Abstract
Aims
In the ischaemia-reperfused heart, transforming growth factor β (TGFβ) proteins trigger the differentiation of cardiac fibroblasts (CFs) contributing to fibrosis. Reoxygenation of the heart, in addition to being a trigger for reperfusion injury, induces tissue remodelling by hyperoxia-sensitive signalling processes involving TGFβ. Here, we sought to characterize the molecular mechanisms responsible for the O2-sensitive transcriptional induction of TGFβ in murine CF and to test the significance of such findings in the infarcted myocardium in vivo using laser capture microdissection.
Methods and results
All three isoforms of TGFβ were induced in the CF-rich peri-infarct tissue as well as in CF exposed to hyperoxic challenge. Reporter studies demonstrated that TGFβ transcription is hyperoxia inducible. Deletion of any one or both of the activating protein-1 (AP-1) binding sites in the TGFβ reporter construct resulted in loss of O2 sensitivity, demonstrating that AP-1 confers O2 sensitivity to TGFβ transcription. Fos-related AP-1 transcription factor (Fra-2) and Ask-1 (apoptosis signal-regulating kinase-1) were identified as key mediators of AP-1-dependent O2-sensitive TGFβ transcription. Knockdown of Fra-2 significantly blunted O2-induced expression of TGFβ1 as well as TGFβ3 in CF. Knockdown of Ask-1 blunted hyperoxia-induced Fra-2 gene expression and nuclear localization in CF. Collectively, these observations point towards a central role of Ask-1 and Fra-2 in O2-inducible AP-1 activation and induction of TGFβ.
Conclusion
Taken together with the observation that Fra-2-regulated genes are implicated in fibrosis, identification of Fra-2 as an O2-sensitive transcriptional regulator of inducible TGFβ expression positions Fra-2 as an important player in reoxygenation-induced fibrosis.
Keywords: Oxygen, Reperfusion, TGF
1. Introduction
Cardiac fibrosis is characterized by the expansion of the interstitial compartment caused by increased deposition of extracellular matrix (ECM) by activated myofibroblasts.1 In the ischaemia-reperfused heart, TGFβ proteins trigger the differentiation of cardiac fibroblasts (CFs) to myofibroblasts, therefore contributing to the development of fibrosis.2 TGFβ directly induces collagen production and ECM contraction by CFs.3 Overexpression of TGFβ in the heart causes myocardial fibrosis.4 TGFβ1, β3 and latent TGFβ-binding protein (LTBP) are increased in patients with cardiac fibrosis, suggesting the direct involvement of active TGFβ in cardiac fibrogenesis.5 The evolutionarily conserved TGFβ proteins are distributed ubiquitously throughout the body and have a role in almost every biological process. In mammals, three structurally homologous isoforms of TGFβ (TGFβ1, β2, and β3) are encoded by three distinct genes. Although each of these isoforms are expressed in a distinct pattern under control of a unique promoter, all three isoforms signal through the same cell surface receptors and have similar cellular targets.6 Differential expression of the isoforms of TGFβ is reported for lung fibrosis7 but remains unknown for cardiac fibrosis.
Studies in our laboratory have demonstrated that reoxygenation of a focally ischaemic site of the heart, in addition to being a trigger for reperfusion injury, induces tissue remodelling.1,8–11 Focal ischaemia in the heart results in a hypoxic area containing a central focus of near-zero O2 pressure bordered by tissue with diminished but non-zero O2 pressures. These border zones extend for several millimetres from the hypoxic core, with the O2 pressures progressively increasing from the focus to the normoxic region.10 Moderate hypoxia is associated with a 30–60% decrease (∼1–3% O2) in pO2.12 During chronic hypoxia in the heart, cells lower their normoxic set-point13 such that the return to normoxic pO2 after chronic hypoxia results in perceived hyperoxic challenge.1,8–10,14,15 Compared with myocytes, CFs are relatively more resistant to oxygen toxicity.16,17 As a result, the infarct site, devoid of myocytes, continues to be populated by CF.14,15 Perceived hyperoxia induces differentiation of CF to myofibroblasts at the infarct site.10 CF, isolated from adult murine ventricle, cultured in 10 or 20% O2 (high O2, relative to the pO2 to which cells are adjusted in vivo), compared with 3% O2 (mildly hypoxic), exhibit reversible growth inhibition and a phenotypic switch indicative of differentiation. TGFβ was noted to play a central role in inducing O2-sensitive differentiation of CF to myofibroblasts.9 In this study, we sought to characterize the molecular mechanisms responsible for the O2-sensitive transcriptional induction of TGFβ in adult murine ventricular CF.
