Abstract
Aims
Tumours secrete proangiogenic factors to induce the ingrowth of blood vessels, the end targets of which are vascular endothelial cells (ECs). The MEOX2 homeoprotein inhibits nuclear factor-κB (NF-κB) signalling and EC activation in response to serum and proangiogenic factors. We hypothesize that MEOX2 interacts with components of this pathway in vascular ECs to modulate NF-κB activity and EC activation and that these interactions depend upon specific domains within the MEOX2 protein.
Methods and results
To test our hypothesis, we transduced ECs with MEOX2 expression constructs. MEOX2 protein localized to the nuclear fraction, as did IκBβ and p65. By co-immunoprecipitation, MEOX2 bound to both p65 and IκBβ. Immunofluorescence demonstrated that MEOX2 colocalizes in the nucleus with both p65 and IκBβ and that this colocalization requires the MEOX2 homeodomain and N-terminal domain. Finally, promoter assays revealed that MEOX2 expression has a biphasic effect on NF-κB-dependent promoters. At low levels, MEOX2 stimulates NF-κB activity, whereas at high levels, it represses, effects that also depend upon the homeodomain and the N-terminal domain.
Conclusion
Our results represent the first report of an interaction between a homeobox protein and IκBβ and suggest that MEOX2 modulates the activity of the RelA complex through direct interaction with its components. These observations implicate MEOX2 as a potentially important regulatory gene inhibiting not only the angiogenic response of ECs to proangiogenic factors, but also their response to chronic inflammatory stimulation that normally activates NF-κB, suggesting MEOX2 as a possible molecular target for the therapy of angiogenesis-dependent diseases such as cancer.
Keywords: Vascular endothelial cell, Angiogenesis, Homeodomain protein, Transcriptional regulation, Nuclear factor-κB
1. Introduction
To grow beyond a diameter of 1 mm, malignancies must be able to induce the host to provide them with a blood supply. In vascular endothelial cells (ECs), angiogenesis involves complex temporally coordinated changes in global gene expression in response to alterations in the balance between pro- and antiangiogenic factors.1 Proangiogenic factors bind to cell surface receptors and activate signalling pathways, the end result of which is the binding of transcription factors to promoters of genes whose modulation is necessary for the angiogenic phenotype. We have previously described one such transcription factor, MEOX2 (mesodermal homeobox-2, also known as GAX, Growth Arrest-specific homeoboX), which encodes a homeodomain-containing transcription factor expressed both in vascular smooth muscle and ECs and has characteristics suggesting it as a master regulatory gene controlling the angiogenic phenotype.2–4 MEOX2 expression, maximal in quiescent ECs but rapidly down-regulated in response to serum and proangiogenic or proinflammatory factors,3,5 inhibits EC proliferation and angiogenesis.3,5 Two main mechanisms have been implicated in MEOX2 activity in ECs, the up-regulation of p21WAF1/CIP1 expression by binding directly to its promoter and an upstream enhancer6,7 and the down-regulation of nuclear factor-κB (NF-κB) signalling.5
NF-κB/Rel proteins are a family of transcription factors composed of five related proteins that exist as homo- and heterodimers.8 The classical NF-κB pathway is composed of p50/p65 heterodimers, bound to the nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB), of which there are two isoforms, IκBα and IκBβ.8 When ECs are stimulated by inflammatory or other stimuli, IκBα is phosphorylated by the IκB kinase (IKK) complex, which leads to IκB ubiquitination and degradation of the isoform IκBα.8 This allows the free p50/p65 complex to translocate to the nucleus, bind to NF-κB DNA-binding sites, and activate the expression of downstream batteries of NF-κB-responsive genes. A ubiquitous, pleiotropic, and critical survival signalling pathway involved in inflammation, angiogenesis, and malignant transformation, among other cellular functions, NF-κB, plays an important role in the activation of ECs during inflammation,9,10 and in general, inhibition of NF-κB signalling has been thought to be antiangiogenic.11
Given our previous results demonstrating that MEOX2 expression in ECs inhibits NF-κB signalling and binding of the p50/p65 complex to NF-κB consensus sequence,5 we wished to identify mechanisms by which MEOX2 might accomplish this down-regulation and hypothesized that (i) MEOX2 interacts with components of this pathway in vascular ECs to modulate NF-κB activity and the angiogenic phenotype and (ii) these interactions depend upon specific domains within the MEOX2 protein, particularly the homeodomain. We found that (i) there is indeed an interaction between components of the NF-κB complex and MEOX2 through its homeodomain and N-terminal domain and (ii) this binding might result in sequestration of p65 and IκBβ in the nucleus with a resultant inhibition of in vitro measures of EC activation and a biphasic effect on NF-κB signalling. Our results also suggest that MEOX2, in addition to its role of inducing cell cycle arrest in ECs, may also have an additional role of modulating the inflammatory and angiogenic activation of this cell type and thus represent a logical molecular target for the antiangiogenic therapy of cancer and/or anti-inflammatory therapy designed to prevent or treat atherosclerosis.
2. Methods
2.1. Cells and cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from BioWhittaker (Walkersville, MD, USA) and cultured according to the manufacturer's instructions.
