Abstract
Human alveolar macrophages were lavaged from surgically resected lungs and from lungs of normal subjects. Macrophages that had been purified by glass adherence were maintained in tissue culture for as long as 54 days. After 3-4 wk in vitro they underwent transformation into multinucleated giant cells. These aged cells had more than 30 times the phagocytic capacity that the same group of cells had had after 1 day in vitro.
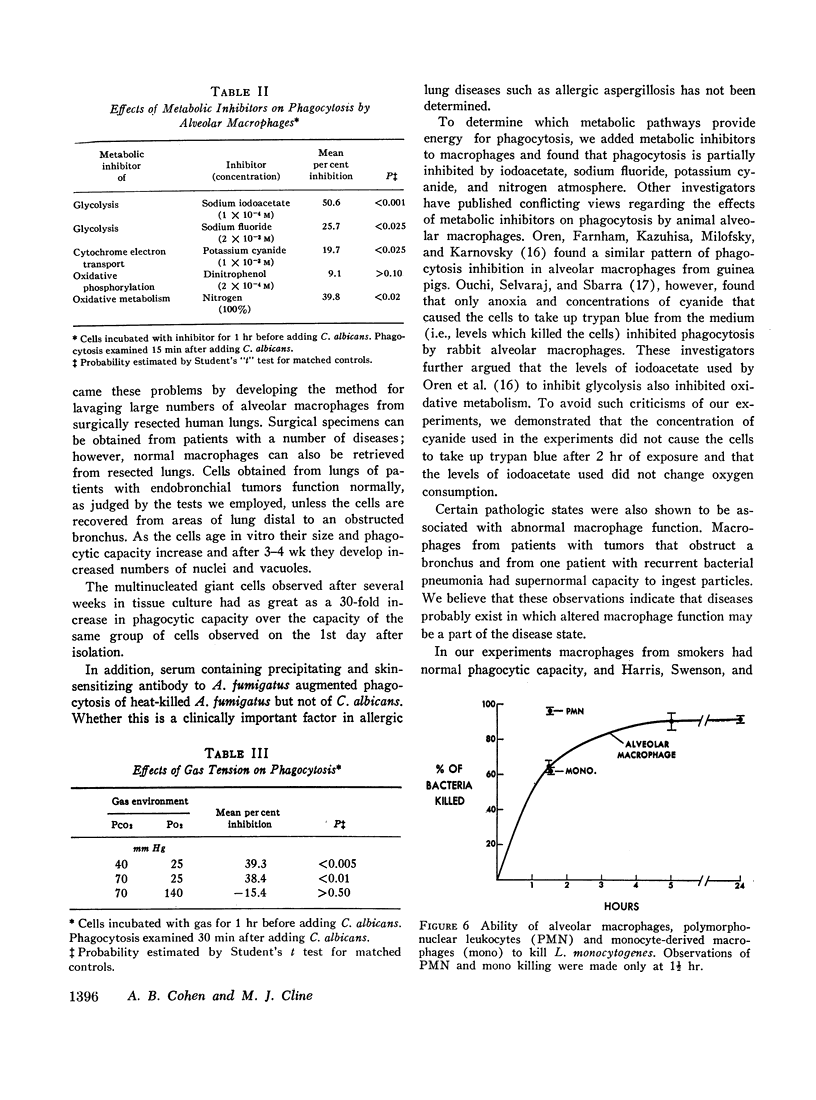
Phagocytosis of heat-killed Candida albicans was inhibited by iodoacetate, sodium fluoride, potassium cyanide, and low partial pressures of oxygen, suggesting that these cells require both oxidative and glycolytic energy sources for maximal particle ingestion. Alveolar macrophages and monocyte-derived macrophages killed Listeria monocytogenes with similar efficiency, but neutrophils were more efficient than either of the other cell types. Bacterial killing is probably not dependent upon myeloperoxidase in the monocyte-derived macrophage or in the alveolar macrophage since histochemical stains for peroxidase do not stain either cell type. C. albicans blastospores, which are killed by neutrophils and monocytes that contain myeloperoxidase, were not killed by human alveolar macrophages during the 4 hr of observation.