2. Methods
2.1. Survival model for coronary artery occlusion and reperfusion
C57BL/6 mice were subjected to ischaemia–reperfusion of the heart as described.1,9,14,18 The studies were approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University and follow the US National Institutes of Health guidelines. Left thoracotomy was performed via the fifth intercostal space to expose the heart. A 60 min occlusion of left anterior descending coronary artery was followed by reperfusion. Laser Doppler flow measurement was used to verify ischaemia and reperfusion. Upon successful reperfusion, the thorax was closed and the negative thoracic pressure was re-established for survival. The mice were killed 7 days after reperfusion. Mean infarct volume at day 7 post-reperfusion was ∼22% of the left ventricle.19 In our laboratory, the survival rate for day 7 post-surgery ranges between 80 and 90%. Using high-resolution (11.7 T) cardiac MRI, we have characterized the temporal loss of cardiac function in C57BL6 mice following IR.20 Detailed haemodynamic data from this model has been also published by our laboratory.11,19 To demonstrate that reoxygenation actually influences TGFβ expression, we utilized an approach of graded reoxygenation. To achieve this, the extent of hypoxia during occlusion was varied by having mice breathe either 0.1 (10%; Group A) or 1 (100%; Group B) FiO2 during the time of occlusion. Reoxygenation was measured as ΔpO2 (i.e. pO2 during reoxygenation − pO2 during occlusion) using EPR oximetry (see Supplementary material online, Figure S3). Hearts were collected frozen in OCT compound for laser capture gene expression analyses and immunohistochemistry. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Laser microdissection and pressure catapulting
Laser microdissection and pressure catapulting was performed using the Microlaser system from PALM Microlaser Technologies AG (Bernreid, Germany) as described.1,14,15,18 Briefly, murine hearts with experimental ischaemia–reperfusion were isolated, frozen in OCT compound, and then cut into 10 μm sections using a cryo-microtome. The sections were placed on polyethylene napthalate membrane glass slides (P.A.L.M. Microlaser Technologies AG, Bernreid, Germany), which had been RNAsin (Ambion, Austin, TX) and UV-treated, for cutting and catapulting as described by our group.1,14,15 Sections were stained using a modified haematoxylin QS procedure14,15 and the infarct site identified as reported. Infarct (I) area was captured in chaotropic RNA lysis solution followed by mRNA quantification as described.14,18,21 RNA extraction and reverse transcription and mRNA quantification using real-time PCR were performed as described.14,18,21
2.3. mRNA quantification
mRNAs were quantified by real-time PCR assay using SYBR green-I as described previously.8,9,18,22 The primer set used for individual genes are listed in Table S1, Supplementary material online.
2.4. Cardiac fibroblast isolation and culture
Experiments were performed using primary CF isolated from adult (5–6-week old) mouse ventricle using procedures described previously.1,8,9
2.5. Multiplex protein assay
TGFβ isoforms were detected simultaneously on a Luminex™ 200 IS apparatus using the Beadlyte® TGFβ1, β2, β3 detection system (Upstate, Temecula, CA, USA) as per manufacturer's instructions.
