Abstract
Aims
We have demonstrated an important role of bone marrow-derived stem cells in preservation of myocardial function. We investigated whether Akt-1 of lin−c-kit+ stem cells preserves ventricular function following myocardial infarction (MI).
Methods and results
Isolated lin−c-kit+ cells were conjugated with anti-c-kit heteroconjugated to anti-vascular cell adhesion molecule to facilitate the attachment of stem cells into damaged tissues. Female severe combined immunodeficient mice were used as recipients. MI was created by ligation of the left descending artery. After 48 h, animals were divided into four groups: (i) sham (n = 5): animals underwent thoracotomy without MI; (ii) MI (n = 5): animals underwent MI and received medium; (iii) MI + wild-type (Wt) stem cells (n = 6): MI animals received 5 × 105 Wt lin−c-kit+ stem cells; (iv) MI + Akt-1−/− stem cells (n = 6): MI animals received 5 × 105 Akt-1−/− lin−c-kit+ stem cells. Two weeks later, left ventricular function was measured in the Langendorff mode. The peripheral administration of Wt armed stem cells into MI animals restored ventricular function, which was absent in animals receiving Akt-1−/− cells. Real-time PCR indicates a decrease in SRY3, a Y chromosome marker in hearts receiving Akt-1−/− cells. An increase in angiogenic response was demonstrated in hearts receiving Wt stem cells but not Akt-1−/− stem cells.
Conclusion
Our results demonstrate that the peripheral administration of Wt lin−c-kit+ stem cells restores ventricular function and promotes angiogenic response following MI. These benefits were abrogated in MI mice receiving Akt-1−/− stem cells, suggesting the pivotal role of Akt-1 in mediating stem cells to protect MI hearts.
Keywords: Akt-1, Myocardial infarction, Stem cells, Heart, Mouse
1. Introduction
Recent advances in the field of cellular cardiomyoplasty have generated enthusiasm for the prospects of stem cell therapy for myocardial regeneration. Adult bone marrow is a rich reservoir of stem and progenitor cells. Bone marrow stromal cells (BMSCs) have many characteristics of mesenchymal stem cells.1 It has been demonstrated that the delivery of primitive bone marrow cells led to the formation of new myocytes generating de novo myocardium.2–4 Administration of stem cell factor and granulocyte colony-stimulating factor (G-CSF) mobilizes pluripotent lin−c-kit+ cells from the bone marrow to the peripheral blood.5 The number of circulating lin−c-kit+ cells increases 250-fold. Introduction of cytokines G-CSF or granulocyte macrophage colony-stimulating factor enhances mobilization of the endothelial progenitors to the ischaemic limbs, augmenting re-endothelialization.6 Primitive bone marrow cells mobilized with cytokines to the damaged myocardium behave as cardiac stem cells, giving rise to myocytes, endothelial cells (ECs), and smooth muscle cells.7 However, others have shown that bone marrow haematopoietic stem cells (HSCs) contribute little to non-haematopoietic tissues.8,9 Recent clinical data have further demonstrated that a multicentre trial of the intracoronary infusion of bone marrow for myocardial infarction (MI) showed an absolute improvement of left ventricular (LV) ejection fraction,10,11 but enthusiasm is tempered by the disparate results.12,13 Nevertheless, clinical studies represent a milestone in this rapidly developing field while serving as a cogent reminder that many important clinical and fundamental questions have yet to be addressed. These beneficial effects of bone marrow stem cells are supported by our own studies in which we have demonstrated that the peripheral delivery of targeted CD34 + HSC with bivalent antibodies directed against myosin light chain antigen significantly increases myocardial functional recovery and angiogenesis in addition to preventing myocardial remodelling.14
The Akt family of intracellular protein kinases regulates cellular growth, proliferation, and metabolism in many systems. Cardiac development and post-natal growth depend on the activation of Akt. Observations from our laboratories demonstrate elevated levels of PI3 and Akt kinases during the proliferative period of cardiac growth.15 It is well known that Akt serves as a powerful survival signal to protect the heart against myocardial injury.16–19 The activation of Akt signalling in bone marrow-derived mesenchymal stem cells resulted in the prevention of cardiac remodelling, an increase in regenerated myocardium, and angiogenesis and restoration of myocardial function.20–22 However, it remains to be determined whether specific Akt-1 of lin−c-kit+ stem cells is essential to produce the beneficial effects after MI. In this study, we utilized a unique and established stem cell-engineered approach to deliver Wt and Akt-1−/− lin−c-kit+ stem cells following MI. We used a mouse gender-mismatched strategy to track delivered cells. We utilized genetically modified mice to further assess the crucial role of Akt-1, a specific Akt isoform in mediating stem cells to preserve cardiac function. Our results demonstrate that the peripheral administration of armed lin−c-kit+ cells restores myocardial function and promotes angiogenic response, which is dependent upon Akt-1 signalling pathway.