Large cells with supernormal phagocytic capacity were recovered from patients with postobstructive pheumonia and from one patient with recurrent bacterial pneumonia, indicating that macrophage function can be altered in certain disease states.
Human alveolar macrophages are unique human phagocytes in their dependence on an oxygen tension greater than 25 mm HG for maximal phagocytosis. Carbon dioxide tensions as high as 70 mm Hg did not alter phagocytosis when the pH of the medium was held constant. These data suggest that the increased susceptibility to pneumonia of patients with chronic bronchitis or atelectasis may be in part related to suboptimal phagocytosis by macrophages in areas of the lung with depressed oxygen tension.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley T. N., Swenson E. W., Curran W. S., Huber G. L., Ladman A. J. Bronchopulmonary lavage in normal subjects and patients with obstructive lung disease. Ann Intern Med. 1967 Apr;66(4):651–658. doi: 10.7326/0003-4819-66-4-651. [DOI] [PubMed] [Google Scholar]
- Firket H. Polyester sheeting (Melinex O), a tissue-culture support easily separable from epoxy resins after flat-face embedding. Stain Technol. 1966 May;41(3):189–191. doi: 10.3109/10520296609116304. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967 Feb 23;276(8):421–427. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- Green L. H., Green G. M. Differential suppression of pulmonary antibacterial activity as the mechanism of selection of a pathogen in mixed bacterial infection of the lung. Am Rev Respir Dis. 1968 Nov;98(5):819–824. doi: 10.1164/arrd.1968.98.5.819. [DOI] [PubMed] [Google Scholar]
- Kass E. H., Green G. M., Goldstein E. Mechanisms of antibacterial action in the respiratory system. Bacteriol Rev. 1966 Sep;30(3):488–497. doi: 10.1128/br.30.3.488-497.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENZI G. A., BERMAN L., FIRST M., KASS E. H. A QUANTITATIVE STUDY OF THE DEPOSITION AND CLEARANCE OF BACTERIA IN THE MURINE LUNG. J Clin Invest. 1964 Apr;43:759–768. doi: 10.1172/JCI104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Grodsky G. M., Caplan J., Craw L. Experimental immune diabetes in the rabbit. Light, fluorescence, and electron microscopic studies. Am J Pathol. 1969 Dec;57(3):597–616. [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi E., Selvaraj R. J., Sbarra A. J. The biochemical activities of rabbit alveolar macrophages during phagocytosis. Exp Cell Res. 1965 Dec;40(3):456–468. doi: 10.1016/0014-4827(65)90226-0. [DOI] [PubMed] [Google Scholar]
- Pratt S. A., Finley T. N., Smith M. H., Ladman A. J. A comparison of alveolar macrophages and pulmonary surfactant(?) obtained from the lungs of human smokers and nonsmokers by endobronchial lavage. Anat Rec. 1969 Apr;163(4):497–507. doi: 10.1002/ar.1091630402. [DOI] [PubMed] [Google Scholar]
- VOISIN C., GUILLAUME J., VAN-MOORLEGHEM C., AERTS C. S'ELECTION ET MISE EN SURVIE IN VITRO DES MACROPHAGES ALV'EOLAIRES DE COBAYE. ASPECTS MORPHOLOGIQUES ET CIN'ETIQUES, ACTIVIT'ES M'ETABOLIQUES ET PHAGOCYTAIRES. Ann Inst Pasteur Lille. 1963;14:183–194. [PubMed] [Google Scholar]
- Ziskind M. M., Schwarz M. I., George R. B., Weill H., Shames J. M., Herbert S. J., Ichinose H. Incomplete consolidation in pneumococcal lobar pneumonia complicating pulmonary emphysema. Ann Intern Med. 1970 Jun;72(6):835–839. doi: 10.7326/0003-4819-72-6-835. [DOI] [PubMed] [Google Scholar]