2.6. DNA-binding activity of activating protein-1 by an enzyme-linked immunosorbent assay
For the assay, a TransAm activating protein-1 (AP-1) family transcription assay ELISA-based kit (Active Motif, Carlsbad, CA, USA) was used. The assay uses 96-well plates to which oligonucleotide containing a 12-O-tetradecanoylphorbol-13-acetate-responsive element (5′-TGAGTCA-3′) is immobilized. Activating protein-1 dimers contained in nuclear extracts bind specifically to this oligonucleotide and are detected by an antibody directed against c-Fos, fos-related antigen (Fra)-1, etc. Secondary antibody is horseradish peroxidase-conjugated and the readout of the assay is colorimetric and quantified by a spectrophotometer. The assay was performed as per manufacturer's instructions.
2.7. Immunofluorescence microscopy
Fra-2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and apoptosis signal-regulating kinase-1 (Ask-1) (Abcam, Cambridge, MA, USA) immunostaining and microscopy (Zeiss Axiovert 200M) were performed as described.1,8,9
2.8. Immunoprecipitation and western blot
Immunoprecipitation and Western blot was performed as described previously.8,13,22 Primary antibodies against Fra-2, c-Jun, JunB, JunD (Santa Cruz Biotechnology, Inc.), Lamin A (St Louis, MO, USA), Ask-1 (Abcam) and pAsk-123 were used to detect the corresponding antigens.
2.9. Histology
Formalin-fixed paraffin-embedded or OCT-embedded frozen specimens were sectioned. Immunohistochemical staining of sections was performed as described earlier18 using anti-Fra-2 (Santa Cruz Biotechnology, Inc.) and anti-alpha sarcomeric actin (Abcam) and anti-TGFβ1 (R&D Systems) antibody. Secondary antibody detection and counterstaining were performed as described previously.18
2.10. TGFβ1-luciferase reporter assay
The mouse TGFβ1-luciferase reporter constructs used in this study has been described previously.24 TGFβ1 promoter–reporter construct was generated by insertion of nucleotides −453 to +11 of the TGFβ1 promoter into the vector pGL3b, which contains the firefly luciferase gene.24 CF were transiently transfected with the TGFβ1–PGL3 constructs using Lipofectamine 2000 (Invitrogen Corporation, Carlsbad, CA, USA) reagent. Luciferase reporter activity was determined using a commercial kit (Stratagene, La Jolla, CA, USA).
2.11. siRNA delivery
Lipofectamine2000 reagent (Invitrogen Corporation) was used to transfect cells with 100 nM siRNA pool (Dharmacon RNA Technologies, Lafayette, CO, USA) for 48 h as described.1,13 For control, siControl non-targeting siRNA pool (mixture of four siRNAs, designed to have four or more mismatches with the gene) was used.
2.12. Statistics
In vitro (cell culture) data are reported as mean ± SD of three to five experiments. Comparisons among multiple groups were made by the analysis of variance (ANOVA). P < 0.05 was considered statistically significant. For animal studies, data are reported as mean ± SD of at least three to four animals. Given the small sample size, Mann–Whitney or Kruskal–Wallis one-way ANOVA tests were performed to test significance (P < 0.05) of difference between means.