2.2. Expression and reporter constructs
Construction of adenoviral vectors expressing rat and human homologs of MEOX2 (Ad.rMEOX2 and Ad.hMEOX2, respectively) conjugated to α-haemagluttinin (HA) and MEOX2 deletion constructs in pcDNA3.1 vectors has been described previously.2,6,7 Replication-deficient adenoviral vector expressing green fluorescent protein (Ad.GFP) was a gift of Dr Daniel Medina (The Cancer Institute of New Jersey, New Brunswick, NJ, USA). Promoter–reporter constructs used included the interleukin-6 (IL-6) promoter, which contains the IL-6 κB DNA-binding consensus sequences in a pGL3 vector upstream from the Luciferase reporter gene.12 Human ID1 and ID3 promoters were similarly cloned in pGL3 after isolation by PCR. The pGL3-p21 reporter plasmid has been described previously,6 and the intercellular adhesion molecule (ICAM) reporter construct was a kind gift of Dennis Hallahan (Vanderbilt University).13
To generate a tamoxifen-inducible MEOX2 construct, a full-length MEOX2 coding sequence was excised from pcDNA3.1-MEOX2 and inserted into pDsRed1-N1 (Clontech, Mountain View, CA, USA) to generate the MEOX2Red fusion plasmid. The MEOX2Red-oestrogen receptor fusion gene (MEOX2RedER) was then generated using PCR amplification to join sequences corresponding to MEOX2Red with the amino acids 281–599 of the oestrogen receptor tamoxifen mutant MOR G525R.14 The resulting plasmid (pDs-MEOX2RedER) drives tamoxifen-inducible expression of MEOX2, and 4-OH tamoxifen (4OHT) was used to drive MEOX2 expression. The p65GFP plasmid was a kind gift from Dr M.R.H. White, Centre for Cell Imaging, University of Liverpool.15
Transfections were carried out using Trans-IT® Jurkat Transfection Reagent (Mirus Bio Corporation, Madison, WI, USA) according to a modification of the manufacturer's instructions.5,6,16 Experiments using the pDs-MEOX2RedER plasmid to express the MEOX2RedER fusion gene were carried out as follows. 4OHT (Sigma Chemical Company, St Louis, MO, USA) at 100 nM was added to cell culture medium 12 h before harvest, with the same volume of vehicle (ethanol) added to control.
2.3. Protein isolation and detection
Protein was isolated from cells as described previously.5,6,16 Depending upon the experiment, whole-cell extracts, nuclear extracts, or cytoplasmic extracts from treated and control HUVECs were subjected to western blotting using the appropriate dilution of primary antibody in blocking solution, either mouse monoclonal anti-FLAG, mouse monoclonal anti-α-tubulin, and mouse monoclonal anti-HA (Sigma Chemical Company) or mouse monoclonal anti-p65, mouse monoclonal anti-p50, rabbit polyclonal anti-IκBα, anti-IκBβ, and mouse monoclonal anti-p-IκBα (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Bands were visualized by chemiluminescence after incubation with appropriate secondary antibody. Band densities were quantified by densitometry on scanned autoradiographs using NIH ImageJ (Macintosh) and were normalized to α-tubulin for cytoplasmic fractions.
2.4. Promoter assays
To measure the effect of MEOX2 expression on the activity of NF-κB-dependent promoters, HUVECs were cotransfected with varying amounts of pcDNA3.1-MEOX2 or one of its deletions plus reporter vectors containing promoters from IL-6, ID1, ID3, or ICAM as described.5,6,16 Promoter activities were measured using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA).6,16 All values are normalized to the control value, and the total mass of DNA was kept constant by adding an empty vector.
2.5. Electrophoretic mobility shift and supershift assays
Electrophoretic mobility shift assays (EMSAs) for NF-κB binding to its consensus sequence were performed as described previously5,6 using double-stranded oligonucleotide containing a consensus κB-site (5′-AGC TTG CTA CAA GGG ACT TTC CGC TGT CTA CTT T-3′) end labelled with 32P.
2.6. Co-immunoprecipitation experiments
Co-immunoprecipitation (co-IP) of MEOX2, p65, and IκBβ proteins was carried out as follows. After transfection or treatment, cells were harvested by gentle scraping and lysed in RIPA buffer containing PMSF and protease inhibitor cocktail. After clearing of particulate debris by centrifugation, supernatants were pre-cleared with blocked protein G beads (Sigma Chemical Company). Target protein–protein complexes were immunoprecipitated by using either anti-MEOX2 antibody, anti-HA antibody, anti-FLAG antibody (Sigma, St Louis, MO, USA), anti-IκBβ antibody, or anti-p65 antibody (Santa Cruz). Immunocomplexes were captured by incubating with blocked protein G agarose/sepharose bead (Sigma Chemical Company), and the immunocomplex capture was enhanced by adding a bridging antibody to the other samples (Pierce Biotechnology, Rockford, IL, USA). Agarose/sepharose beads were collected by centrifugation and immunoprecipitated antibody–protein–protein complexes eluted with sample buffer and dissociated by boiling. Supernatants were then transferred to fresh microcentrifuge tubes for gel electrophoresis and appropriate western blotting.
2.7. Protein localization by confocal microscopy
Protein localization and colocalization were assessed using confocal microscopy with anti-FLAG antibody (Sigma) and either anti-IκBβ antibody or anti-p65 antibody (Santa Cruz), depending upon the experiment. Cells were plated on cover slips in growth medium, allowed to attach overnight, and then transfected with plasmids as described for each experiment, after which they were fixed in 4% paraformaldehyde, permeabilized with 1% Triton X-100 in phosphate-buffered saline, and blocked with 5% goat serum. Immunostaining was carried out using primary antibody to specific target proteins and proteins of interest visualized using Alexa Fluor488-labelled anti-mouse IgG or Alexa Fluor555-labelled anti-rabbit IgG (Molecular Probes, OR, USA). Cell nuclei were stained with TO-PRO-3 iodide (Molecular Probes). Fluorescence was analysed by using Nikon C1 Digital Eclipse confocal microscope system.
