39 | 6.53 | 23.98 | |
| VPA | 38.80 | 10.65 | 5.73 | 20.25 | ||
| S | Control | 28.93 | 36.54 | 27.13 | 33.53 | |
| VPA | 25.93 | 32.88 | 32.65 | 14.66 | ||
| G2/M | Control | 28.81 | 52.79 | 65.07 | 40.30 | |
| VPA | 33.84 | 55.33 | 60.31 | 62.63 |
Cells were pretreated with or without 500 μM of VPA for 16 hours before γ-radiation (4 Gy). Cells were then harvested at different time points, stained with propidium iodide, and analyzed by flow-cytometry. Data are presented as an average from two experiments.
IR, ionizing radiation; VPA, valproic acid.
We also examined the effects of VPA on the levels of cell-cycle–related proteins. IR alone resulted in an upregulation of p21CIP/WAF1 and cyclin B1 within 24 hours in all three cell lines (Fig. 3B). IR alone also increased p53 stabilization in LS174T and HCT116p53+/+. The addition of VPA to IR had no major observable effect on the level of these proteins, although a modest increase in p21CIP/WAF1 protein levels at 24 hours was observed in HCT116/p53−/− cells. Although p53 is primarily considered a G1-phase regulatory protein,23,24 studies indicate that p53 can also regulate G2 arrest through p21CIP/WAF1 after DNA damage,22 which may, in part, explain the changes in G2/M subpopulations observed at 24 hours with the addition of VPA to IR.
These results indicate that the observed differences in VPA-enhanced radioresponse are not fully explained by changes in cell-cycle redistribution or arrest of cells in more sensitive phases of the cell cycle.
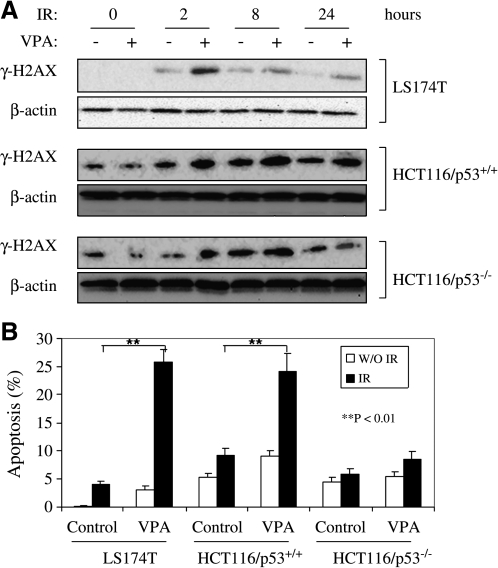
VPA increases DNA double-strand breaks and apoptotic response to irradiation
The occurrence of DNA DSBs after IR represents a potentially lethal lesion to the cell. One of the earliest known responses to radiation-induced DSB formation is phosphorylation of the C-terminal tail of variant H2AX (γ-H2AX) in nucleosomes located in the vicinity of the break.25 Exposure to VPA prior to IR resulted in an increase in γ-H2AX, compared to IR alone, in LS174T and HCT116p53+/+. This difference peaked at 2 hours and remained detectable up to 24 hours. For HCT116/p53−/−, VPA enhancement of IR-induced γ-H2AX induction was seen at 2 hours, but was not observed at 8 and 24 hours (Fig. 4A). These data suggest that exposure to VPA increased the formation of irradiation-induced DNA damage, the magnitude of which may be dependent on p53 status.
FIG. 4.
Effects of valproic acid (VPA) on ionizing radiation (IR)-induced γ-H2AX duration and apoptosis. (A) LS174T, HCT116/p53+/+, and HCT116/p53−/− cells were pretreated with 500 μM of VPA or no VPA for 16 hours prior to 4-Gy irradiation. Cells were then harvested at indicated times, and cell lysates were prepared for immunoblot analysis of γ-H2AX. β-actin was included to show equivalent protein loading. The data are representative of three independent experiments. (B) Apoptosis after IR and VPA. Cells were first exposed to 500 μM of VPA or left untreated for 16 hours before irradiation (4 Gy). Cells were then collected 48 hours after irradiation and apoptosis assayed by Annexin-V–fluorescein isothiocyanate (FITC) staining and flow cytometric analysis, as described in Materials and Methods. Data represent the average of three experiments. Error bars represent one standard deviation.