3. Results
Using the laser capture microdissection approach, we present first evidence that all three isoforms of TGFβ are significantly induced in the myocyte-silent CF-rich peri-infarct tissue (Figure 1A and B, Supplementary material online, Figure S1A and B). Next, we sought to characterize the O2-sensitive transcriptional regulatory mechanism of TGFβ using CF isolated from adult murine ventricles. Cells isolated from a 3–5% O2 normoxic environment of the heart8–10 were either cultured under matched pO2 conditions (i.e. 5% O2) or subjected to hyperoxic challenge by exposure to 20% O2 ambience. Hyperoxic challenge resulted in significant induction of all three isoforms of TGFβ over time. While TGFβ1 and TGFβ3 demonstrated a progressive increase in gene induction over time, TGFβ2 responded differently. Hyperoxic challenge rapidly, but transiently, induced TGFβ2 gene expression (Figure 1C and D). Multiplex protein analyses performed on day 3 after hyperoxic challenge demonstrated that the expression of all three isoforms of total and active TGFβ were significantly O2-sensitive (Figure 1D and E). Similar outcomes were noted when cells were subjected to hypoxia at 1% O2 followed by reoxygenation with 5% O2 (Supplementary material online, Figure S2). To demonstrate that reoxygenation actually influences TGFβ expression, a graded reoxygenation approach was utilized. The model has been described in Methods. Reoxygenation insult in this model was lower in the 100% O2 group compared with the 10% O2 group (Supplementary material online, Figure S3). In such setting, induction of TGFβ was significantly more in the 10% O2 group establishing that TGFβ induction was dependent on the ΔpO2 (Supplementary material online, Figure S3). These observations laid the rationale to characterize the O2-sensitive transcriptional regulation of the TGFβ gene. To address this goal, we utilized a TGFβ1 promoter construct containing a cis-regulatory element in the 5′-flanking region of the TGFβ1 gene. The two AP-1 binding sites are indicated by dark squares. Filled circles show GC boxes. In deletion mutant constructs, the AP-1 binding sites A and B were mutated by a change of two bases in the consensus sequence (Figure 2A). Hyperoxic challenge resulted in TGFβ transactivation demonstrating that indeed TGFβ transcription is O2-sensitive (Figure 2B). Deletion of any one or both of the AP-1 binding sites in the TGFβ reporter construct resulted in the loss of O2-sensitivity demonstrating that AP-1 confers O2-sensitivity to TGFβ transcription. Deletion of AP-1 binding sites resulted in higher basal TGFβ reporter activity under conditions of 5% O2 suggesting a de-repression of TGFβ expression in the absence of AP-1 binding (Figure 2C). The observation is interesting and warrants characterization of underlying molecular mechanisms.
Figure 1.
Oxygen-induced TGFβ induction. (A and B) In vivo induction of TGFβ isoform gene expression in CF+myocyte− infarct region of the ischaemia-reperfused murine heart. (A) Frozen sections (10 µm) from the affected site of the heart were stained with haematoxylin/eosin to histologically define the infarct (I) area. (A) (i) Representative stained frozen section. (ii) The same section as shown in (i) after the tissue elements from I region has been marked and (iii) cut/captured. Scale bar = 150 µm. The captured tissue was used for quantification of mRNA levels of TGFβ isoforms. (B) TGFβ1, β2, and β3 mRNA levels were determined using real-time PCR and normalized against GAPDH expression detected in the same samples. Data are mean ± SD (n = 4); *P < 0.05 compared with day 0. (C and D) In vitro oxygen-induced TGFβ isoform expression in isolated cardiac fibroblasts. Cardiac fibroblasts were cultured for 5 days at 5% O2. Following splitting, cells were either exposed to 5% (in vivo normoxia) or 20% O2 (hyperoxia) ambience for the indicated periods of time. (C) Changes in TGFβ1, β2, and β3 mRNA levels in CF at 20% O2 compared with cells grown at 5% O2. TGFβ mRNA levels were determined using real-time PCR and normalized against GAPDH expression detected in the same samples. Data shown are % change compared with control CF grown at 5% O2. Mean ± SD (n = 4). *P < 0.05 compared with cells in 5% O2. (D and E) Multiplex protein analysis of total (D) and active (E) TGFβ isoforms was performed using luminex technology. Bar graph shown is the quantitative assessment of TGFβ protein levels in media on day 3 of exposure to 20% O2. Data were normalized to the cell count. Data shown are mean ± SD (n = 3). **P < 0.01 compared with cells in 5% O2.
Figure 2.
