Abstract
The mechanisms for NK cell activation during infection by intracellular bacterial pathogens are not clearly defined. To dissect how Listeria monocytogenes infection elicits NK cell activation, we evaluated the requirements for activation of naïve splenic NK cells by infected bone marrow-derived dendritic cells (BMDC). We found that NK cell activation in this setting required infection of BMDC by live wild-type bacteria. NK cells were not activated when BMDC were infected with a live hemolysin deficient (Δhly) strain. Neutralization of IL-12, TNFα, or caspase-1 each dramatically reduced NK cell IFNγ production in response to live wt L. monocytogenes infection. Addition of recombinant IL-18, but not IL-1β, reversed the effects of caspase-1 inhibition. Recombinant IL-18 also restored NK cell activation by BMDC infected with Δhly L. monocytogenes, which produced IL-12 but not IL-18. IL-18 acted on NK cells, since MyD88 expression was required in responding NK cells, but not infected BMDC. However, secreted cytokines were not sufficient for activation of naïve NK cells by infected BMDC. Rather, NK cell activation additionally required contact between infected BMDC and NK cells. These data suggest that the activation of NK cells during L. monocytogenes infection requires both secreted cytokines and ligation of NK activating receptors during direct contact with infected DCs.
Introduction
NK cells play an important role in innate immune responses to tumors, viruses, and bacteria. Host cells, such as tumor cells, lacking MHC I are targeted by NK cells due to ‘missing self’ recognition (1). NK cells also recognize up-regulated self and non-self molecules induced in response to stress or infection, such as the NKG2D ligands RAE1 and MULT1 in mice (2). Certain viral proteins also activate NK cells. For example, the MCMV viral protein m157 is presented on the surface of virally infected cells and recognized by the NK receptor Ly49H (2). In addition lysis of infected and tumor cells, NK cells produce IFNγ. IFNγ promotes inflammatory and antibacterial responses by inducing other inflammatory chemokines and cytokines and eliciting nitric oxide and reactive oxygen species in IFNγ-responsive cells (3).
Several cytokines are known to promote NK cell effector mechanisms. For example, viral induction of Type I IFNs (IFNα and β) promotes NK cell cytotoxicity (4). Dendritic cell trans-presentation of IL-15 has also recently been implicated in the priming of NK cells to become fully activated for lysis and IFNγ secretion (5). In addition, IL-12 promotes NK and Th1 type T cells to produce IFNγ in part via induction of the transcription factor Tbet (6). TNFα and TLR signaling, through IRFs and NFκB, enhances NK cell production of IFNγ in concert with IL-12 to enhance NK cell activation (7, 8). NFκB is also activated by IL-1β and IL-18 through a MyD88-dependent pathway (9, 10). IL-1β and IL-18 are synthesized as pro-cytokines that are processed into their active forms by caspases 1 and 11 (11). Caspase-1 deficient mice exhibit decreased IFNγ levels in response to infection with the bacterial pathogen L. monocytogenes (Lm) (12), further implicating IL-1β, IL-18, or possibly other recently identified caspase-1 substrates in NK cell responses to Lm infection (13).
Lm is a facultative intracellular bacterium that replicates within the host cell cytosol and uses host actin machinery to spread from cell to cell (14). Lm requires a hemolysin, LLO, to escape from phagosomes and enter into the host cell cytosol. Mice infected with LLO-deficient Lm (Δhly Lm) do not produce IFNγ 15). Mice lacking the ability to produce or respond to IFNγ fail to control Lm expansion and succumb to normally sub-lethal doses as early as four days after systemic inoculation (16, 17). Early production of IFNγ is thought to promote a Th1 response required for efficient clearance of the pathogen (18). Cells involved in the early production of IFNγ include NK cells and T cells (19), with the NK cells being the major source of this cytokine during the first two days of infection in C57BL/6 mice (20). However, previous data from our lab and others suggest that activation of NK cells by Lm may promote virulence (20, 21). It is thus important to determine how NK cells are activated during infection with live wt Lm.
One challenge in dissecting the requirements for NK cell activation during in vivo infection is defining whether various cytokines primarily act on NK cells or other specific cell types. Although cell culture models can be used to shed light on this issue, previous in vitro studies have largely used NK cells expanded or cultured with IL-15 or IL-2 prior to stimulation with killed infectious agents (8, 22, 23). To determine the factors necessary for activation of naïve NK cells during infection with live Lm and to characterize the respective effects of such factors on NK cells or other cell types, we developed a novel in vitro NK cell activation assay. Using fresh NK cells isolated from naïve mouse spleens and infected bone marrow-derived dendritic cells (BMDC) we were able to reproducibly induce activation of a large fraction of NK cells. With this assay system we confirmed that cytokines such as IL-12 and IL-18 are essential for potent NK cell activation by Lm infection. We further demonstrated that LLO expression by Lm is required to trigger NK cell activation via activation of caspase-1 and the subsequent production of IL-18. However, our findings also revealed that cytokines alone are not sufficient to drive NK cell activation by live Lm infection. Rather, we found that cell contact between naïve NK cells and infected BMDC was essential for efficient Lm-induced NK cell production of IFNγ. This requirement for NK cell contact with infected DCs is not explained by MHC I down-regulation or IL-15 trans-presentation by BMDC, and instead appears to reflect the involvement of an infection-induced NK cell activating ligand(s).