2. Methods
Animals: adult male C57BL6 wild-type (Wt) and Akt-1 knockout mice were bred and maintained; severe combined immunodeficient (SCID) female recipient mice were supplied by Charles River Laboratories (Wilmington, MA, USA). All animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital, which conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.1. In vivo MI
The mouse MI model was created following thoracotomy by applying permanent ligation of the left anterior descending artery as described previously.23 Briefly, mice were anaesthetized with an intraperitoneal (ip) injection of sodium pentobarbital at a dose of 50 mg/kg; additional doses of pentobarbital were given as needed during the procedure to maintain an anaesthetized state. Mice were placed in a supine position and were intubated with an endotracheal tube. Ventilation was achieved with a mini rodent ventilator (Harvard, MA, USA). Thoracotomy was performed with tenotomy scissors. A 7-0 nylon suture was passed with a tapered needle under the left anterior descending coronary artery. The suture was tied to create coronary occlusion. Upon completion of ligation, the chest was closed in a layered fashion and air was evacuated to prevent pneumothorax. Mice in the sham group were anaesthetized and underwent thoracotomy without coronary ligation.
2.2. Langendorff's isolated heart perfusion
The methodology of Langendorff's perfused heart preparation and measurement of LV function has been described previously in detail.24 Briefly, mice were anaesthetized with a lethal ip injection of sodium pentobarbital (120 mg/kg). Hearts were rapidly excised and arrested in ice-cold Krebs–Henseleit buffer. They were then cannulated via the ascending aorta for retrograde perfusion by the Langendorff method using the Krebs–Henseleit buffer containing (mM): 110 NaCl, 4.7 KCl, 1.2 MgSO4·7H2O, 2.5 CaCl2·2H2O, 11 glucose, 1.2 KH2PO4, 25 NaHCO3, and 0.5 EDTA. The buffer, aerated with 95% O2:5% CO2 to give a pH of 7.4 at 37°C, was perfused at a constant pressure of 55 mmHg. A water-filled latex balloon, inserted into the LV and attached to the tip of polyethylene tubing, was then inflated sufficiently to provide a left ventricular end-diastolic pressure (LVEDP) of about 10 mmHg measured by means of a disposable Gould pressure transducer. LV functional analysis was performed using software and a computer-based recording system (BIOPAC, Goleta, CA, USA). The measured parameters included LV systolic pressure, LVEDP, heart rate, and LV developed pressure (LVDP), where DP is systolic pressure minus LVEDP. LV dP/dtmax and dP/dtmin were obtained.
2.3. Cell purification and production of bispecific antibodies and cell injection
Bone marrow from male donor mice was collected by crushing the tibiae, femurs and iliac crests in phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA) supplemented with 5% heat-inactivated foetal bovine serum (Invitrogen) using a sterile mortar and pestle. Lin−c-kit+ cells were isolated by immunomagnetic separation using a sequence of negative and positive selection. The lin+ cells were magnetically labelled with a cocktail of biotin-conjugated antibodies/Anti-Biotin MicroBeads specific for the following haematopoietic lineages: CD5, CD45R (B220), CD11b, anti-Ly-6G (GR-1), 7-4, and Ter-19 (Lineage Cell Depletion Kit, Miltenyi Biotec, Auburn, CA, USA). This magnetic cell fraction containing the lin+ cells was eluted using an autoMACS Separator. Subsequently, the remaining lin− cells were directly labelled with c-kit MicroBeads and sorted, with the positive fraction containing the lin−c-kit+ cells.