Next, we assessed the apoptotic response to IR or the combination of VPA and IR in vitro. Exposure to 4-Gy IR or 500-μM VPA alone did not significantly induce apoptotic death in all three colorectal cancer cell lines evaluated. However, when cells were first exposed to 500-μM VPA followed by IR, a significant increase in the percentage of cells undergoing apoptosis was detected in LS174T (from 4.03 ± 0.61 to 25.90 ± 2.17; p < 0.01) and HCT116/p53+/+ (from 9.25 ± 1.27 to 24.21 ± 3.11; p < 0.01), but not in HCT116/p53−/− cells (from 5.79 ± 1.03 to 8.52 ± 1.33; p > 0.05) (Fig. 4B). These data demonstrate that VPA not only enhances IR-induced γ-H2AX, but also augments apoptotic response to IR in a p53-dependent way, which seems to account for the radiosensitization observed by clonogenic assay.
Effects of VPA exposure on modulation of DNA-repair and Bcl-2 family member gene expression after irradiation
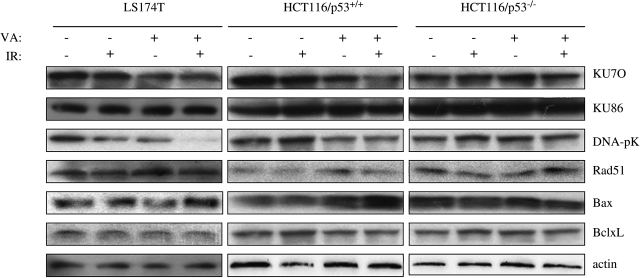
We next examined the effect of VPA exposure on the expression of proteins known to be involved in the repair of radiation-induced DSBs (e.g., KU70, KU86, and DNA-pK abd Rad51) and in apoptotic response (e.g., Bax and Bcl-xL). As shown in Figure 5, VPA exposure decreased the expressions of KU70 and DNA-pKin LS174T and HCT116/p53+/+, but not in HCT116/p53−/−, cells, whereas VPA also induced Bax expression in HCT116/p53+/+ cells. The combination of VPA and IR significantly reduced DNA-pK protein levels only in LS174T cells and increased Bax protein level in HCT116/p53+/+ cells. Therefore, the changes in expression of these apoptotic-related proteins in response to VPA seem to play some role in enhanced apoptosis; however, they do not easily explain the differences in VPA enhancement of radiation-induced apoptotic response observed in the various colorectal cancer cell lines.
FIG. 5.
Changes in expression of apoptosis-related proteins after valproic acid (VPA) and ionizing radiation (IR). LS174T, HCT116/p53+/+ and HCT116/p53−/− cells were pretreated with 500 μM of VPA or left untreated for 16 hours, followed by 4-Gy irradiation. Proteins were extracted and lysed in RIPA buffer 24 hours later. Immunoblot assays were performed to detect the expression of DSB-related proteins (KU70, KU86, DNA-pK, and Rad51) and Bcl-2 family proteins (Bax and Bcl-xL) in cells after VPA and IR. β-actin was included to show equivalent protein loading. DSB, double-strand break.
Exposure to VPA increases IR-induced mitochondrial membrane depolarization
Recent studies have demonstrated that mitochondrial dysfunction plays a critical role in HDIs-induced apoptosis, as well as in HDIs-enhanced apoptotic response to IR.26–28 To further understand the mechanism of VPA-mediated enhancement of apoptosis after IR, we examined the effects of VPA on mitochondrial membrane potential and the modulation of IR-induced mitochondrial localization of Bcl-2 family proteins.