Materials and Methods
Mice
Rag1−/−IL15Rα−/− and MyD88−/− mice crossed onto the B6 background for at least 10 generations were used as sources for bone marrow stem cells to cultivate bone marrow-derived dendritic cells (BMDC), isolate spleen NK cells, and for in vivo mouse infections. C57BL/6 mice were obtained from Jackson Labs. Rag1−/−IL15Rα−/−mice were obtained from Dr. Steve Jameson (University of Minnesota). MyD88−/− mice were obtained from Dr. Christina Leslie (National Jewish Health). Bone marrow and spleens from IL-12R−/− mice were obtained from Dr. Ross Kedl (University of Colorado). Mice were housed in the National Jewish Health Biological Resource Center, and all animal studies were approved by the NJH IACUC.
Mouse infections
Female C57BL/6 and MyD88−/− mice between 8 and 10 weeks of age were used for all in vivo experiments. Mice were infected with 3 ×104 cfu of log phase mouse-passaged L. monocytogenes strain 10403S via intra-peritoneal injection. At the indicated times after infection, sera and spleens were harvested for analysis. Spleens were processed into single cell suspensions for staining and flow cytometry.
Adoptive cell transfers
NK cells were isolated from C57BL/6 mouse spleens via nylon-wool column enrichment followed by depletion of CD3, CD19, CD11c, and CD11b cells using PE-conjugated antibodies (BD Bioscience) and anti-PE magnetic beads (Miltenyi). The resulting NK cell population was ~85% pure. 1.5 × 106 purified NK cells were injected into recipient MyD88−/− mice at the indicated time before infection.
Cell culture
For BMDC, bone marrow cells were flushed from both femurs of one mouse and cultured for 7 days in RPMI media supplemented with 10% FBS, 1% sodium pyruvate, 1% L-glutamine, 1% penicillin/streptomycin, 2-mercaptoethanol, and ~2% GM-CSF (B78hi hybridoma supernatants). During culture, media was changed at day 2 and 4. Non-adherent cells were plated on day 7 for use in in vitro experiments.
Infection and stimulation of cultured BMDC
Following overnight culture in antibiotic-free media, BMDC were infected with L. monocytogenes strain 10403S or an isogenic L. monocytogenes strain with an in-frame deletion of the hly gene coding for LLO (Δhly). Strains were originally obtained from Dr. Daniel Portnoy (UC Berkeley). BMDC were infected with wt L. monocytogenes at an MOI of 1 and Δhly L. monocytogenes at an MOI of 20, unless otherwise indicated. LPS was used in some experiments at 10ng/ml. At 1 hour post-infection, fresh media and 10μg/ml gentamicin was added to each well to kill extracellular bacteria.
Isolation of NK cells
Spleens were harvested from uninfected naïve mice and processed into single cell suspensions. Cell suspensions were added to nylon wool columns and incubated for 1hr at 37°C. Column elutions were collected, and cells stained for NK 1.1 (PK136) and CD3 (145-2C11). NK cells were ~6% of the cell population collected from the columns, compared to 2% from total spleen preparations. For pure NK cells, NWNA were sorted using NK1.1 (PK136) and CD3 (145-2C11) antibodies on a MoFlo XDP sorter to achieve >97% NK cell enrichment. All antibodies were obtained from BD Biosciences and eBioscience.
Co-culture of NK cells and BMDC
BMDC were infected with the indicated L. monocytogenes strains. At 2hpi, NK cells were added to the infected BMDC at a ratio of 0.1:1 and allowed to incubate in co-culture for up to 19hrs. At the indicated time points, cells and/or supernatants were collected for analysis. For cell staining, NK1.1 (PK136), CD3 (145-2C11), and IFNγ XMG1.2) antibodies were used. Cells were fixed and permeabilized using saponin and paraformaldehyde (PFA) buffers. ELISAs were performed using kits for murine IL-1β, TNFα, IL-12p70, and IFNγ BD Bioscience), and IL-18 (MBL). Anti-IL-12 (C17.8), anti-TNFα XT.11), rIL-18 (MBL), rIL-1β R&D Systems), control mouse IgG2a (BD Bioscience), control caspase inhibitor FA-FMK (R&D Systems), caspase-1 specific inhibitor Z-WHED-FMK (R&D Systems), and pan-caspase inhibitor ZVAD-FMK (R&D Systems) were used at the indicated concentrations. Anti-mouse IL-18 (93-10C) (MBL) and anti-mouse ICAM (R&D Systems) were also used at the indicated concentrations.
Statistics
All conditions were tested in triplicate in each experiment, and each experiment was repeated three times. Data shown are a representative experiment. Statistics were done for each experiment using a Student’s t-test. Significance was determined to be p ≤ 0.05.
Results
Co-culture of naïve NK cells and BMDC infected with live, cytosolic Lm elicits IFNγ production
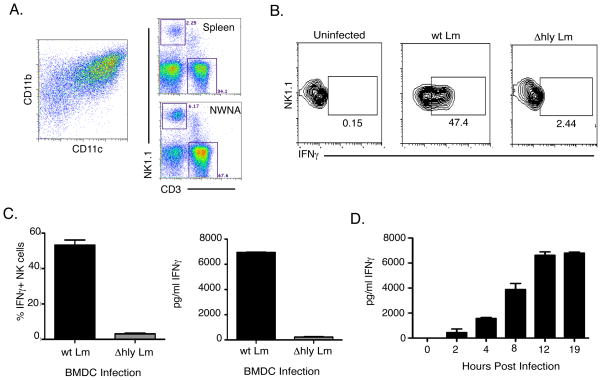
We previously showed that up to 60% of splenic NK1.1+CD3− NK cells produce IFNγ within 24h of wt Lm infection in C57BL/6 (20). We sought to evaluate the mechanism for this bulk activation of NK cells. We used freshly isolated naïve splenic lymphocytes (NWNA) as our source of NK cells. CD11b+CD11c+ BMDCs were used as the infected cell population (Fig. 1A). BMDCs were infected with wt Lm at an MOI of 1. After infection, NWNA cells were added into the culture. At 19hpi, the cultures were analyzed for IFNγ production by both intracellular cytokine staining and ELISA of cell supernatants. BMDC infected with wt Lm triggered over 40% of the NK cells in the NWNA population to produce IFNγ Fig. 1B–C). However, heat-killed wt Lm (data not shown) and LLO-deficient Lm (Δhly Lm) used at an MOI of 20 failed to induce significant IFNγ in parallel co-cultures. IFNγ production by the NK cells peaked by 12hrs of co-culture with infected BMDC (Fig. 1D). These data indicate that cytosolic Lm infection of BMDC is both necessary and sufficient to activate naïve splenic NK cells to produce IFNγ during in vitro co-cultures.