Transcriptional activation of TGFβ promoter by oxygen: essential role of AP-1 binding sites. (A) TGFβ1 promoter construct. cis-Regulatory elements in the 5′-flanking region of the TGFβ1 gene in pGL3basic vector. The two activating protein-1 binding sites are indicated by dark rhombus, and filled circles show GC boxes. (B and C) TGFβ1 reporter constructs (B) wild-type: TGFβ1 fragment −453/+11 fused to the luciferase gene or (C) mutated: AP-1 binding sites A and B were mutated by a change of two bases in the consensus sequence were transiently transfected in CF cells using lipofectamine 2000 reagent. After transfection, cells were cultured in 5% O2 for 24 h following which the CFs were exposed to either 5 or 20% O2 for 24 h. Luciferase activity as a marker of promoter activation was determined from lysed cells. Results of reporter activities were normalized for the amount of the protein in cell lysates. Data are mean ± SD (n = 7). *P < 0.001 compared with cells in 5% O2. (D–F) An ELISA-based method was used to analyse DNA-binding activity of the proteins of AP-1 family. After isolation, cells were cultured at 5% O2 for 5 days following which the CFs were split and were exposed to either 5% (solid bars) or 20% O2 (empty bars) for 1–3 days. DNA-binding activities of Fra-2, Fra-1, c-Fos, and c-Jun in nuclear extracts were detected using Tans-AM (Active Motif) AP-1 assay kit. Data are mean ± SD; n = 4. *P < 0.05 compared with cells at 5% O2.
Activation of the AP-1 transcription factor complex is a universal response of a wide variety of mammalian cells to a broad range of external stimuli, including growth factors, chemokines and ECM.25 An ELISA-based approach was adopted to analyse the DNA-binding activity of the proteins of AP-1 family. Whereas c-Fos, c-Jun and Fra-1 were observed to be not sensitive to hyperoxic challenge, Fra-2 was identified as the O2-sensitive AP-1 family transcription factor (Figure 2D–F). Exposure of CF to hyperoxic challenge resulted in increased abundance of Fra-2 in the nucleus indicating that expression and nuclear localization of Fra-2 is O2-sensitive (Figure 3A–C). Fos family members including Fra-2 are known to form stable heterodimers with the members of Jun family (c-Jun, JunB or JunD) to form a functional transcription factor AP-1. Among Jun family members, Fra-2 protein was observed to bind with JunB in CF subjected to hyperoxic challenge (Figure 3C). Under these conditions, Fra-2 did not bind with c-Jun or JunD (not shown). Immunohistochemical staining of myocardial tissue subjected to ischaemia–reperfusion demonstrate that Fra-2 is abundantly expressed in the SMA-positive myofibroblast-rich peri-infarct region and that the expression is low in the non-infarct control region of the heart (Figure 3D–E).
Figure 3.
Fra-2: oxygen-inducible and abundant in the reoxygenated heart. (A–C) In vitro after isolation, CFs were cultured at 5% O2 and then transferred to either 5 or 20% O2 for 3 days. (A and B) Fos-related antigen 2 expression was determined in nuclear protein extracts using the western blot. Lamin A was used to normalize Fra-2 protein in nuclear fractions. (A) A representative western blot image of Fra-2 and lamin A protein in nuclear protein extracts of CF cultured at 5 or 20% O2 for 3 days. (B) Densitometric data of blot shown in (A). Data shown are mean ± SD (n = 3). *P < 0.01 compared with cells in 5% O2. (C). Cardiac fibroblasts lysates were subjected to immunoprecipitation with Fra-2 antibody. Immunoprecipitates were subjected to SDS–PAGE and subjected to immunoblotting for the detection of JunB. (D and E) In vivo increased Fra-2 expression in the infarct region of heart. Mice were subjected to left anterior descending coronary artery ligation for 30 min followed by reperfusion. Representative images of infarct (D) or non-infarct (E) regions showing SMA (red) and Fra-2 (green) expression in a section of mouse heart subjected to 7 days post-ischaemia–reperfusion. The sections were counter-stained with DAPI (nuclear, blue). Merged (red, green and blue) images show increased expression of Fra-2 in SMA-positive myofibroblast-rich infarct region. Scale bar = 50 µm.