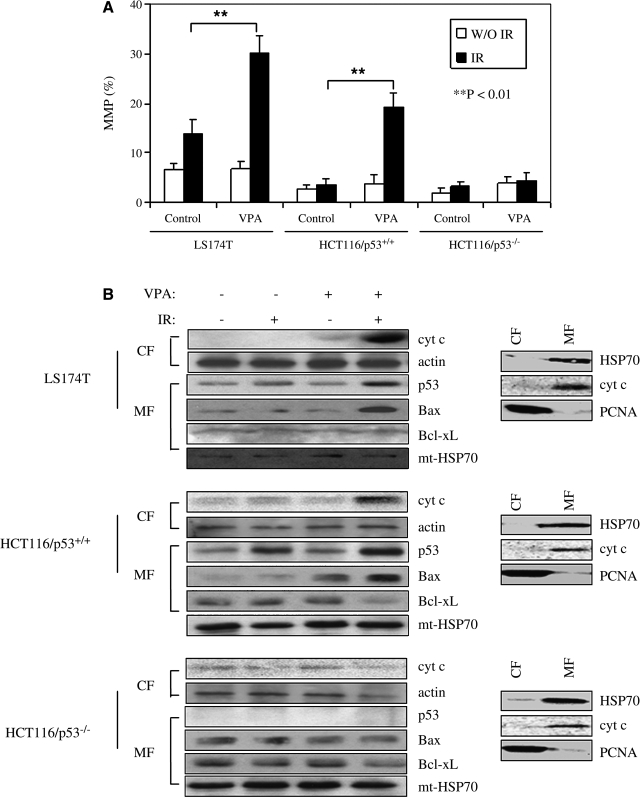
Significant changes in MMP were detected after exposure to the combination of VPA and IR, compared to IR or VPA alone, in LS174T and HCT116/p53+/+ cells (Fig. 6A). This was not observed in HCT116/p53−/− cells. Consistent with this was an observed increase in cytochrome c release from mitochondria detected only in LS174T and HCT116/p53+/+ cells treated with the combination of VPA and IR (Fig. 6B).
FIG. 6.
Effects of valproic acid (VPA) exposure on ionizing radiation (IR)-induced changes in mitochondrial membrane potential and mitochondrial accumulation of p53 and Bcl-2 family membrane proteins. (A) Cells were pretreated with or without 500 μM of VPA for 16 hours before 4-Gy IR. Cells were then collected and stained with JC-1, as described in Materials and Methods. Mitochondrial membrane potential was quantified by the measurement of JC-1 fluorescence intensity. Data represent the average of three independent assays. Error bars represent one standard deviation. (B) Cytochrome c release and mitochondrial accumulation of p53 and Bcl-2 family proteins in response to VPA and IR exposure. Cells were pretreated with or without 500 μM of VPA for 16 hours before 4-Gy IR. Cytosolic (CF) and mitochondrial (MF) fractions were prepared 24 hours after irradiation. Immunoblot assay with antibodies against cytochrome c, p53, Bax, and Bcl-xL was then performed. β-actin and mt-Hsp70 were included to show equivalent protein loading. The data are representative of three independent experiments. To assess the integrity of purified CFs and MFs, equal amounts of proteins (10 μg) were loaded, and mitochondrial proteins cytochrome c (cyto c), HSP70, and the cytosolic/nuclear protein proliferating cell nuclear antigen (PCNA) were assessed by immnunoblotting (right panel).
Figure 6B shows that the combination of IR and VPA increased p53 protein level in mitochondria in LS174T and HCT116/p53+/+ cells, compared to IR or VPA alone. The combination of VPA and IR also increased mitochondrial levels of Bax over IR and VPA alone in LS174T and HCT116/p53+/+, but not in HCT116/p53−/−. No significant changes were observed in Bcl-xL mitochondrial levels.
Taken together, these results suggest that p53 and its localization at the mitochondria may play a role in VPA enhancement of apoptosis after IR and radiosensitivity of colorectal cancer cells.
p53-dependent radiosensitization by VPA in vivo
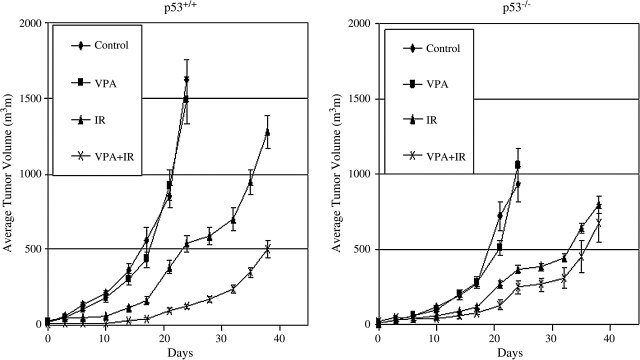
Studies were extended to an in vivo model. We investigated the effects of VPA, IR, or VPA combined with IR on tumor growth of HCT116 xenografts. VPA significantly increased tumor growth suppression, compared to IR alone, in HCT116/p53+/+, but not in isogenic HCT116/p53−/− (Fig. 7). These results are consistent with results seen in vitro.
FIG. 7.