The methodology for stem cell arming has been described in our previous studies.25 For the studies presented here, anti-c-kit (BD Pharmingen, San Diego, CA, USA) was cross-linked with Traut's reagent and anti-vascular cell adhesion molecule (VCAM) was cross-linked with sulfoSMC. After elution to remove unbound cross-linkers, antibodies were combined and allowed to heteroconjugate overnight to produce anti-c-kit X anti-VCAM. The proportion of dimers, multimers, and monomers were determined by non-reducing SDS–PAGE. The specific antibody was used at a pre-titrated, saturating arming dose of 50 ng/106 purified lin−c-kit+ cells. Two days after MI, either 5 × 105 armed Wt or Akt-1−/− lin−c-kit+ stem cells or 0.15 mL RPMI was injected intravenously via tail vein. Animals receiving the stem cells or RPMI medium were euthanized 2 weeks later for evaluation of cardiac performance.
2.4. Immunofluorescent staining
Cardiac tissues were snap-frozen in pre-chilled 2-methylbutane and embedded in TBS (Pittsburgh, PA, USA). Ten micrometre-thick sections were cut with a cryostat (Thermo Shandon, Pittsburgh, PA, USA) at −22°C, mounted on plus charge slides (Fisher Scientific), and air-dried at room temperature. Sections were fixed in 3.7% (vol/vol) paraformaldehyde for 15 min and permeabilized in 0.5% Triton X-100 in PBS for 10 min. The sections were then incubated with anti-α-smooth muscle actin (α-SMA) monoclonal antibody or anti-von Willebrand factor antibody (vWF, Sigma, St Louis, MO, USA) for 1 h. Secondary antibodies Texas Red horse anti-mouse IgG (H + L) and/or Fluorescein anti-rabbit IgG (H + L) (Vector Laboratories, Burlingame, CA, USA) were applied at room temperature. Fluorescent imaging was performed using a high-resolution Nikon E2000 fluorescence microscope equipped with MetaVue software for image analysis. The numbers of α-SMA and vWF-positive vessels were counted in six randomized fields of the tissue sections, which were taken in the middle plane of each heart and contained infarct and border regions. The total number of vessels from each group was calculated and normalized to tissue area.
2.5. Tunel staining
The sections were assessed by terminal dUTP nick end-labelling (TUNEL) using the In Situ Cell Death Kit, Fluorescein from Roche (Indianapolis, IN, USA) according to the manufacturer's instructions. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei and photographed with a Nikon E2000 fluorescence microscope. The number of TUNEL-positive nuclei in the border zone of infarcted heart was counted.
2.6. Detection of SRY3 by real-time PCR
Genomic DNA was extracted from pooled sections of female recipient hearts that had received male Lin−c-kit+ stem cells. The sections were extracted in 50 µL DNA extraction solution consisting of 100 mM Tris–HCl, 2 mM EDTA, 1% Tween 20, and 500 µg/mL proteinase K at 56°C overnight. The extracted DNA was then boiled for 8 min before performing real-time PCR. Real-time PCR was performed on an ABI Prism 7000 series machine.26 TaqMan gene expression primers and probes for mouse actin and mouse SRY3 were purchased as the Assays on Demand™ from ABI. Heart genomic DNA samples were run in duplicate or triplicate for 40 cycles. PCRs were 25 µL reactions using 5 µL of the gDNA, 1.25 µL primer/probe, 12.5 µL TaqMan Universal PCR 2× Master Mix, and distilled water.
2.7. Measurement of myocardial infarct size and myocyte size
Ten micrometre-thick frozen sections were prepared as before and sections from the apex, mid-LV, and base were stained with Masson's trichrome staining according to the manufacturer's protocol (Sigma). Images of three sections from each heart were taken using a Nikon Eclipse 80i microscope with Spot Advanced software. Infarct scar area and the total area of LV were traced manually and measured using image software (NIH Image J). Infarct size, expressed as a percentage, was calculated by dividing the sum of infarct areas from all sections by the sum of LV areas from all sections (including those without infarct scar) and multiplying by 100. Myocyte cross-sectional area was measured from images captured from the sections obtained mid-distance from the base to the apex. Suitable cross-sections were defined as having nearly circular-to-oval myocyte sections. The outline of myocytes was traced in the LV of each animal, using Image J to determine myocyte cross-sectional area. A value from each heart was calculated by use of the measurements of 30–40 cells of remote area from infarction of an individual heart. The mean area was calculated for the LV in each animal, and the group mean was calculated for each region and group.
2.8. Statistics
All data are expressed as mean ± SEM. Differences among the groups were analysed by one-way analysis of variance, followed by Bonferroni's correction or Student's unpaired t-test for two groups. A value of P < 0.05 was considered a significant difference.