2.8. EC tube formation assays
Tube formation assays were carried out as we have described previously.3,6,16,17 Briefly, HUVECs were transfected with either pcDNA3.1-MEOX2, one of the deletion constructs (see Supplementary material online, Figure S1), or empty vector. Eighteen hours later, 2 × 105 cells were plated on six-well plates on reconstituted basement membrane (Low Growth Factor Matrigel, BD Biosciences, San Jose, CA, USA) and incubated overnight in the presence of serum and 10 ng/mL VEGF165 (R&D Systems, Minneapolis, MN, USA). The number of tubes, defined as projections that connect two cell bodies, per low-powered field (×50) was determined for each well for at least five fields per well by an observer blinded to experimental groups.
2.9. Statistics and data analysis
All experiments were repeated at least three times. In addition, assays producing quantitative data were run in triplicate. Statistical significance was determined by one-way ANOVA or the unpaired Student's t-test, as appropriate.
3. Results
3.1. MEOX2 expression results in increased levels of IκBβ and p65 in the nuclear fraction of ECs
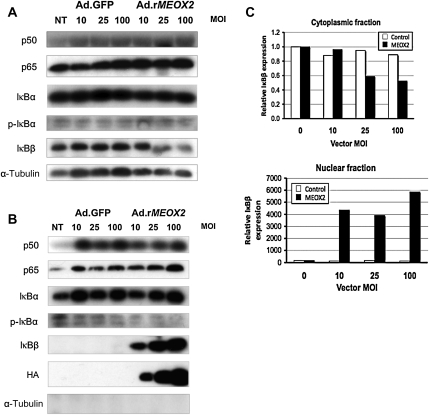
We first wished to determine whether MEOX2 expression alters the expression or localization of components of the NF-κB complex and noted that MEOX2 expression had little effect on the overall cytoplasmic or nuclear levels of p50 (Figure 1). In contrast, although it demonstrated little effect on the cytoplasmic level of p65 (Figure 1A), MEOX2 expression did result in a modest increase in nuclear p65 (Figure 1B) by western blot. More surprisingly, MEOX2 expression resulted in a decrease in the nuclear factor-κB inhibitor, β (IκBβ).8
Figure 1.
MEOX2 expression results in the nuclear accumulation of p65 and IκBβ. Cytoplasmic and nuclear fractions were isolated from HUVECs transduced with either Ad.rMEOX2 or Ad.GFP (control) and subjected to western blot as described in Section 2. (A) Cytoplasmic fraction. IκBβ levels decrease slightly in response to MEOX2 expression, whereas p65 levels remain nearly constant. (B) Nuclear fraction. Nuclear IκBβ levels increase markedly in response to increasing expression of MEOX2 driven by Ad.rMEOX2. Nuclear p65 increases more modestly. No α-tubulin was detectable in the nuclear extract, indicating a lack of contamination with cytoplasmic extract, and MEOX2, a nuclear protein, was undetectable in the cytoplasm (data not shown). Blots were independently loaded using the same protein samples from the same experiment. (C) Densitometry for IκBβ, cytoplasmic, and nuclear fractions, from (A) and (B).
IκBβ levels in the cytoplasm by western blot (Figure 1A) and densitometry (Figure 1C) while increasing MEOX2 expression resulted in an impressive increase in the level of the IκBβ isoform in the nucleus (Figure 1B and C).
3.2. MEOX2 colocalizes with p65 in the nucleus
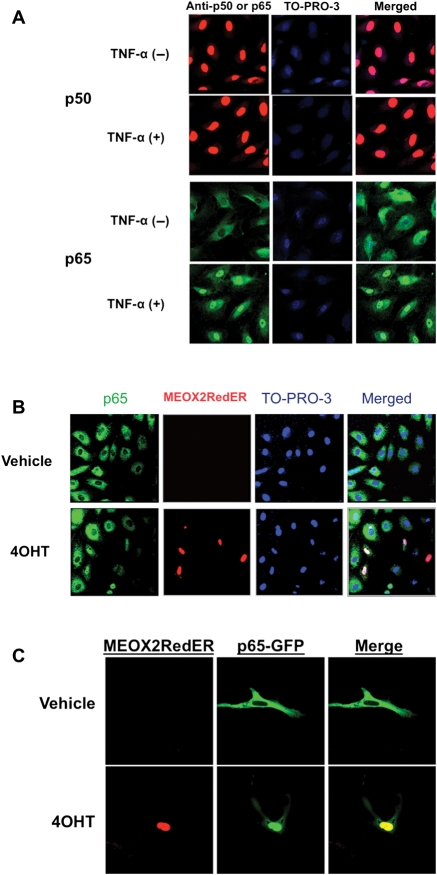
We next examined the localization of NF-κB components with MEOX2 expression driven by an exogenous vector. HUVECs were stimulated with TNF-α (20 ng/mL for 30 min), and p50 was detected in the nucleus at a relatively high basal level in both stimulated and unstimulated HUVECs. In unstimulated cells, p65 remained cytoplasmic but translocated into the nucleus after stimulation (Figure 2A). Next, we wished to determine whether MEOX2 expression alters the localization of the major components of the NF-κB complex. To this end, we cotransfected HUVECs with pMEOX2RedER, which produces tamoxifen-inducible expression of a fusion protein of MEOX2 and red fluorescent protein, and p65GFP, which expresses a fusion of p65 and GFP. Colocalization of MEOX2 and p65 was observed in the nuclei of cells induced with the tamoxifen metabolite 4OHT to produce exogenous MEOX2 (Figure 2B and C). In the cells not expressing MEOX2, p65 remained cytoplasmic. We were surprised to observe that MEOX2 expression was associated with colocalization with p65 in the nucleus in the absence of stimulation with activators of NF-κB.