Tumor-growth delay after valproic acid (VPA) and ionizing radiation (IR) exposure in vivo. Athymic nude mice bearing isogenic HCT 116/p53+/+ and HCT 116/p53−/− xenograft tumors were treated with VPA (300 mg/kg × 6) administered intraperitoneally every 12 hours for 3 days and/or 10-Gy irradiation. In the combined treatment group, IR was delivered after the third injection of VPA. The growth curves represent the average value in each group of 5–8 mice. Error bars represent one standard error.
Discussion
Colorectal cancer is the second leading cause of cancer mortality in the United States. Radiation therapy plays an important role in the treatment of this disease, as adjuvant therapy after surgery for locally advanced rectal cancer or as neoadjuvant therapy often combined with chemotherapy.29–31 When utilized in the preoperative setting, radiotherapy, in many cases, can convert an initially unresectable cancer to one resectable by surgery or result in reduction of tumor size to permit sphincter preservation and avoidance of a permanent colostomy. Therefore, strategies that can further sensitize tumors to IR have potential significant clinical importance.
While tumor cells of hematopoietic or lymphatic origin undergo significant apoptosis after irradiation in vitro, and are often highly radioresponsive clinically,32,33 solid tumor cells tend to be relatively refractory to the induction of apoptosis by radiation. The initial response to irradiation in these cells is, instead, growth arrest.34 When cell death does occur, this is usually subsequent to one or more divisions (i.e., so-called reproductive cell death or mitotic catastrophe).35,36
Results from the present study demonstrate that VPA blocked HDAC activity in a dose-dependent manner in all colorectal cell lines studied (LS174T and HCT116). However, in cells exposed to VPA prior to IR, clonogenic survival decreased and radiation-induced DSBs and apoptosis increased, compared to IR exposure alone, only in those cells that expressed wild-type p53 (LS174T and HCT116/p53+/+), but not in cells with the absence of wild-type p53 lines (HCT116/p53−/−). Mice bearing HCT116 wild-type p53 tumor xenografts demonstrated increased tumor growth suppression with the combination of VPA and IR, compared to IR alone, which was not observed in p53 null isogenic tumors. Taken together, these data strongly suggest that VPA may enhance radiation-induced apoptosis and serve as radiosensitizer in a p53-dependent manner in colorectal cancer cells.
The growth-arrest and cell-death pathways, which are activated in response to DNA-damaging agents, such as IR, appear to be closely intertwined, in that both are p53 dependent. The results from this study indicate that augmented apoptotic cell death seems to account for most of the observed radioenhancement secondary to VPA.
The tumor-suppressor protein, p53, is one of the most commonly altered genes in human cancer and plays an important role in cellular response to genotoxic stress.37,38 The role of p53 in apoptosis induced by a variety of stress stimuli was thought, initially, to center on its role as a transcription factor modulating gene expression. In cells with functional p53 protein, a post-translational increase in levels of p5339,40 occurs in response to irradiation, which appears to be mediated through the ATM proteins,41,42 and, ultimately, results in an increase in the level of the cyclin-dependent kinase inhibitory protein, p21Waf1/cip1, and the proapoptotic protein, Bax.39,43,44
Recent work has also provided evidence that p53 can contribute to the induction of apoptosis via non-nuclear pathways through the initiation of proapoptotic mechanisms that involve the physical interaction of the core DNA-binding domain of p53 with apoptosis regulators, such as Bcl-2 and Bcl-XL, at the mitochondria.45–48 Bcl-2 protein binds to other proteins with which it shares amino-acid sequence homology, including Bax, Bcl-XL, Bcl-XS, Mcl-1, Bik, and Bad. Bax (and Bad) and Bcl-2 (and Bcl-xL) are, respectively, pro- and antiapoptotic proteins that influence mitochondrial integrity and membrane potential. Studies have demonstrated that mitochondrial translocation of p53 in response to DNA damage triggers cell death. This appears to involve the localization of p53 to the mitochondria and its interaction with Bcl-xl and Bcl-2, and activation of Bax to increase mitochondrial membrane permeability.46,49,50
Results from the present study show that VPA exposure also leads to significant accumulation of mitochondrial p53 and Bax proteins in response to IR, which occurred in parallel with observed enhancement of mitochondrial membrane potential, and correlated with the observed radiosensitization, in LS174T and HCT116/p53+/+ cells. In addition, the pattern of mitochondrial Bax accumulation did not seem to correlate well with overall expression level, but rather more with the degree of p53 translocating to the mitochondria. This suggests that p53 might directly or indirectly interact and enhance Bax protein accumulation in mitochondria in response to the combination of IR and VPA.