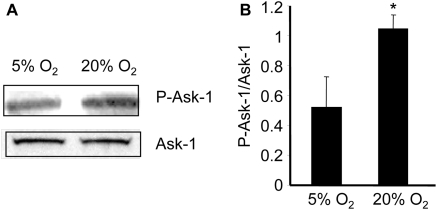
Next, we sought to examine the significance of Fra-2 in regulating TGFβ transcription. To achieve this goal, knockdown of Fra-2 in CF was achieved by the use of siRNA. The knockdown protocol employed was successful in significantly lowering endogenous Fra-2 mRNA as well as protein (Figure 4A–C). Fos-related antigen-2 knockdown significantly blunted O2-induced expression of TGFβ1 as well as TGFβ3 (Figure 4D). TGFβ2 expression, as induced by hyperoxic challenge, was not affected. This finding indicates that Fra-2 is implicated in O2-induced expression of TGFβ1 and TGFβ3. Apoptosis signal-regulating kinase-1 is a stress-responsive redox-sensitive mitogen-activated protein kinase kinase kinase (MAP3K) regulating p38 MAPK and JNK cascades. Ask1-JNK/p38 MAPK-dependent pathways are known to induce AP-1 activation.26 Therefore, we sought to test whether O2-inducible AP-1 is mediated by Ask-1. Apoptosis signal-regulating kinase-1 is typically found in the inactive form, bound to reduced thioredoxin. When oxidized, thioredoxin dissociates from Ask-1. The Ask-1, which is found as a homo-oligodimer, autophosphorylates and becomes an active MAP3K. Exposure of CF to hyperoxic challenge resulted in phosphorylation of Ask-1 demonstrating that Ask-1 activation is O2-inducible (Figure 5). To test the significance of Ask-1 in regulating Fra-2, Ask-1 knockdown was performed in CF using the siRNA approach. The knockdown approach adopted resulted in significant downregulation of Ask-1 mRNA as well as protein expression in CF (Figure 6A and B). Interestingly, knockdown of Ask-1 blunted hyperoxia-induced expression of Fra-2 indicating that Ask-1 regulates inducible Fra-2 transcription in CF (Figure 6C). Studies aimed at immunolocalizing Fra-2 in the CF demonstrated that under basal conditions Fra-2 abundance in CF is minimal. Exposure of the CF to hyperoxic challenge clearly showed a higher abundance of Fra-2 in the nuclear compartment (Figure 6D). Such O2-inducible Fra-2 expression and nuclear localization was markedly attenuated in CF subjected to Ask-1 knockdown (Figure 6D). Collectively, these observations point towards a central role of Ask-1 and Fra-2 in O2-inducible AP-1 activation and induction of TGFβ.
Figure 4.
Fra-2 plays a major role in oxygen-induced TGB1 & β3 expression in CFs. After isolation, CFs were cultured at 5% O2 and then subjected to Fra-2 knockdown (Fra-2 siRNA) or not (control siRNA). Successful knockdown of Fra-2 mRNA and protein following Fra-2 siRNA transfection of CFs. (A) Fos-related antigen 2 mRNA levels were determined using real-time PCR. Data ± SD (n = 4). (B) A representative western blot image of Fra-2 and β-actin protein in protein extracts of CF transfected with control or Fra-2 siRNA. (C) Densitometric data of the blot shown in (B). Data shown are mean ± SD (n = 3). *P < 0.01 compared with cells transfected with control siRNA. (D) mRNA expression of TGFβ isoforms in CF transfected with Fra-2 or control siRNA and then exposed to 20% O2 for 3 days. Data mean ± SD (n = 3). *P < 0.01 compared with cells transfected with control siRNA exposed to 20% O2.
Figure 5.
Oxygen-mediated activation of Ask-1. After isolation, CF were cultured at 5% O2 and then exposed to 20% O2 for 24 h. Apoptosis signal-regulating kinase-1 and pAsk-1 proteins were detected using the western blot. (A) A representative blot of pAsk-1 and Ask-1 proteins. (B) Densitometric data of blot shown in (A). Data shown are mean ± SD (n = 3). *P < 0.05 compared with cells grown at 5% O2.