Figure 2.
MEOX2 colocalizes with the p65 subunit of NF-κB in ECs. (A) Stimulation with TNF-α results in the translocation of NF-κB to the nucleus. HUVECs show a high basal level of p50 in the nucleus, but p65 remains primarily cytoplasmic. However, stimulation with TNF-α (20 ng/mL for 30 min) results in translocation of p65 into the nucleus. Note that the secondary antibody used for the lower panels uses a green fluorescent marker rather than red. (B) p65 translocates into the nucleus in cells expressing MEOX2. HUVECs were cotransfected with pDs-MEOX2RedER and p65GFP under basal conditions (unstimulated with TNF-α). MEOX2 expression was then induced with 100 nM 4OHT. In cells expressing MEOX2, p65 colocalizes in the nucleus, whereas little p65 is observed in the nuclei of cells not expressing MEOX2. (C) An image of an individual cell from one experiment identical to that described in (B). Note that cytoplasmic p65 decreases markedly as MEOX2 and p65 colocalize in the nucleus in response to MEOX2 induction with 4OHT. All images were photographed at a magnification of ×600 using an oil immersion lens.
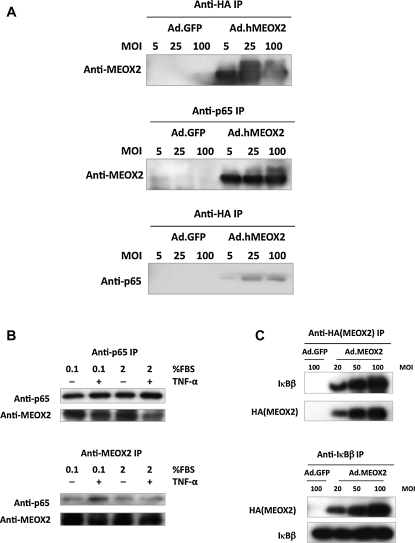
3.3. MEOX2 co-immunoprecipitates with NF-κB components
Next, we performed a series of co-IP experiments to test whether there might be a physical interaction between the proteins involved. HUVECs were transduced with Ad.hMEOX2 at different multiplicity of infections (MOIs) and immunoprecipitation performed with anti-HA antibody and anti-p65 antibody. MEOX2 coprecipitated with p65, but there was no detectable signal in cells transduced with Ad.GFP at an MOI equal to the highest MOI of the Ad.hMEOX2 control (Figure 3A). From this result, we conclude that there is likely a physical interaction between exogenously expressed MEOX2 and endogenous p65. To support this result, we attempted to detect an interaction between endogenous MEOX2 and p65. HUVECs were grown under conditions designed to maximize MEOX2 expression (low serum) or stimulated with TNF-α to induce NF-κB. Under these conditions, MEOX2 and p65 coprecipitated (Figure 3B), suggesting that the interaction observed between endogenous p65 and exogenous MEOX2 can also occur under physiological conditions. Finally, given the large increase in nuclear IκBβ observed in ECs transduced with MEOX2, we were interested in whether MEOX2 and IκBβ also co-immunoprecipitate. Testing this possibility, we observed that MEOX2 and IκBβ also coprecipitated (Figure 3C), suggesting a physical interaction between these two proteins as well.
Figure 3.
MEOX2 coprecipitates with p65 and IκBβ. (A) Exogenous MEOX2 co-immunoprecipitates with p65. MEOX2 and p65 coprecipitate in MEOX2-transduced HUVECs, whereas coprecipitation is not observed in cells transduced with control virus. (B) Endogenous MEOX2 co-immunoprecipitates with p65. HUVECs were treated as described in order to induce either endogenous MEOX2 expression or NF-κB activity. For TNF-α treatment, they were exposed to TNF-α (20 ng/mL) for 30 min, after which cells were harvested for co-IP. (C) MEOX2 co-immunoprecipitates with IκBβ. HUVECs were transduced in the same manner as in (A), after which co-IPs were performed with anti-HA and anti-IκBβ antibodies. MEOX2 also coprecipitates with IκBβ.