3. Results
3.1. Ventricular function of post-infarction mouse heart in vitro
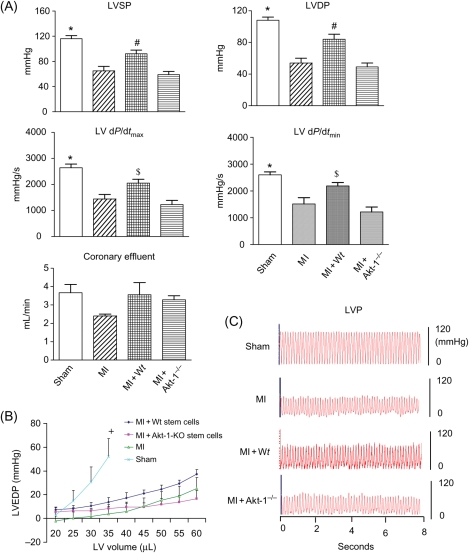
Ventricular function is illustrated in Figure 1A. A significant reduction in LV systolic pressure and DP was observed in infarcted hearts when compared with sham control heart. Administration of lin−c-kit+ stem cells into MI mice restored LV systolic pressure, LVDP, LV dP/dtmax, and LV dP/dtmin. These improvements in ventricular function were not seen following the infusion of Akt-1−/− lin−c-kit+ stem cells. In addition, the diastolic pressure–volume curves resulted in a rightward shift in MI hearts when compared with control sham hearts. Infarcted hearts receiving Wt stem cells show a leftward shift in the pressure–volume relative to infarcted control animals and MI + Akt-1−/− stem cell-treated hearts (Figure 1B). There was no significant difference in heart rate between the groups (data not shown). Therefore, peripheral delivery of armed stem cells increased cardiac functional restoration in post-ischaemic hearts, which was dependent on intact Akt-1 in donor stem cells.
Figure 1.
(A) The effects of infusion of the armed lin−c-kit+ on ventricular function in LV systolic pressure (LVSP), LVDP, LV dP/dtmax, LV dP/dtmin, coronary effluent, and heart rate. Values represent mean ± SE. *P < 0.001 vs. MI, MI + Akt-1−/−, #P < 0.05 vs. MI, MI + Akt-1−/− group; $P < 0.05 vs. MI + Akt-1−/−; MI, myocardial infarction, Wt, wild-type. (B) LV end-diastolic pressure–volume relationship for sham, MI, MI + Wt stem cells, and MI + Akt-1−/− stem cell-treated hearts. LV pressure–volume relationships were then generated. From a balloon volume of zero, the balloon was filled in increments of 5 µL and subsequent pressures recorded. LVEDP, left ventricular end-diastolic pressure. Values are mean ± SEM. +P < 0.05 vs. MI, MI + Akt-1−/−. (C) Representative left ventricular pressure records in sham and different MI hearts. LVP, left ventricular pressure.
3.2. Prevention of myocardial hypertrophy and the reduction of infarct size
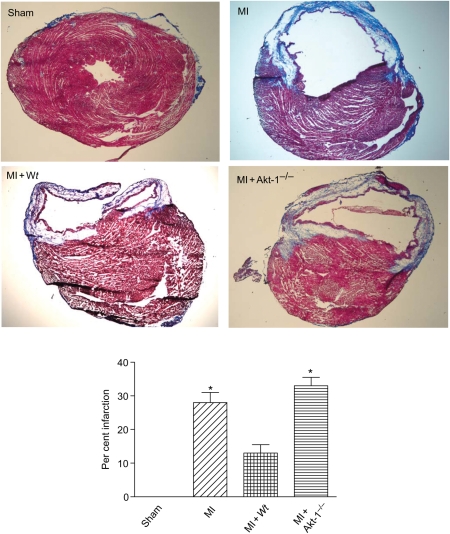
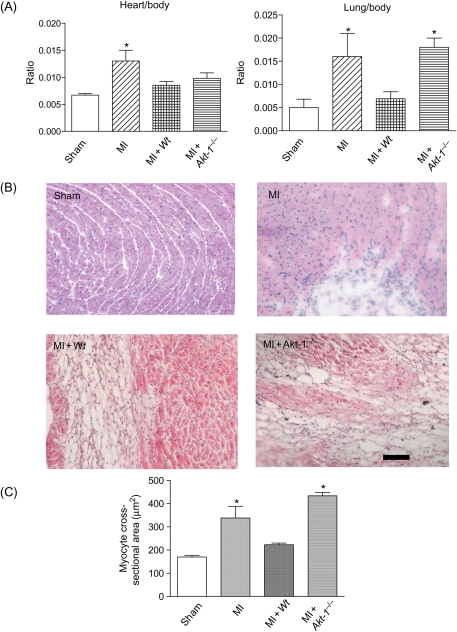
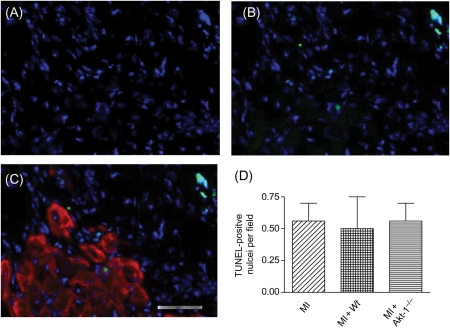
MI hearts exhibited enlarged chamber diameters compared with non-infarcted control hearts. In addition, they showed a decrease in infarct wall thickness, suggesting characteristic post-MI scar thinning and infarct expansion. Administration of the Wt lin−c-kit+ stem cells reduced the infarct size (Figure 2). The reduction of infarct size was markedly decreased in hearts receiving Akt-1−/− stem cells. Figure 3A illustrates that