Figure 6.
Ask-1 regulates oxygen-induced Fra-2. After isolation, CFs were cultured at 5% O2 and then subjected to Ask-1 knockdown (Ask-1 siRNA) or not (control siRNA). (A) Successful knockdown of Ask-1 mRNA following Ask-1 siRNA transfection of CFs. Apoptosis signal-regulating kinase-1 mRNA levels were determined using real-time PCR. Data ± SD (n = 4); **P < 0.01 compared with cells transfected with control siRNA. (B) A representative western blot image of Fra-2 and GAPDH protein in CF transfected with control or Ask-1 siRNA. (C) Densitometric data of the blot shown in (B). Data shown are mean ± SD (n = 3). **P < 0.01 compared with cells transfected with control siRNA at 20% O2. (D) Cardiac fibroblasts were stained with anti-Fra-2 antibody (FITC, green) and DAPI (blue nucleus).Increased Fra-2 staining in the nucleus and cytosol of cells grown at 20% O2 transfected with control siRNA can be seen. Scale bar = 50 µm.
4. Discussion
A characteristic feature of CF is their ability to differentiate forming myofibroblasts.27 TGFβ represents a central driver of CF differentiation during the course of myocardial fibrosis.2 The current study provides novel mechanistic insight into how relative hyperoxic shock, as noted in the peri-infarct region during ischaemia–reoxygenation of the heart, may serve as a trigger for the induction of TGFβ. Over 25 years after the discovery of the cytokine,28 the transcriptional control of TGFβ expression remains poorly characterized.29 The TGFβ1 promoter contains AP-1 binding sites where proteins of the AP-1 family bind to and stimulate TGFβ1 production.30–32 The regulation of TGFβ2 and TGFβ3 expression is distinct from that of TGFβ1 and is thought to be mostly under the control of developmental or hormonal signals.33 Hyperoxic challenge is commonly associated with ischaemia–reperfusion insult.10 Furthermore, ischaemia–reperfusion is commonly followed by TGFβ-dependent fibrosis.2 This work presents maiden evidence demonstrating that hyperoxic challenge may induce the expression of all three isoforms of TGFβ.
The mature TGFβ is derived from pre-pro-TGFβ by proteolytic cleavage. The pre-region consists of a signal peptide. The pro-TGFβ processing occurs in the Golgi complex by a furin-like peptidase,34 where the N terminus of the immature protein, the propeptide, is removed. A homodimer of this new protein, called the latency-associated protein (LAP), is noncovalently associated with a homodimer of mature TGFβ. This association forms the latent TGFβ or the small latent complex. For secretion, the small latent complex associates with LTBP to form the large latent complex. Latent TGFβ-binding protein plays an important role in targeting TGFβ to the ECM.35 In order to be functionally active, TGFβ needs to be released from the LAP and LTBP. In vivo, the mechanisms for activation are less clear, but several models have been proposed, including proteolytic activation by transglutaminase, conformational change of LAP through physical interaction with thrombospondin and mechanical traction.36 Consistent with the literature, our previous work has identified that TGFβ may be activated by oxidation and is sensitive to hyperoxic challenge.8,37 Findings of the current work categorically establish that hyperoxic challenge induces the transcription of all three isoforms of TGFβ. Taken together, hyperoxia emerges as a major signal capable of both inducing as well as activating TGFβ. Ischaemia–reoxygenation commonly leads to tissue fibrosis across a variety of organs.38–40 Given that hyperoxic insult represents an integral component of ischaemia–reperfusion,10 the significance of O2-inducible mechanisms in fibrosis of reoxygenated tissue deserves attention.