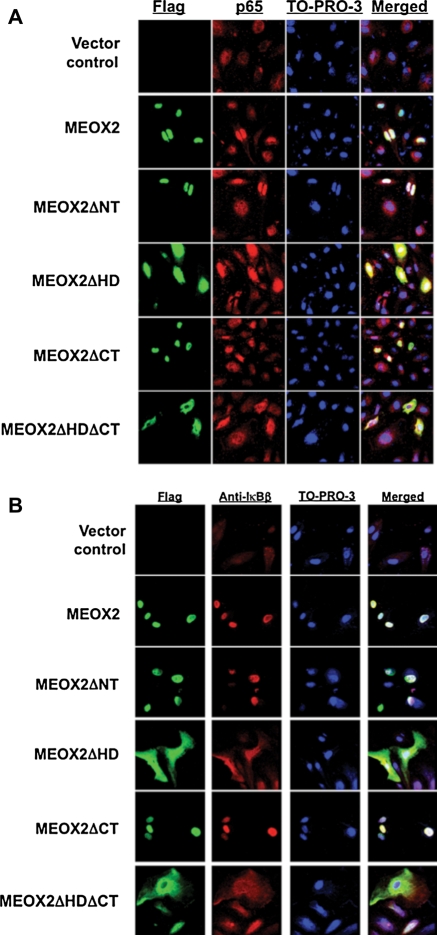
Finally, we wished to attempt to determine which domains of the MEOX2 protein are important in mediating the interaction between MEOX2 and NF-κB components. Consequently, we made deletion constructs of the MEOX2 cDNA in pcDNA3.1 (see Supplementary material online, Figure S1),6 transfected HUVECs with them as described in Section 2, and observed that colocalization of MEOX2 and p65 in the nucleus depends upon the MEOX2 homeodomain. MEOX2 protein lacking its homeodomain colocalized in the cytoplasm with p65 rather than in the nucleus, although an MEOX2 deletion lacking the N-terminal domain colocalized in the nucleus as wild-type MEOX2 does (Figure 4A). Co-IP using anti-FLAG and anti-p65 antibodies demonstrated again MEOX2 coprecipitation with p65, but this coprecipitation was abolished if the homeodomain or N-terminal domain was not present (data not shown; see Supplementary material online, Figure S2). Finally, we wished to test whether IκBβ also colocalizes with MEOX2 as p65 did under these conditions. We therefore repeated the experiment using anti-IκBβ antibody and observed a very similar result, namely that IκBβ and MEOX2 colocalized, and this colocalization appeared to depend upon the presence of the MEOX2 homeodomain (Figure 4B).
Figure 4.
Colocalization of MEOX2 and p65 in the nucleus depends upon the MEOX2 homeodomain. (A) Deletion of the homeodomain abrogates the colocalization of MEOX2 and p65 in the nucleus. HUVECs were transduced with MEOX2 deletion constructs (Figure 1) and subjected to immunofluorescence with anti-FLAG and anti-p65 as described in Section 2. Results were visualized using confocal microscopy. MEOX2 and p65 only colocalize when the MEOX2 homeodomain is present. (B) MEOX2 colocalizes with IκBβ in a homeodomain-dependent manner. HUVECs were transduced with one of the five MEOX2 constructs illustrated and then subjected to immunofluorescence with anti-FLAG and anti-IκBβ. MEOX2 and IκBβ only colocalize when the MEOX2 homeodomain is present. All images were photographed at a magnification of ×600 using an oil immersion lens.
3.4. MEOX2 exhibits a biphasic effect on NF-κB-dependent promoter activity
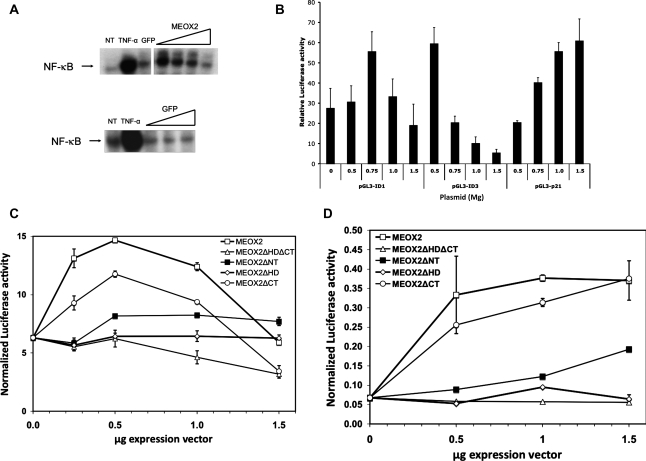
Next, we wished to study in more detail the effect of MEOX2 expression on an NF-κB-dependent promoter. First, we examined the effect of MEOX2 expression on NF-κB binding to its consensus sequence by EMSA (Figure 5). After verifying that p65 was in the NF-κB complex binding to the κB consensus sequence from IL-6 by supershift assay (data not shown), we transduced HUVECs with either Ad.GFP or with Ad.rMEOX2 (Figure 5A). For comparison, we also included HUVECs treated with TNF-α 20 ng/mL for 30 min. MEOX2 expression resulted in a biphasic effect, in which NF-κB binding to its consensus sequence first increased at low MOI but then decreased as the MOI increased. Consistent with this, we noted similar biphasic effects due to increasing levels of MEOX2 expression in reporter assays for the ID1 and ID3 promoters (Figure 5B), the former of which has been implicated as an inducer of the angiogenic phenotype in ECs.18 Of note, the peak of each curve appeared to occur at different levels of MEOX2 expression in different NF-κB-dependent promoters; for instance, ID3 promoter activity peaked at a lower dose of pcDNA3.1-MEOX2 than did the ID1 promoter (Figure 5B).
Figure 5.