MI resulted in an increase in the ratios of heart/body and lung/body. Myocyte cross-sectional area following MI was also attenuated by Wt, but not Akt-1−/− stem cells, indicating the anti-hypertrophic effects of stem cells (Figure 3B and C). In addition, as shown in Figure 4, there were detectable apoptotic signals in infarcted myocardium, but we did not demonstrate any differences between hearts receiving Wt or Akt-1−/− stem cells.
Figure 2.
Percentage of myocardial infarct size for sham, MI, MI + Wt stem cells, and MI + Akt-1−/− stem cell-treated hearts. Upper panel shows representative images of different groups, and low panel shows the percentage of infarct size among the groups. Values are mean ± SEM (n = 3 per group). *P < 0.05 vs. sham and MI + Wt stem cells.
Figure 3.
(A) The effects of infusion of the armed lin−c-kit+ on heart/body ratio (left) and lung/body ratio (right) in infarcted myocardium. Values represent mean ± SE (n = 3 per group). *P < 0.05 vs. sham group. MI, myocardial infarction; Wt, wild-type. (B) Representative images in sham, MI, MI + Wt stem cells, and MI + Akt-1−/− stem cell-treated hearts. (C) Myocyte cross-sectional areas were compared between the groups. Bar represents 50 µm. Values are mean ± SEM (n = 3 per group). *P < 0.05 vs. sham and MI + Wt stem cells.
Figure 4.
TUNEL-positive cells in the border zone of infarcted myocardium in hearts receiving Wt lin−c-kit+ cells. Nuclei were stained with DAPI (A) and (B) DAPI + TUNEL-positive nuclei. (C) Myocytes were stained with α-sarcomeric actinin (red). (D) Values represent mean ± SE (n = 3 per group). MI, myocardial infarction; Wt, wild-type.
3.3. Evidence of lin−c-kit+ in post-infarction heart
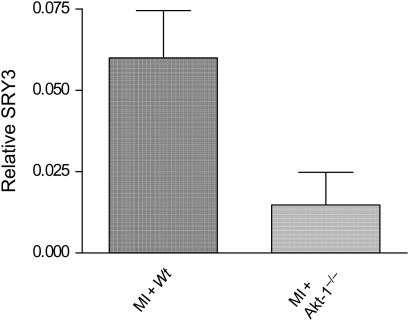
Real-time PCR was used to detect SRY3 in order to quantify the ratio of SRY3 (male only) genomic DNA vs. β-actin (male and female) genomic DNA, which infers the number of male cells at day 14 following intravenous injection of stem cells into female recipient infarcted hearts. As shown in Figure 5, SRY3 was detectable in the infarcted hearts receiving stem cells. Interestingly, SRY3 decreased in hearts receiving Akt-1−/− stem cells, indicating that Akt-1 is critical for stem cell migration, stem cell survival, or cell proliferation.
Figure 5.
Detection of male SRY3 in female recipient hearts by real-time PCR. Values represent mean ± SE (n = 5 per group). The amount of genomic DNA sequence for SRY3 (male only) was expressed relative to the amount of genomic DNA for actin (male plus female) using the ΔCt method. MI, myocardial infarction; Wt, wild-type.