Figure 1.
NK cells produce IFNγ in vitro in response to co-culture with Lm-infected BMDCs. (A) CD11c+CD11b+ BMDC were derived from BM cells cultured in GM-CSF. Spleen cells were processed via nylon wool columns to enrich for lymphocytes. These nylon wool non-adherent (NWNA) cells were co-cultured with wt Lm or Δhly Lm-infected BMDC for 19hrs. (B and C) NK cells produced IFNγ only in response to wt Lm infection as measured by intracellular staining and ELISA. (D) Kinetics of IFNγ production by NWNA responding to wt Lm in infected BMDC as measured by ELISA.
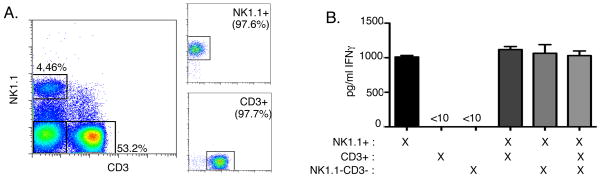
Cell staining revealed that NWNA cell preparations contained NK cells (~6%), T cells (~70%), few contaminating B cells and monocytes (Fig. 1A and data not shown). Depletion of CD3+ cells from NWNA using magnetic bead separation did not affect IFNγ production in co-cultures with infected BMDC (data not shown), suggesting T cells did not significantly contribute to the IFNγ produced in response to infected BMDC. To confirm that only NK cells within the NWNA populations produced IFNγ in response to live wt Lm infection, we sorted NWNA into three populations: NK1.1+CD3− (NK cells), CD3+ (T cells), and CD3−NK1.1− (other cells). All sorted populations were over 97% pure for their respective cell type (Fig. 2A and data not shown). Each sorted cell type was then co-cultured with wt Lm-infected BMDC for 19hrs. Neither CD3+ nor CD3−NK1.1− cells produced IFNγ in co-culture. In contrast, sorted NK1.1+CD3− cell populations produced significant amounts of IFNγ Fig. 2B). Furthermore, addition of purified CD3+ or CD3−NK1.1− cells to the sorted NK cells did not affect the ability of NK cells to produce IFNγ (Fig. 2B). Since NK cell activation did not require help from T cells or other cell populations present in the NWNA preparations, we used this in vitro system to further study the requirements for activation of naïve NK cells during live Lm infection.
Figure 2.
Purified NK cells are solely responsible for IFNγ production in response to Lm-infected BMDC. (A) NWNA were isolated as in Fig. 1 and sorted into three populations: NK1.1+CD3− (NK), CD3+NK1.1− (T), and CD3−NK1.1− (other), each population >90% pure. (B) Sorted NK cells produce IFNγ in response to co-culture with wt Lm-infected BMDC. T cells or other cells alone do not produce IFNγ. Addition of either of the other sorted populations does not enhance IFNγ production in co-cultures.
Pro-inflammatory cytokines produced by infected BMDC contribute to NK cell activation
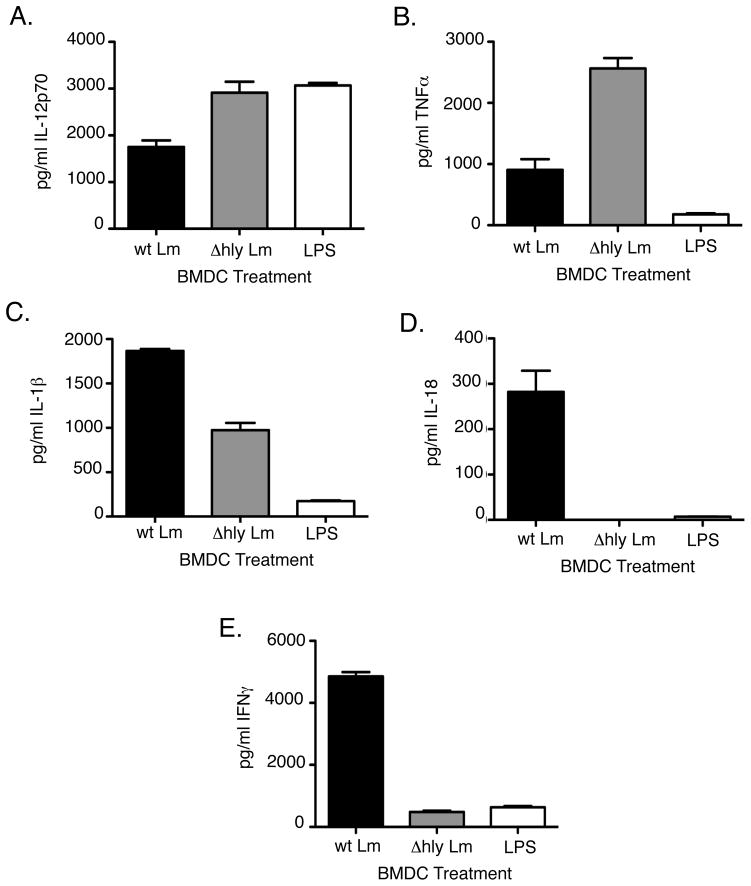
Several pro-inflammatory cytokines previously implicated in the induction of IFNγ by NK cells were also produced by Lm-infected co-cultures. As measured by ELISA, wt Lm infection of BMDC induced secretion of ng concentrations of IL-12p70, TNFα, IL-1β, and pg concentrations of IL-18 by 19hrs of co-culture with NWNA (Fig. 3A–D). In contrast, Δhly Lm infection of BMDC, which failed to induce IFNγ Fig. 1C), induced high levels of IL-12p70 and TNFα, but less IL-1β and no IL-18 (Fig. 3A–D).