The effect of MEOX2 expression on NF-κB binding to its consensus sequence depends upon the level of expression of MEOX2 protein. HUVECs were stimulated with TNF-α (20 ng/mL) for 30 min, after which nuclear extracts were harvested for EMSA and supershift assays as described in Section 2. p65 was detected in the shifted complex. (A) The effect of MEOX2 expression on NF-κB binding to its consensus sequence behaves in a biphasic manner. (Upper panel) HUVECs were transduced either with Ad.rMEOX2 or with Ad.GFP overnight and then subjected to EMSA with the NF-κB probe as described in Section 2 (NT, no treatment control). For comparison, uninfected cells stimulated for 30 min with TNF-α were also harvested. When HUVECs are transduced with MEOX2 at a low MOI, NF-κB binding is activated but then decreases gradually at higher MOIs. (Lower panel) In a separate experiment, HUVECs were transduced with Ad.GFP and then treated identically to cells in the upper panel. GFP causes little or no change in NF-κB binding to the probe. (B) MEOX2 expression results in a biphasic effect on other NF-κB-dependent promoters. HUVECs were cotransfected with 0.5–1.5 µg pcDNA3.1-MEOX2 (listed on the X-axis) and 0.5 µg reporter constructs containing the NF-κB-dependent promoters ID1 and ID3 linked to Luciferase and subjected to dual Luciferase assay after an overnight incubation. Increasing MEOX2 expression resulted in a biphasic effect on both of these promoters. A p21 promoter construct is included to show MEOX2 up-regulating its activity. (C) MEOX2 regulates the activity of an NF-κB-regulated promoter in a biphasic fashion that depends upon the presence of its homeodomain. HUVECs were cotransfected with p-IL6-Luciferase and an MEOX2 deletion construct at different doses of MEOX2 plasmid. MEOX2 expression demonstrates a biphasic effect, with stimulation of IL-6 promoter activity at low doses of plasmid, and a decrease beyond 0.5 µg. In contrast, MEOX2ΔHD and MEOX2ΔNT demonstrate nearly flat dose–response curves. (D) The same experiment carried out with a reporter construct driven by the ICAM promoter. The same experiment as in (C) was carried out using an ICAM reporter construct with similar results, except that the peak of the biphasic response is found at a higher dose of pcDNA3.1-MEOX2 than it is for the other reporter constructs.
Next, we tested the effect of expressing MEOX2 or its deletions on the activity of the IL-6 promoter (Figure 5C). Again, consistent with the observed binding to the NF-κB consensus sequence (Figure 5A and B), we noted a biphasic effect due to increasing MEOX2 expression, with a peak at 0.5 µg pcDNA3.1-MEOX2. In contrast, the effect of MEOX2 on the ICAM promoter produced a later peak (Figure 5D). For comparison, we note that overexpressing MEOX2 using adenoviral vectors down-regulates ICAM expression,5 suggesting that ICAM also shows a biphasic response to increasing MEOX2 expression but that the level of expression of MEOX2 required for peak ICAM promoter activity is likely beyond what can be achieved with plasmid-based transfection methods. Consistent with results showing no co-IP in the absence of the homeodomain or N-terminal domain, expression of MEOX2ΔHD, MEOX2ΔNT, or MEOX2ΔHDΔCT resulted in a nearly flat dose–response curve for both IL-6 and ICAM (Figure 5C and D). In contrast, deleting the C-terminal end of MEOX2 (MEOX2ΔCT) resulted in a slight attenuation, but not abrogation, of MEOX2 activity, producing a biphasic response curve similar to that of wild-type MEOX2. These results suggest different but overlapping functions for each MEOX2 domain.
3.5. The loss of the homeodomain or the N-terminal domain results in impairment of the ability of MEOX2 to inhibit EC activation and angiogenesis
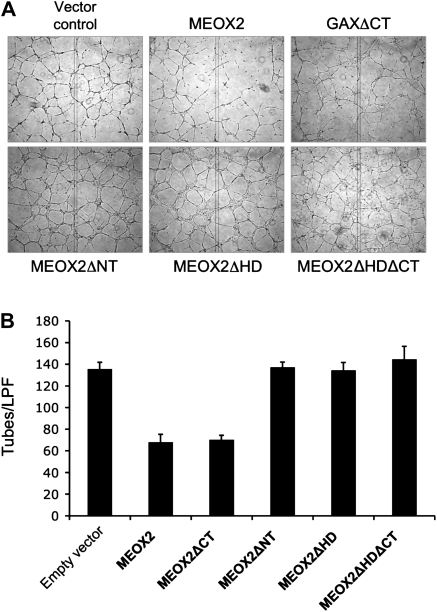
In order to determine whether there is a functional consequence of deleting domains of the MEOX2 protein involved in interaction with p65 and IκBβ, we next transfected HUVECs with the full-length MEOX2 expression construct as well as the four deletion constructs (Figure 6). Consistent with our previous observations (Figure 5), deletion of the C-terminal domain did not abolish MEOX2-mediated inhibition of tube formation on reconstituted basement membrane. However, deletion of the N-terminal domain or the homeodomain attenuated MEOX2-mediated inhibition of tube formation. We conclude from this that the ability of MEOX2 to inhibit this in vitro functional measure of angiogenesis correlates with the presence of both the N-terminal domain and homeodomain.
Figure 6.
Inhibition of tube formation on reconstituted basement membrane by MEOX2 requires the presence of both the N-terminal domain and the homeodomain. (A) Tube formation. HUVECs were transfected with pcDNA3.1-MEOX2 and the four MEOX2 deletion constructs as described and then plated on reconstituted basement membrane in Materials and Methods. Cells were incubated overnight and then photographed. (B) Quantification of tube formation. Tube formation was quantified as described 9,10,22 and the mean ± standard deviation for tubes/low powered field (LPF) were calculated. In spite of a transfection efficiency of only 30% (not shown), MEOX2 and MEOX2ΔCT reduced tube formation by nearly 50% (P < 0.01).