Conclusions
In summary, radiation-induced DNA DSBs, apoptosis, and clonogenic death increased in colorectal cancer lines exposed to VPA, a known HDAC inhibitor. This effect was p53 dependent and observed only in cell lines with wild-type p53. Results also suggest that p53 and its localization at the mitochondria may play a critical role in the VPA enhancement of apoptosis after IR and are consistent with the findings of others, which suggest that localization of p53 to the mitochondria and its interaction with Bcl-xl and Bcl-2, and activation of Bax to increase mitochondrial membrane permeability, may be involved in apoptosis.46,49,50 The observed VPA enhancement of radiation-induced tumor growth suppression in vivo further supports the fact VPA, and possibly other HDAC inhibitors, may be potentially useful in improving tumor response to radiotherapy in the clinical setting, especially for cancers with the wild-type p53 genotype.
Acknowledgments
This work was supported by an NIH Cancer Center grant (CA 33572).
Dislosure Statement
No competing financial interests exist.
References
- 1.Howell SB. Resistance to apoptosis in prostate cancer cells. Mol Urol. 2000;4:225. [PubMed] [Google Scholar]
- 2.Gordon AT. McMillan TJ. A role for molecular radiobiology in radiotherapy? Clin Oncol (R Coll Radiol) 1997;9:70. doi: 10.1016/s0936-6555(05)80443-1. [DOI] [PubMed] [Google Scholar]
- 3.Ward JF. The complexity of DNA damage: Relevance to biological consequences. Int J Radiat Biol. 1994;66:427. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 4.Kastan MB. Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 5.Rogakou EP. Pilch DR. Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 6.Lee KK. Workman JL. Histone acetyltransferase complexes: One size doesn't fit all. Nat Rev Mol Cell Biol. 2007;8:284. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 7.Gregoretti IV. Lee YM. Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Johnstone RW. Licht JD. Histone deacetylase inhibitors in cancer therapy: Is transcription the primary target? Cancer Cell. 2003;4:13. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 9.Piekarz RL. Sackett DL. Bates SE. Histone deacetylase inhibitors and demethylating agents: Clinical development of histone deacetylase inhibitors for cancer therapy. Cancer J. 2007;13:30. doi: 10.1097/PPO.0b013e31803c73cc. [DOI] [PubMed] [Google Scholar]
- 10.Michishita E. Park JY. Burneskis JM, et al. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy S. Tenniswood M. Site-specific acetylation of p53 directs selective transcription complex assembly. J Biol Chem. 2007;282:4765. doi: 10.1074/jbc.M609588200. [DOI] [PubMed] [Google Scholar]
- 12.Florenes VA. Skrede M. Jorgensen K, et al. Deacetylase inhibition in malignant melanomas: Impact on cell cycle regulation and survival. Melanoma Res. 2004;14:173. doi: 10.1097/01.cmr.0000129576.49313.26. [DOI] [PubMed] [Google Scholar]
- 13.Momparler RL. Cancer epigenetics. Oncogene. 2003;22:6479. doi: 10.1038/sj.onc.1206774. [DOI] [PubMed] [Google Scholar]
- 14.Dewey WC. Noel JS. Dettor CM. Changes in radiosensitivity and dispersion of chromatin during the cell cycle of synchronous Chinese hamster cells. Radiat Res. 1972;52:373. [PubMed] [Google Scholar]
- 15.Bedford JS. Dewey WC. Radiation Research Society. 1952–2002. Historical and current highlights in radiation biology: Has anything important been learned by irradiating cells? Radiat Res. 2002;158:251. doi: 10.1667/0033-7587(2002)158[0251:hachir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Chinnaiyan P. Cerna D. Burgan WE, et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clinical Cancer Res. 2008;14:5410. doi: 10.1158/1078-0432.CCR-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinnaiyan P. Vallabhaneni G. Armstrong E, et al. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62:223. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y. Adachi M. Zhao X, et al. Histone deacetylase inhibitors FK228, N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)amino-methyl]benzamide, and m-carboxycinnamic acid bis-hydroximade augment radiation-induced cell death in gastrointestinal adenocarcinoma cells. Cancer. 2004;110:301. doi: 10.1002/ijc.20117. [DOI] [PubMed] [Google Scholar]
- 19.Camphausen K. Burgan WE. Cerra M, et al. Enhanced radiation-induced cell killing and prolongation of gammaH2AX foci expression by the histone deacetulase inhibitor MS-275. Cancer Res. 2004;64:316. doi: 10.1158/0008-5472.can-03-2630. [DOI] [PubMed] [Google Scholar]