Figure 3.
Inflammatory cytokines produced during in vitro co-culture of BMDC and NWNA in response to wt Lm, Δhly Lm, or LPS. (A) BMDC were infected with wt Lm,Δhly Lm, or treated with 10ng/ml LPS. IL-12p70, TNFα, IL-1β, and IL-18 were measured in supernatants after 19hrs. Both Δhly Lm and LPS failed to induce IL-18, but did induce varying levels of each of the other cytokines. (B) Compared to wt Lm infection, Δhly Lm and LPS fail to induce significant levels of IFNγ in co-cultures.
LPS stimulation of BMDC induces a similar pro-inflammatory cytokine profile toΔhly Lm infection of BMDC (24). We thus compared LPS stimulation of BMDC to Lm infection of BMDC to determine which cytokines were necessary for NK activation. After 19hrs of stimulation, LPS induced high levels of IL-12p70, but TNFα and IL-1β concentrations were low at this time point when compared wt Lm infection (Fig. 3A–D). However, there was a substantial level of TNFα ng concentrations) after 6hrs of LPS treatment (data not shown). LPS stimulation of BMDC also poorly induced IFNγ Fig. 3E). These data indicate that neither LPS nor a non-cytosolic Lm infection were sufficient to induce naïve NK cells to make IFNγ.
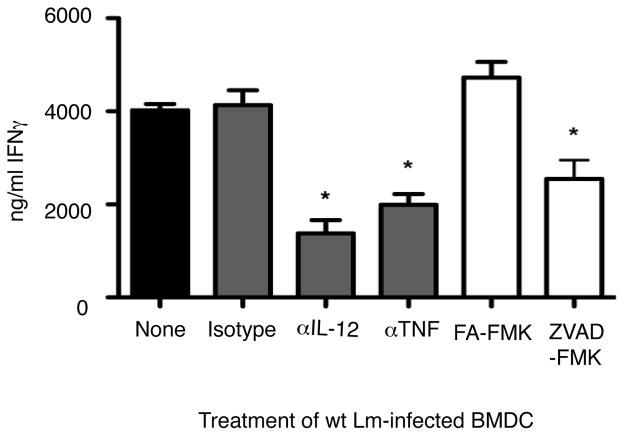
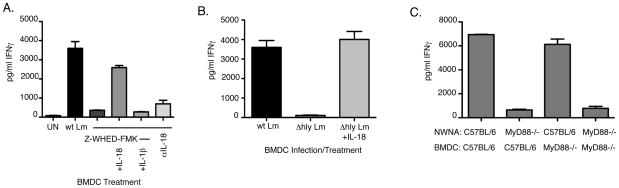
To further determine the relative importance of BMDC-derived cytokines for naïve NK cell activation we inhibited IL-12 and TNFα using neutralizing mAbs at a concentration of 10ug/ml. We blocked caspase activation with the pan-caspase inhibitor Z-VAD-FMK. In each case, IFNγ production was significantly attenuated (Fig. 4). Neutralization of IL-12 did not affect the levels of TNFα produced by infected BMDC, and vice versa (data not shown). These results suggest that IL-12, TNFα, and caspase-1 activation are all involved in naïve NK cells production of IFNγ. However, eliminating individual cytokines or caspases failed to prevent NK cell activation.
Figure 4.
Effects of cytokine inhibition on IFNγ production in response to wt Lm infected BMDC. Control IgG, anti-IL-12 and anti-TNFα were used at 10ug, and the caspase inhibitor ZVAD-FMK and control FA-FMK were used at 10μM. Inhibition of IL-12, TNFα, and caspases reduced the level of IFNγ produced in response to wt Lm infected BMDC, indicating that each of these cytokines is necessary, but not sufficient for activationof naïve NK cells.