4. Discussion
When we made our original observation that MEOX2 down-regulates of NF-κB signalling,5 the role of NF-κB in regulating angiogenesis was thought to be mainly permissive, based on a series of observations in various model systems that inhibiting NF-κB signalling was antiangiogenic while stimulation of NF-κB signalling was not proangiogenic.9,19,20 However, recent evidence hints at a more complex story, where, under some circumstances, NF-κB signalling appears to be antiangiogenic.21,22 That is why perhaps it was not surprising that our results suggested not only that the regulation of a κB-dependent promoter by MEOX2 is more complex than a simple monotonic down-regulation,5 but also that MEOX2 interacts with key components of the NF-κB pathway through protein–protein interactions. Even more surprisingly, one of these components is IκBβ but not IκBα consistent with a protein–protein interaction between MEOX2 and IκBβ. Instead of the expected monotonic decrease in NF-κB binding to its consensus sequence with increasing levels of MEOX2 expression and a resultant decline in NF-κB-dependent promoter activity, we observed a biphasic response. Moreover, mechanistically, the MEOX2 homeodomain was critically important to the binding and colocalization of p65 and IκBβ with MEOX2, their colocalization to the nucleus, and the biphasic response of NF-κB-dependent promoters to increasing levels of MEOX2 expression.
One model of NF-κB activity postulates IκB constantly shuttling in and out of the nucleus via nuclear import and export sequences.23 Because IκB nuclear export is more efficient than its nuclear import process, at equilibrium, the NF-κB/IκB complex remains mostly in the cytoplasm of unstimulated cells. Upon stimulation by proinflammatory cytokines, IκB is phosphorylated by the IKK complex and subsequently degraded in the proteosome pathway,23 allowing NF-κB to enter the nucleus and activate downstream genes, including IκB. According to this model, newly synthesized IκB protein moves to the nucleus, removes NF-κB from its target genes, and shuttles it back to the cytoplasm as the classic inactive IκB-NF-κB complex. On the basis of our results, it is tempting to speculate that MEOX2 binds to p65, either alone or while it is bound to p50, possibly shifting the equilibrium to the nucleus, at the same time preventing the NF-κB complex from binding to its consensus binding sites and in the process increasing nuclear levels of IκBβ. This latter observation suggests that MEOX2 may have a role in regulating the binding of nuclear NF-κB to its consensus binding sites, with differential effects depending upon its level.
There is a precedent for an interaction between a homeodomain protein and p65, specifically Oct-1 a POU-domain-containing homeoprotein. MEOX2 behaves similarly to Oct-1, which interacts with p65 to inhibit the transcription of NF-κB-dependent promoters in a DNA-binding-independent manner.24 However, in contrast to Oct-1, MEOX2 expression is down-regulated, not up-regulated, by stimuli that activate NF-κB.5 There is also a precedent for an interaction between a homeodomain protein and an IκB protein, specifically the interaction of HOXB7 with IκBα.25,26 However, in marked contrast, the protein–protein interaction between IκBα and HOXB7 appears to be more important for activating transcription from HOXB7-dependent promoters than it is for modulating NF-κB activity25 and is independent of NF-κB. IκBα has been reported to mediate its enhancement of the transcriptional activity of homeodomain-containing proteins through cytoplasmic sequestration of HDAC1 and HDAC3.27 It is thus tempting to speculate in addition that a similar mechanism might be at play with MEOX2 and IκBβ, with IκBβ potentially playing a role in MEOX2-mediated transcription. To the best of our knowledge, our observations represent for the first time such an interaction has been observed between a homeodomain-containing protein and IκBβ. Although the details of the mechanism remain to be worked out, this interaction between MEOX2 and p65, coupled with the interaction between MEOX2 and IκBβ, may represent a potentially interesting novel regulatory mechanism.
Taken as a whole, this study also raises the question of whether at physiological levels, MEOX2 stimulates or inhibits NF-κB activity in ECs. The evidence is very clear and compelling that MEOX2 induces G0/G1 cell cycle arrest through the p53-independent up-regulation of the cyclin kinase inhibitor p21WAF1/CIP1, 6,7 and MEOX2 expression at levels attainable with plasmid transfection is clearly antiangiogenic.16 However, although we have never observed MEOX2 to exhibit anything other than antiangiogenic activity in ECs from peripheral vasculature,2,3,5–7,16 it has been reported that MEOX2 expression has proangiogenic activity in the cerebral vasculature,28 implying that phenotypic changes due to MEOX2 activity might vary depending upon factors specific to ECs in different vascular beds. Extrapolating beyond levels of expression achievable with plasmid transfection to what can be achieved with adenoviral vectors, our current results probably also explain why we originally observed only inhibition of NF-κB signalling by MEOX2 and suggest that MEOX2 may modulate EC phenotype in a different manner in different vascular beds.5
Finally, we have previously suggested MEOX2 as a possible molecular target for the antiangiogenic therapy of cancer. Our current results reinforce the conclusion that MEOX2, in addition to its role in causing cell cycle arrest in ECs, may also have a role in modulating the activation of this cell type and thus represents a logical molecular target for the antiangiogenic therapy of cancer and/or anti-inflammatory therapy designed to prevent or treat atherosclerosis. Taken together with previous results, our results implicate MEOX2 as a potential major, even ‘master’, regulatory gene inhibiting not only the angiogenic response of ECs to tumour-secreted proangiogenic factors, but also the vascular response to chronic inflammatory stimulation that normally activates NF- κB.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (R01 CA111344 to D.H.G.) and the U.S. Department of Defense (DAMD17-03-1-0292 to D.H.G.).