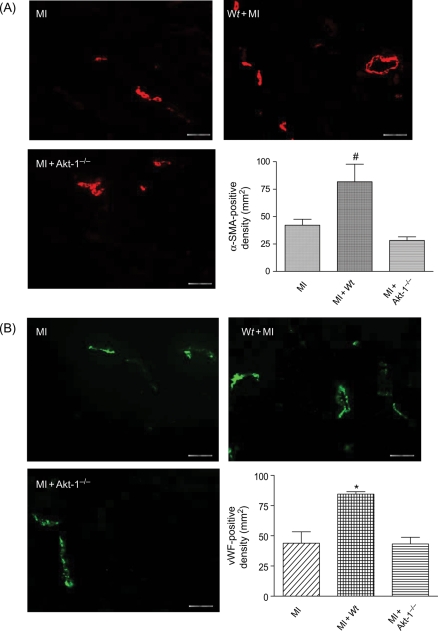
3.4. Akt-1 and angiogenesis
Angiogenic responses were examined by immunofluorescent staining for vascular SMA and vWF. In the border and centre of the infarct zone, α-SMA and vWF-positive stainings were observed (Figure 6). As shown in Figure 6, infusion of the Wt lin−c-kit+ stem cells significantly increased α-SMA- and vWF-stained vessels and vascular density in the border zones of infarcted hearts when compared with MI alone. This angiogenic response was significantly diminished by disruption of Akt-1 in administered stem cells.
Figure 6.
(A) Immunofluorescent staining for α-SMA-positive cells in hearts receiving medium, Wt or Akt-1−/− lin−c-kit+ stem cells, and average number for α-SMA-positive cells. (B) Immunofluorescent staining for vWF-positive cells and average number for vWF-positive cells. Values represent mean ± SE (n = 3 per group), #P < 0.05 vs. MI and Akt-1−/−, *P< 0.05 vs. MI and Akt-1−/− groups. MI, myocardial infarction; Wt, wild-type. Bar represents 50 µm.
4. Discussion
Bone marrow-derived stem cells are demonstrated to form functionally competent cardiomyocytes and vascular structures that exert beneficial effects on infarcted myocardium.2,4 In this study, we have demonstrated: (i) the peripheral administration of armed lin−c-kit+ cells led to significant restoration of ventricular function in infarcted myocardium; (ii) these effects were eliminated by disruption of Akt-1 of stem cells; (iii) the administration of lin−c-kit+ stem cells prevented hypertrophic response and reduced myocardial infarct following MI; (iv) an increased angiogenic response elicited by the delivery of lin−c-kit+ stem cells into infarcted myocardium was absent in the mice receiving Akt-1−/− stem cells; (v) real-time PCR showed an increase in SRY3 in gender-mismatched infarcted hearts, which was reduced by disruption of Akt-1 of stem cells, indicating the essential role of Akt-1 in modulating cell migration, cell survival, and cell proliferation following the peripheral administration of stem cells.
We have previously shown by using a unique approach that BMSCs could be armed with bi-specific antibodies and delivered in increased numbers to injured cardiac target tissue.25 In the present study, we utilized this approach to deliver stem cells into female SCID recipient after creation of an experimental MI. Y chromosome was used to demonstrate cardiac chimerism when a male host received a transplanted heart from a female donor.27 We carried out real-time PCR to detect and quantify the SRY3 genomic marker from male stem cells.28 SRY3 exhibited a mild increase in the female damaged heart receiving male lin−c-kit+ cells, suggesting that the injected male lin−c-kit+ stem cells had migrated to and resided in the recipient hearts following the peripheral delivery of stem cells. Given that the same amount of stem cells was successfully delivered into infarcted recipient mice following MI, we could exclude the contribution of cell loss to account for cardiac functional recovery and SRY3 signals. The higher SRY3 signals in MI hearts of animals receiving Wt lin−c-kit+ cells indicate the importance of Akt-1 in stem cells to migrate towards the infarct zone and mediate survival signalling as well as potential proliferation. However, it is possible, but not established, whether disruption of Akt-1 of lin−c-kit+ cells was susceptible to apoptotic and cell death signalling following in vivo delivery of stem cells after MI. This is worthy to investigate in the future by examining cell accumulation in the infarcted hearts shortly following stem cell injection. In addition, provision of different doses of Wt lin−c-kit+ stem cells into infarcted hearts and comparison of their physiological functions with infarcted animals receiving Akt-1−/− stem cells would suggest an optimal dose of stem cells to achieve cardioprotective effects and the precise beneficial effect of Akt-1 of lin−c-kit+ cells in infarcted hearts.