Role of Il-18 in Lm-induced NK cell activation
Given that IL-18 was only produced during wt Lm infection of BMDC, and only wt Lm potently induced IFNγ production by naïve NK cells, we hypothesized that caspase-1 activation and release of IL-18 may act as a limiting factor in naïve NK cell activation. To test this, we pre-treated wt Lm-infected BMDC with the irreversible caspase-1 specific inhibitor Z-WHED-FMK. Z-WHED-FMK treatment of infected BMDC reduced IL-1β levels in cell culture supernatants as measured by ELISA (data not shown). The BMDC were washed after inhibitor treatment and incubated with NWNA cells. Inhibition of caspase-1 activity in the BMDC prior to co-culture reduced NWNA IFNγ production to basal levels (Fig. 5A). Furthermore, addition of recombinant IL-18 reversed the effects of Z-WHED-FMK on IFNγ production while addition of recombinant IL-1β had no effect (Fig. 5A). Specific inhibition of IL-18 with a neutralizing mAb also dramatically reduced IFNγ production in co-cultures (Fig. 5A). In the context of these results and our above findings that Δhly Lm infection induces IL-12p70 and TNFα comparable to wt Lm, but reduced IL-1β and no measurable IL-18 (Fig. 3), we hypothesized that lack of IL-18 might be responsible for the failure of Δhly Lm to induce NK cell IFNγ. Indeed, when recombinant IL-18 was added to Δhly Lm infected co-cultures, IFNγ secretion by NWNA was restored to levels seen with wt Lm infected BMDC co-cultures (Fig. 5B). Hence, caspase-1 dependent release of IL-18 is crucial for NK cell IFNγ production and the failure of Δhly Lm to induce IL-18 secretion explains why infection with this attenuated Lm strain fails to activate naïve NK cells.
Figure 5.
IL-18 is necessary IFNγ production by naïve NK cells. (A) wt Lm-infected BMDC co-cultures were treated with the caspase-1 specific inhibitor Z-WEHD-FMK at 100μM. Caspase-1 inhibition abrogated IFNγ induction. Addition of 500 pg/ml exogenous IL-18 restored IFNγ induction, but 2.5 ng/ml of exogenous IL-1β did not. Specific inhibition of IL-18 (1μg/ml of αIL-18) significantly reduced IFNγ induction in co-cultures infected with wt Lm. (B) Addition of recombinant IL-18 to Δhly Lm infected co-cultures restored IFNγ induction to levels seen with wt Lm infection. (C) wt Lm induction of IFNγ requires MyD88 expression on NWNA. C57BL/6 and MyD88−/−BMDC were infected with wt Lm and co-cultured with either C57BL/6 or MyD88−/−NWNA. Only co-cultures with MyD88−/− NWNA failed to induce IFNγ in response to Lm infection.
MyD88 signaling in NK cell activation
MyD88 is a well-known adaptor molecule for many TLRs. In addition, IL-1R and IL-18R utilize MyD88 as an adaptor molecule for downstream signaling (8). MyD88−/− mice exhibit reduced levels of IL-12 and TNFα and fail to make IFNγ in response to Lm infection (10). We sought to directly test whether the reduced IFNγ production in MyD88−/− mice was due to a requirement for TLR or IL-18 signaling in DCs, naïve NK cells, or both. We thus infected C57BL/6 and MyD88−/− BMDC with wt Lm and co-cultured them with either C57BL/6 or MyD88−/− NWNA. Wt Lm infection induced similar IFNγ production by NWNA regardless of MyD88 expression by the infected BMDC (Fig. 5C). Conversely, NWNA lacking MyD88 did not produce IFNγ in response to wt Lm-infected BMDC, even when BMDC expressed MyD88 (Fig. 5C). These data revealed that signaling via MyD88 is only necessary in responding NK cells during infection, likely due to its role in the NK cell response to IL-18 produced by infected DCs.
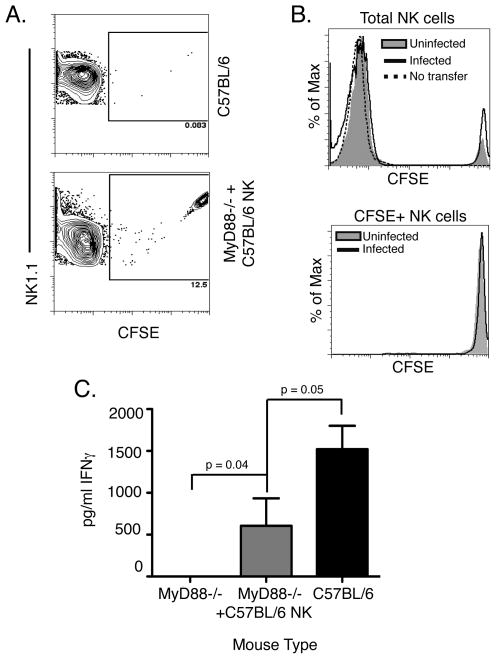
To further examine the requirement and sufficiency of MyD88 signaling in NK cells during in vivo Lm infection, we established an adoptive transfer system using MyD88−/− mice. On day 0 we isolated NK cells from the spleens of C57BL/6 mice by negative selection, labeled them with CFSE, and transferred them into recipient MyD88−/− mice. The purity of the transferred NK cell population was ~84% (data not shown). Recipient and control mice were infected with Lm on day 1, and all mice were analyzed on day 2. At the time of harvest, we detected a significant population of CFSE-labeled NK cells in the spleens of infected mice that had received the adoptive transfer (Fig. 6A), indicating that the transferred cells were able to populate this organ. Analysis of the CFSE staining levels in the transferred NK cells suggested that these cells failed to proliferate within the first day of the Lm infection (Fig. 6B). However, the sera of infected MyD88−/− mice that had received C57BL/6 NK cells exhibited a significantly increased IFNγ concentration when compared to control MyD88−/− mice (Fig. 6C). These findings confirm that expression of MyD88 by naïve NK cells is both necessary and sufficient to permit their activation during Lm infection. Further, our data suggest the requirement for MyD88 is due to its role in the response to IL-18 rather than IL-1β or TLR agonists.
Figure 6.