Supplementary Material
References
- 1.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 2.Gorski DH, LePage DF, Patel CV, Copeland NG, Jenkins NA, Walsh K. Molecular cloning of a diverged homeobox gene that is rapidly down-regulated during the G0/G1 transition in vascular smooth muscle cells. Mol Cell Biol. 1993;13:3722–3733. doi: 10.1128/mcb.13.6.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorski DH, Leal AJ. Inhibition of endothelial cell activation by the homeobox gene Gax. J Surg Res. 2003;111:91–99. doi: 10.1016/s0022-4804(03)00042-8. doi:10.1016/S0022-4804(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 4.Grigoriou M, Kastrinaki MC, Modi WS, Theodorakis K, Mankoo B, Pachnis V, et al. Isolation of the human MOX2 homeobox gene and localization to chromosome 7p22.1-p21.3. Genomics. 1995;26:550–555. doi: 10.1016/0888-7543(95)80174-k. doi:10.1016/0888-7543(95)80174-K. [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Leal AD, Gorski DH. The homeobox gene Gax inhibits angiogenesis through inhibition of nuclear factor-{kappa}B-dependent endothelial cell gene expression. Cancer Res. 2005;65:1414–1424. doi: 10.1158/0008-5472.CAN-04-3431. doi:10.1158/0008-5472.CAN-04-3431. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Leal AD, Patel S, Gorski DH. The homeobox gene GAX activates p21WAF1/CIP1 expression in vascular endothelial cells through direct interaction with upstream AT-rich sequences. J Biol Chem. 2007;282:507–517. doi: 10.1074/jbc.M606604200. doi:10.1074/jbc.M606604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RC, Branellec D, Gorski DH, Guo K, Perlman H, Dedieu JF, et al. p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 1997;11:1674–1689. doi: 10.1101/gad.11.13.1674. doi:10.1101/gad.11.13.1674. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. doi:10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 9.Klein S, de Fougerolles AR, Blaikie P, Khan L, Pepe A, Green CD, et al. Alpha 5 beta 1 integrin activates an NF-kappa B-dependent program of gene expression important for angiogenesis and inflammation. Mol Cell Biol. 2002;22:5912–5922. doi: 10.1128/MCB.22.16.5912-5922.2002. doi:10.1128/MCB.22.16.5912-5922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oitzinger W, Hofer-Warbinek R, Schmid JA, Koshelnick Y, Binder BR, de Martin R. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97:1611–1617. doi: 10.1182/blood.v97.6.1611. doi:10.1182/blood.V97.6.1611. [DOI] [PubMed] [Google Scholar]
- 11.Ko HM, Seo KH, Han SJ, Ahn KY, Choi IH, Koh GY, et al. Nuclear factor kappaB dependency of platelet-activating factor-induced angiogenesis. Cancer Res. 2002;62:1809–1814. [PubMed] [Google Scholar]
- 12.Kim KE, Gu C, Thakur S, Vieira E, Lin JC, Rabson AB. Transcriptional regulatory effects of lymphoma-associated NFKB2/lyt10 protooncogenes. Oncogene. 2000;19:1334–1345. doi: 10.1038/sj.onc.1203432. doi:10.1038/sj.onc.1203432. [DOI] [PubMed] [Google Scholar]
- 13.Hallahan DE, Virudachalam S, Kuchibhotla J. Nuclear factor kappaB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endothelium. Cancer Res. 1998;58:5484–5488. [PubMed] [Google Scholar]
- 14.Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. doi:10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. doi:10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. doi:10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goydos JS, Mann B, Kim HJ, Gabriel EM, Alsina J, Germino FJ, et al. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg. 2005;200:362–370. doi: 10.1016/j.jamcollsurg.2004.10.032. doi:10.1016/j.jamcollsurg.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama K, Takaji K, Kataoka K, Kurihara Y, Yoshimura M, Kato A, et al. Id1 gene transfer confers angiogenic property on fully differentiated endothelial cells and contributes to therapeutic angiogenesis. Circulation. 2005;112:2840–2850. doi: 10.1161/CIRCULATIONAHA.104.516898. doi:10.1161/CIRCULATIONAHA.104.516898. [DOI] [PubMed] [Google Scholar]
- 19.Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, et al. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16:4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Invest Ophthalmol Vis Sci. 1999;40:1624–1629. [PubMed] [Google Scholar]
- 21.Tabruyn SP, Griffioen AW. A new role for NF-kappaB in angiogenesis inhibition. Cell Death Differ. 2007;14:1393–1397. doi: 10.1038/sj.cdd.4402156. doi:10.1038/sj.cdd.4402156. [DOI] [PubMed] [Google Scholar]
- 22.Tabruyn SP, Griffioen AW. NF-kappa B: a new player in angiostatic therapy. Angiogenesis. 2008;11:101–106. doi: 10.1007/s10456-008-9094-4. doi:10.1007/s10456-008-9094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl.):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 24.dela Paz NG, Simeonidis S, Leo C, Rose DW, Collins T. Regulation of NF-kappaB-dependent gene expression by the POU domain transcription factor Oct-1. J Biol Chem. 2007;282:8424–8434. doi: 10.1074/jbc.M606923200. [DOI] [PubMed] [Google Scholar]
- 25.Chariot A, Gielen J, Merville MP, Bours V. The homeodomain-containing proteins: an update on their interacting partners. Biochem Pharmacol. 1999;58:1851–1857. doi: 10.1016/s0006-2952(99)00234-8. doi:10.1016/S0006-2952(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 26.Chariot A, Princen F, Gielen J, Merville MP, Franzoso G, Brown K, et al. IkappaB-alpha enhances transactivation by the HOXB7 homeodomain-containing protein. J Biol Chem. 1999;274:5318–5325. doi: 10.1074/jbc.274.9.5318. doi:10.1074/jbc.274.9.5318. [DOI] [PubMed] [Google Scholar]
- 27.Viatour P, Legrand-Poels S, van Lint C, Warnier M, Merville MP, Gielen J, et al. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J Biol Chem. 2003;278:46541–46548. doi: 10.1074/jbc.M306381200. doi:10.1074/jbc.M306381200. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.