Mesenchymal stem cells have been shown to restore greater myocardial volume, which was significantly augmented and genetically enhanced by Akt-1 and prevented cardiac remodelling.20 In addition, the role of Akt has been well documented in mediating cardiac growth, protecting the heart against injury as well as preventing cell death.15–18 However, the role of Akt-1 of lin−c-kit+ stem cells in mediating cardiac function of post-infarcted myocardium has not yet been elucidated. In this study, we used lin−c-kit+ stem cells as the donor and delivered them into mice with infarcted myocardium. Lin−c-kit+ stem cells have been shown to regenerate de novo myocardium, which increases myocardial functional recovery.2 In this study, we have performed non-pacing isovolumetric isolated hearts to measure LV functional parameters, which are widely used by us and others.14,20,24 It was also reported that the pacing approach was used to detect ventricular function in the isolated perfused hearts.29 However, our works document that pacing of mouse hearts generated a cardioprotective effect, which is associated with the activation of phosphatidylinositol 3 kinase-eNOs pathway.30 In this regard, we conducted non-pacing isolated perfused hearts to determine LV function. Administration of the armed Wt lin−c-kit+ stem cells significantly improved the restoration of ventricular function recovery in infarcted myocardium, but these beneficial effects on ventricular function were absent in mice receiving the Akt-1−/− lin−c-kit+ cells which is consistent with an observation from Dr Dzau's lab.20 It is also interesting in the future to analyse the force–frequency relationship in isolated myocytes to further investigate the mechanism by which cardiac contractility is mediated.
The beneficial effects of stem cells on the damaged hearts were attributable to anti-hypertrophy and reduction of myocardial infarct size.20 Myocardial infarct size was also reduced with provision of Wt stem cells, but not in hearts receiving Akt-1−/− stem cells, suggesting the role of Akt-1 in the reduction of myocardial necrosis mediated by stem cells in injured myocardium. It will be interesting to investigate the role of Akt-1 on stem cells in regulating myocardial remodelling following MI in future studies. Furthermore, the irregular myocyte structure after MI was ameliorated by administration of stem cells. It is likely that the beneficial effects were due to the paracrine secretory effects of stem cells. Our results are in line with the observations that Akt-1 has been regarded as an important mechanism whereby stem cells generate these protective effects.22,31
Angiogenesis is a complex physiological process consisting of the local degradation of surrounding capillaries and migration of ECs to angiogenic stimuli following proliferation, leading to the formation of a three-dimensional capillary network. We have shown that angiogenesis following CD34 + HSC delivery is associated with the restoration/preservation of myocardial function following MI.14 Coronary effluent was increased by the administration of stem cells compared with that of control MI, but a difference in coronary effluent was not observed between animals receiving Wt and Akt-1−/− stem cells. It will be interesting to perform a long-term follow-up for the measurement of coronary effluent. We observed a more robust angiogenic response after administration of the armed lin−c-kit+ stem cells, which was significantly mitigated by disruption of Akt-1. It is known that pathological cardiac hypertrophy with reduced contractility is accompanied by impaired coronary angiogenesis.32 It is likely that the increase in angiogenic response in stem cell-treated infarcted hearts contribute to the improvement in contractile performance in our studies. This emphasizes the importance of Akt-1 of stem cells in the regulation of angiogenic response in MI.
Activation of Akt has been shown to dramatically inhibit the apoptosis of hypoxic cardiomyocyte and ischaemic myocardium.17 Akt-1 is likewise demonstrated to possess the anti-apoptotic properties of mesenchymal stem cells to protect ischaemic myocardium.20 The apoptotic index was observed in ischaemic myocardium among all groups, but a marked anti-apoptotic effect was not observed after provision of Wt stem cells and/or Akt-1−/− cells, suggesting that anti-apoptosis was not a major mechanism by which Akt-1 initiates cardioprotective effects of lin−c-kit+ stem cells in infarcted hearts. It is not clear from the results, but it will be interesting to see whether the expression of Akt-1 by stem cells affects the ability to prevent apoptosis long term following MI.