C57BL/6 NK cells can restore IFNγ production in infected MyD88−/− mice. 1.5 × 106 CFSE-labeled C57BL/6 NK cells were adoptively transferred into MyD88−/− mice before wt Lm infection. (A) At 24hpi, the transferred CFSE-labeled NK cells were clearly visible in the spleens of Lm-infected mice. (B) Transferred NK cells did not show evidence of proliferation with the first day after infection. In the total NK cell population infected MyD88−/− mice that received C57BL/6 NK cells (black line) did not exhibit NK cell proliferation compared to uninfected MyD88−/− mice that received C57BL/6 NK cells (shaded). This was apparent in both the total NK cell population and the population of NK cells gated as CFSE+. (C) IFNγ was undetectable in MyD88−/− and significantly higher in the sera of MyD88−/− mice that received the transferred C57BL/6 NK cells, as determined by the t-test (p=0.04).
NK-DC contact is required for IFNγ production in response to infection
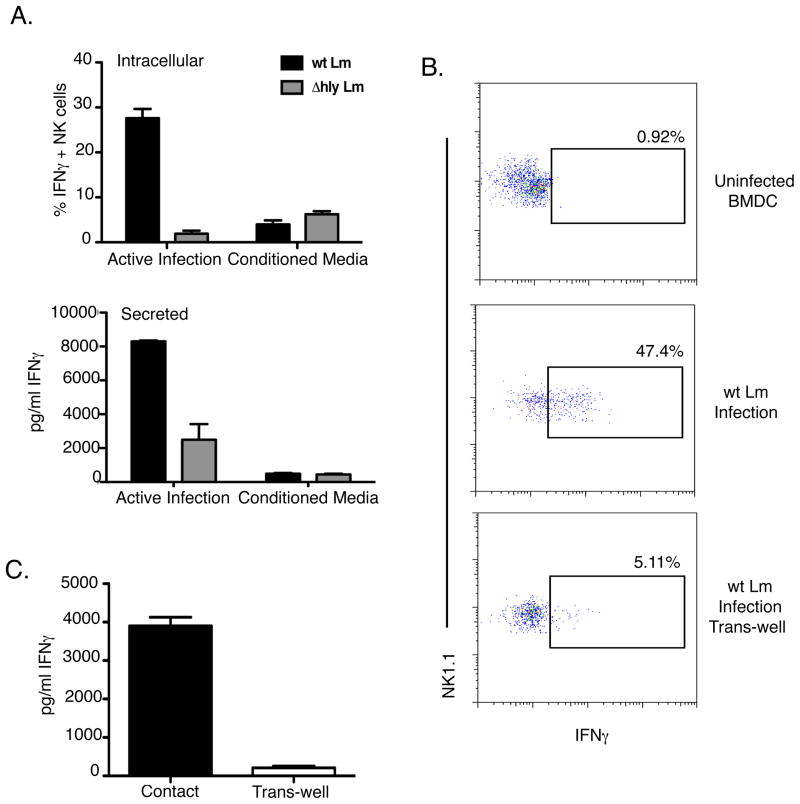
The data above indicated roles for IL-12, TNFα, IL-1β, and IL-18 in the activation of naïve NK cells by wt Lm infection. To test if these secreted cytokines were sufficient for Lm-induced NK cell activation, we transferred conditioned media from infected BMDC onto uninfected BMDC cultured with NWNA. Surprisingly, the transfer of conditioned media from infected BMDC failed to induce IFNγ production and secretion (Fig. 7A). Furthermore, when a 0.5μM membrane was used to separate infected BMDC from responding NWNA for the 19hr co-culture, the NWNA also failed to produce IFNγ, both by intracellular staining and by ELISA (Fig. 7B and C). ELISAs confirmed that pro-inflammatory cytokines such as IL-12 and IL-18 were present in these cultures at concentrations similar to those obtained during co-cultures with direct contact of infected BMDC and NWNA cells (data not shown). Thus we concluded that both cytokines and cell contact were necessary for NK cell IFNγ production in response to wt Lm infection.
Figure 7.
IFNγ induction by naïve NK cells requires cell contact between NWNA and BMDC. (A) BMDC were infected with wt and Δhly Lm for 8hrs. Media was collected from the infected BMDC and added to cultures of uninfected BMDC and NWNA. IFNγ production was measured after 19hrs by ELISA and intracellular staining. Infected BMDC directly co-cultured with NWNA induced IFNγ. Infected BMDC separated from NWNA by a 0.4μM trans-well did not induce significant levels of IFNγ, either by (B) intracellular staining or (C) ELISA.
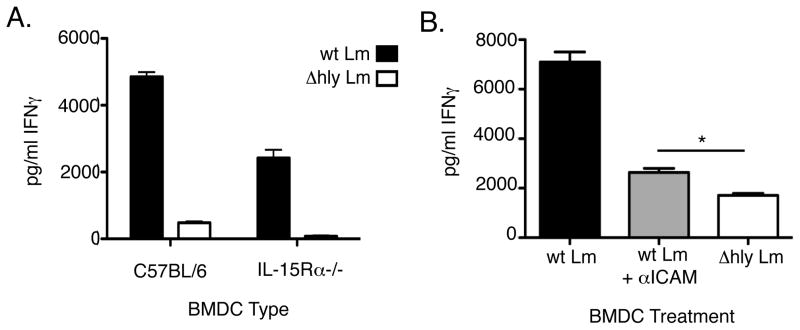
Given a previous report suggesting that cell contact was required in DC priming of NK cells by IL-15 (25), we asked whether IL-15 trans-presentation might account for the contact-dependent interaction in our infection system. Consistent with the previous work, we found that IL-15 trans-presentation was important for NK cell priming since infected IL-15Rα−/− BMDC induced lower amounts of NK cell IFNγ production than did control C57BL/6 BMDC. However, wt Lm infection of the BMDCs still activated NK cell IFNγ production well above the background level seen with the control ΔHly Lm infection (Fig. 8A). Thus, IL-15Rα expression by BMDC contributed to priming of naïve NK cells in our system, but failed to fully account for the contact-dependent induction of IFNγ. To examine cell contact requirements further, we asked whether blocking cell-cell interactions using a mAb against ICAM reduced IFNγ production in the co-cultures. We found that blocking ICAM reduced NK cell activation and IFNγ production to a very low level. However, such blockade failed to fully abrogate production of IFNγ, which was still modestly but significantly higher in the anti-ICAM treated wt Lm-infected cultures than that seen in the ΔHly Lm infected cultures for this experiment (Fig. 8B). These data suggest that either ICAM-specific DC-NK interactions are directly involved in the activation of naïve NK cells or that αICAM interferes with the ability of naïve NK cells to interact with the infected BMDCs in a manner that permits NK cell receptors to bind ligands induced by the wt Lm infection.