- 20.Fertil B. Dertinger H. Courdi A, et al. Mean inactivation dose: A useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73. [PubMed] [Google Scholar]
- 21.Chen X. Shen B. Xia L, et al. Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocytes. Cancer Res. 2002;62:1213. [PubMed] [Google Scholar]
- 22.Bunz F. Dutriaux A. Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 23.Yonish-Rouach E. The p53 tumour-suppressor gene: A mediator of a G1 growth arrest and of apoptosis. Experientia. 1992;52:1001. doi: 10.1007/BF01920109. [DOI] [PubMed] [Google Scholar]
- 24.Mercer WE. Cell cycle regulation and the p53 tumor suppressor protein. Crit Rev Eukaryot Gene Expr. 1992;2:251. [PubMed] [Google Scholar]
- 25.Sedelnikova OA. Pilch DR. Redon C, et al. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y. Adachi M. Kawamura R, et al. Bmf contributes to histone deacetylase inhibitor-mediated enhancing effects on apoptosis after ionizing radiation. Apoptosis. 2006;11:1349. doi: 10.1007/s10495-006-8266-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XD. Gillespie SK. Borrow JM, et al. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther. 2004;3:425. [PubMed] [Google Scholar]
- 28.Peart MJ. Tainton KM. Ruefli AA, et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003;63:4460. [PubMed] [Google Scholar]
- 29.Kapiteijn E. Marijnen CA. Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. NEJM. 2001;345:638. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 30.Sauer R. Becker H. Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. NEJM. 2004;351:1731. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 31.Habr-Gama A. Perez RO. Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg. 2004;240:711. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe SW. Bodis S. McClatchey A, et al. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 33.Rupnow BA. Murtha AD. Alarcon RM, et al. Direct evidence that apoptosis enhances tumor responses to fractionated radiotherapy. Cancer Res. 1998;58:1779. [PubMed] [Google Scholar]
- 34.Scott SL. Earle JD. Gumerlock PH. Functional p53 increases prostate cancer cell survival after exposure to fractionated doses of ionizing radiation. Cancer Res. 2003;63:7190. [PubMed] [Google Scholar]
- 35.Chang WP. Little JB. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991;60:483. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- 36.Szumiel I. Ionizing radiation-induced cell death. Int J Radiat Biol. 1994;66:329. doi: 10.1080/09553009414551271. [DOI] [PubMed] [Google Scholar]
- 37.Haupt S. Berger M. Goldberg Z, et al. Apoptosis—the p53 network. J Cell Sci. 2003;116:4077. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 38.Norbury CJ. Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- 39.Gudas J. Nguyen H. Li T, et al. Effects of cell cycle, wild-type p53, and DNA damage on p21CIP1/Waf1 expression in human breast epithelial cells. Oncogene. 1995;11:253. [PubMed] [Google Scholar]
- 40.Kastan MB. Onyekwere O. Sidransky D, et al. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304. [PubMed] [Google Scholar]
- 41.Pawlik TM. Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Sarkaria JN. Eshleman JS. ATM as a target for novel radiosensitizers. Semin Radiat Oncol. 2001;11:316. doi: 10.1053/srao.2001.26030. [DOI] [PubMed] [Google Scholar]
- 43.Bargonetti J. Manfredi JJ. Multiple roles of the tumor suppressor p53. Curr Opin Oncol. 2002;14:86. doi: 10.1097/00001622-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 44.el Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 45.Leu JI. Dumont P. Hafey M, et al. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 46.Chipuk JE. Kuwana T. Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 47.Mihara M. Erster S. Zaika A, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 48.Manfredi JJ. p53 and apoptosis: It's not just in the nucleus anymore. Mol Cell. 2003;11:552. doi: 10.1016/s1097-2765(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 49.Roy S. Packman K. Jeffrey R, et al. Histone deacetylase inhibitors differentially stabilize acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Differ. 2005;12:482. doi: 10.1038/sj.cdd.4401581. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y. Chaiswing L. Velez JM, et al. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005;65:3745. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]