4.1. Summary
Our results demonstrated that the intravenous injection of armed lin−c-kit+ stem cells resulted in restoration of ventricular function of post-infarcted myocardium, which was associated with the prevention of cardiac hypertrophy and reduction of myocardial infarct. The beneficial effects of Wt stem cells were abrogated with the disruption of Akt-1 of stem cells. The decreased angiogenic response associated with Akt-1 also indicates the contribution of Akt-1 to neovascularization and myocardial functional recovery. Our results demonstrate that Akt-1 plays an essential role in stem cell-mediated cardioprotection.
Funding
This work was supported by the National Institutes of Health Grant NIH 1P20RR01872802, R01 HL089405 and the National American Heart Association (AHA) and Medical Research Grant 20052634 (RIF).
Acknowledgements
We thank W.C. Sessa from Yale University for providing us Akt-1−/− mice during our study.
Conflict of interest: none declared.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. doi:10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 3.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: a study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. doi:10.1161/01.CIR.0000061910.39145.F0. [DOI] [PubMed] [Google Scholar]
- 4.Lee RJ, Fang Q, Davol PA, Gu Y, Sievers RE, Grabert RC, et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cells. 2007;25:712–717. doi: 10.1634/stemcells.2005-0602. doi:10.1634/stemcells.2005-0602. [DOI] [PubMed] [Google Scholar]
- 5.Bodine DM, Seidel NE, Gale MS, Nienhuis AW, Orlic D. Efficient retrovirus transduction of mouse pluripotent hematopoietic stem cells mobilized into the peripheral blood by treatment with granulocyte colony-stimulating factor and stem cell factor. Blood. 1994;84:1482–1491. [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. doi:10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. doi:10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. doi:10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 9.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. doi:10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 10.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. doi:10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 11.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. doi:10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 12.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. doi:10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig A. Cardiac cell therapy-mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. doi:10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 14.Zhao TC, Tseng A, Yano N, Tseng Y, Davol PA, Lee RJ, et al. Targeting human CD34+ hematopoietic stem cells with anti-CD45 x anti-myosin light chain bispecific antibody preserves cardiac function in myocardial infarction. J Appl Physiol. 2008;104:1793–1800. doi: 10.1152/japplphysiol.01109.2007. doi:10.1152/japplphysiol.01109.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng YT, Yano N, Rojan A, Stabila JP, McGonnigal BG, Ianus V, et al. Ontogeny of phosphoinositide 3-kinase signaling in developing heart: effect of acute beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2005;289:H1834–H1842. doi: 10.1152/ajpheart.00435.2005. doi:10.1152/ajpheart.00435.2005. [DOI] [PubMed] [Google Scholar]
- 16.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, et al. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. doi:10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 17.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 19.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. doi:10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 20.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. doi:10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37:1041–1052. doi: 10.1016/j.yjmcc.2004.09.004. doi:10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. doi:10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. doi:10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao TC, Kukreja RC. Protein kinase C-delta mediates adenosine A3 receptor-induced delayed cardioprotection in mouse. Am J Physiol Heart Circ Physiol. 2003;285:H434–H441. doi: 10.1152/ajpheart.00095.2003. [DOI] [PubMed] [Google Scholar]
- 25.Lum LG, Fok H, Sievers R, Abedi M, Quesenberry PJ, Lee RJ. Targeting of Lin-Sca+ hematopoietic stem cells with bispecific antibodies to injured myocardium. Blood Cells Mol Dis. 2004;32:82–87. doi: 10.1016/j.bcmd.2003.09.019. doi:10.1016/j.bcmd.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Freeman WM, Walker SJ, Vrana KE. Quantitative RT–PCR: pitfalls and potential. Biotechniques. 1999;26:112–122. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- 27.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. doi:10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 28.Haqq CM, King CY, Ukiyama E, Falsafi S, Haqq TN, Donahoe PK, et al. Molecular basis of mammalian sexual determination: activation of Mullerian inhibiting substance gene expression by SRY. Science. 1994;266:1494–1500. doi: 10.1126/science.7985018. doi:10.1126/science.7985018. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PB, Cerny FJ. Evaluation of the isolated paced rat heart. J Appl Physiol. 1976;41:328–331. doi: 10.1152/jappl.1976.41.3.328. [DOI] [PubMed] [Google Scholar]
- 30.Zhao TC, Kukreja RC. Rapid pacing-induced preconditioning in mouse heart: role of phosphatidylinositol 3 kinase-eNOs pathway. Circulation (Suppl.) 2002;106:II57. [Google Scholar]
- 31.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. doi:10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 32.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]