Figure 8.
Inhibition of receptor-ligand binding impairs NK cell activation. (A) C57BL/6 or Rag1−/−/IL-15Rα−/− BMDC were infected with wt or Δhly Lm and co-cultured with C57BL/6 NWNA for 19hrs. IFNγ in supernatants was measured by ELISA. (B) C57BL/6 BMDC were infected with wt or Δhly Lm and co-cultured with NWNA. In parallel, BMDC infected with wt Lm were also treated with anti-mouse ICAM1 at 1μg/ml prior to addition of NWNA. Inhibition of ICAM1 severely attenuated the IFNγ response to infection as measured by ELISA.
Discussion
Our results help to clarify the mechanisms for activation of naïve NK cells early after Lm infection. We show that infection of BMDCs with live wt Lm potently activates naïve NK cells to release IFNγ. The mechanism for such activation involves the activation of caspase-1 and release of IL-18 by infected BMDCs. Caspase-1 activation in response to Lm infection is known to require the pore-forming Lm LLO protein (26). Our data show that Δhly Lm-infection of BMDC is not sufficient to trigger activation of naïve NK cells. However, the Δhly Lm infection was sufficient when exogenous IL-18 is added. We also show that TNFα and caspase-1 activity contribute to NK cell activation in this system. While inhibition of either of these molecules impairs IFNγ production, neither is essential for wt Lm-induced NK cell activation.
The precise roles of IL-12 and IL-18 in activation of naïve NK cells are not clear. Mice deficient in both IL-12 and IL-18 have significantly impaired NK cell IFNγ activity compared to mice deficient for either cytokine alone (27). Furthermore, previous in vitro studies have indicated that IL-18 can act in synergy with IL-12 to induce NK cell IFNγ production by Lm and other stimuli (9, 23, 28). One study suggested that IL-12 induces IFNγ transcription while IL-18 stabilizes these transcripts and promotes translation of IFNγ 9). Our results reveal that MyD88 signaling is essential in naïve NK cells, likely due to its role in the response of these cells to IL-18. Although Lm infection of MyD88−/−mice fails to induce NK cell IFNγ production (10), we show that transfer of C57BL/6 NK cells is sufficient to partially restore IFNγ production. MyD88 expression by NK cells was also necessary and sufficient for their IFNγ production in our co-culture system. In contrast to MyD88, our in vitro data reveal that IL-12R expression is required on both DCs and NK cells for efficient induction of IFNγ data not shown). We speculate that IL-12 may likewise have roles on both infected and responding cells during the activation of naïve NK cells during in vivo Lm infection.
An additional conclusion from our studies is that soluble pro-inflammatory cytokines are not sufficient for activation of naïve NK cells in response to wt Lm infection. Although naïve NK cells do secrete IFNγ in our hands after extended culture in recombinant IL-12 and IL-18 (data not shown), the rapid NK cell IFNγ production seen in response to live wt Lm additionally requires direct cell contact between the infected BMDC and responding NK cells. Cell contact has also recently been implicated as a requirement for activation of human NK cells by macrophages infected with Salmonella (22) and RBCs infected with Plasmodium (29). In the case of in vivo infection, cell contact may explain why only 30–60% of splenic NK cells are activated in response to wt Lm (20). Cell contact likely occurs within the lymphoid follicles of the spleen, as recent work suggests that NK cells clustered near DCs and Lm organisms in these areas prior to producing IFNγ 30). The requirement for cell contact may be a way for the immune system to localize NK cell activation within the lymphoid follicles. Such localization may help reduce immunotoxicity and damage to neighboring uninfected cells, and could indicate an important role for NK cells in regulating the function of other lymphocytes.
The specific mechanisms of cell contact in infection-induced NK cell activation are still unclear. Some previous studies have suggested that IL-12 is secreted across a synapse between NK cells and DCs (31). It has also been published that NK/DC interactions can result in the directed release of IL-18 to NK cells (32). Such directed release of cytokines may increase their effective concentrations in cultures where NK cells and DCs are allowed to intimately associate with one another. Thus, one possible mechanistic explanation for the cell contact requirement to activate NK cells is that the low level of IL-18 produced by wt Lm infection (<500pg/ml in vitro) is more efficiently presented to NK cells that are in close proximity to the infected DCs.
Numerous receptor-ligand interactions have been implicated in the activation of NK cells. NKG2D is expressed by nearly all NK cells responding to Lm infection (data not shown) and is associated with cytokine production and cytotoxicity in NK cells (33–37). However, our studies argue against a role for NKG2D in NK cell activation, as blocking NKG2D had no effect on IFNγ production in response to wt Lm infection (data not shown). Adhesion molecules such as ICAM have previously been associated with NK cell activation during M. tuberculosis infection (38), and we also found that blocking cell contact with an antibody to ICAM1 significantly reduced NK cell IFNγ production in our co-culture system. These findings suggest that proximity between infected and responding cells is not sufficient for NK cell activation. Rather, NK cell activation in response to wt Lm infection requires an association between the NK cell and the infected BMDC that can be disrupted by blockade of ICAM1. Whether the actual activating stimulus received by the NK cell is due to ligation of ICAM1 or other specific receptor-ligand pair(s) remains to be determined.
In summary, our findings reveal that regulation of the NK cell response involves a complex combination of priming, pro-inflammatory cytokine stimulation, and contact-dependent “firing” in response to contact with Lm infected DCs. Our in vitro assay system can be used as a tool to further examine the precise mechanisms of NK cell activation by this, and possibly other, intracellular pathogens.
Acknowledgments
The authors would like to thank Rebecca Schmidt for critical reading of the manuscript. We also thank the National Jewish Cytometry Core for help with NK cell sorting experiments.
These studies were supported by grant #AI065638 (to LLL) and training grants AI07505 and AI5206606, which partially supported JH.
References
- 1.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 4.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman LA, Hunter CA. Regulatory pathways involved in the infection-induced production of IFN-gamma by NK cells. Microbes Infect. 2002;4:1531–1538. doi: 10.1016/s1286-4579(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 8.Sawaki J, Tsutsui H, Hayashi N, Yasuda K, Akira S, Tanizawa T, Nakanishi K. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol. 2007;19:311–320. doi: 10.1093/intimm/dxl148. [DOI] [PubMed] [Google Scholar]
- 9.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 11.Mueller NJ, Wilkinson RA, Fishman JA. Listeria monocytogenes infection in caspase-11-deficient mice. Infect Immun. 2002;70:2657–2664. doi: 10.1128/IAI.70.5.2657-2664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji NM, Tsutsui H, Seki E, Kuida K, Okamura H, Nakanishi K, Flavell RA. Roles of caspase-1 in Listeria infection in mice. Int Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 13.Scott A, Saleh M. The inflammatory caspases: guardians against infections and spesis. Cell Death Differ. 2007;14:23–31. doi: 10.1038/sj.cdd.4402026. [DOI] [PubMed] [Google Scholar]
- 14.Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 15.Hara H, Kawamura I, Nomura T, Tominaga T, Tsuchiya K, Mitsuyama M. Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement. Infect Immun. 2007;75:3791–3801. doi: 10.1128/IAI.01779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai WJ, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 17.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 18.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 19.Thale C, Kiderlen AF. Sources of interferon-gamma (IFN-gamma) in early immune response to Listeria monocytogenes. Immunobiology. 2005;210:673–683. doi: 10.1016/j.imbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Humann J, Bjordahl R, Andreasen K, Lenz LL. Expression of the p60 autolysin enhances NK cell activation and is required for listeria monocytogenes expansion in IFN-gamma-responsive mice. J Immunol. 2007;178:2407–2414. doi: 10.4049/jimmunol.178.4.2407. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira HC, Kaufmann SH. Role of NK1.1+ cells in experimental listeriosis. NK1+ cells are early IFN-gamma producers but impair resistance to Listeria monocytogenes infection. J Immunol. 1994;152:1873–1882. [PubMed] [Google Scholar]
- 22.Lapaque N, Walzer T, Meresse S, Vivier E, Trowsdale J. Interactions between Human NK Cells and Macrophages in Response to Salmonella Infection. J Immunol. 2009;182:4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 23.Walker W, Aste-Amezaga M, Kastelein RA, Trinchieri G, Hunter CA. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. J Immunol. 1999;162:5894–5901. [PubMed] [Google Scholar]
- 24.Kolb-Maurer A, Kammerer U, Maurer M, Gentschev I, Brocker EB, Rieckmann P, Kampgen E. Production of IL-12 and IL-18 in human dendritic cells upon infection by Listeria monocytogenes. FEMS Immunol Med Microbiol. 2003;35:255–262. doi: 10.1016/S0928-8244(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 25.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 26.Hara H, Tsuchiya K, Nomura T, Kawamura I, Shoma S, Mitsuyama M. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J Immunol. 2008;180:7859–7868. doi: 10.4049/jimmunol.180.12.7859. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 28.Nomura T, Kawamura I, Tsuchiya K, Kohda C, Baba H, Ito Y, Kimoto T, Watanabe I, Mitsuyama M. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect Immun. 2002;70:1049–1055. doi: 10.1128/IAI.70.3.1049-1055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ing R, Stevenson MM. Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infect Immun. 2009;77:770–782. doi: 10.1128/IAI.00994-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, Galy A, Caignard A, Zitvogel L. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 32.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secrtion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 33.Draghi M, Pashine A, Sanjanwala B, Gendzekhadze K, Cantoni C, Cosman D, Moretta A, Valiante NM, Parham P. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 34.Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J Immunol. 2007;179:3472–3479. doi: 10.4049/jimmunol.179.6.3472. [DOI] [PubMed] [Google Scholar]
- 35.Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 2007;8:1345–1352. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- 36.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 37.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 38.Schierloh P, Aleman M, Yokobori N, Alves L, Roldan N, Abbate E, del CSM, de la Barrera S. NK cell activity in tuberculosis is associated with impaired CD11a and ICAM-1 expression: a regulatory role of monocytes in NK activation. Immunology. 2005;116:541–552. doi: 10.1111/j.1365-2567.2005.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]