Activating protein-1 transcription factor complex consists of heterodimer partners from fos family (c-Fos, FosB, Fra-1, and Fra-2) with Jun family (c-Jun, JunB and JunD) of transcription factors. Such heterodimer complexes are able to activate or suppress the expression of many genes involved with regulation of invasion and metastasis; proliferation, differentiation, and survival; genes associated with hypoxia; and angiogenesis. Fos-related antigen 2 is a member of the Fos family of immediate-early serum-inducible genes.41 Fos-related antigen 2 possesses significantly lower transforming activity compared with c-Fos.41 Fos-related antigen 2 forms stable heterodimer complexes with Jun family members. Once formed, these complexes bind to AP-1 sites. Phosphorylation of Fra-2 causes increased DNA-binding activity of this protein.42 Like Fra-1, the Fra-2 protein lacks the C-terminal transactivating domain. In vitro, Fra-2 lacks ability to stimulate artificial AP-1-responsive promoters due to lack of the transactivation domain. Regardless of these in vitro observations, several recent studies indicate that Fra-1 and Fra-2 might play an important role in the progression of various human tumor types in vivo.43 In this study, Fra-2 was noted to dimerize with JunB in response to hyperoxic challenge, suggesting a role of this complex in the transcriptional activation of TGFβ1. That Fra-2 may induce transcription is evident from experiments where overexpression of Fra-2 resulted in the activation of osteopontin, CD44 and thrombospondin.44 Of note, all three of these Fra-2 targets are directly related to TGFβ. Osteopontin is a bone morophogenetic protein which represents a group of structurally related proteins in the TGFβ family that is implicated in cardiac fibrosis.45 The CD44 protein is a TGFβ inducible cell surface glycoprotein involved in cell–cell interactions, cell adhesion, and migration.46 It is a receptor for hyaluronic acid and can also interact with other ligands, such as osteopontin, collagens, and matrix metalloproteinases.47 Thrombospondin is a potent activator of TGFβ.48 The current work recognizes Fra-2 as a protein that is abundant in the infarcted myocardial tissue and is implicated in inducing TGFβ transcription. Because all of these known transcriptional targets of Fra-2 are known to be upregulated in the context of tissue fibrosis,40,45,49,50 Fra-2 may serve as an important driver of myocardial fibrosis.
While the downstream targets of TGFβ signalling have been extensively studied, characterization of the transcriptional control of TGFβ remains sketchy.29 TGFβ function depends on both the transcription of the gene and the activation of the protein. The current work presents first evidence demonstrating that hyperoxic challenge, as evident during reoxygenation of ischaemic tissue, may trigger the induction of all three isoforms of TGFβ. The functional significance of Fra-2, especially in the heart, remains poorly developed. Taken together with the observation that Fra-2 regulated genes are implicated in cardiac fibrosis, identification of Fra-2 as a O2-sensitive transcriptional regulator of inducible TGFβ expression hypothetically positions Fra-2 as an important player in reoxygenation-induced fibrosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
The work was supported by NIH R01 HL073087 and GM077185.
Supplementary Material
References
- 1.Roy S, Khanna S, Rink T, Radtke J, Williams WT, Biswas S, et al. P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Mol Biol Cell. 2007;18:4837–4846. doi: 10.1091/mbc.E07-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74:207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol. 2003;25:79–86. doi: 10.1358/mf.2003.25.2.723680. [DOI] [PubMed] [Google Scholar]
- 4.Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, et al. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 5.Waltenberger J, Lundin L, Oberg K, Wilander E, Miyazono K, Heldin CH, et al. Involvement of transforming growth factor-beta in the formation of fibrotic lesions in carcinoid heart disease. Am J Pathol. 1993;142:71–78. [PMC free article] [PubMed] [Google Scholar]
- 6.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Wen FQ, Kohyama T, Skold CM, Zhu YK, Liu X, Romberger DJ, et al. Glucocorticoids modulate TGF-beta production. Inflammation. 2002;26:279–290. doi: 10.1023/a:1021412601538. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, et al. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1) Circ Res. 2003;92:264–271. doi: 10.1161/01.res.0000056770.30922.e6. [DOI] [PubMed] [Google Scholar]
- 9.Roy S, Khanna S, Wallace WA, Lappalainen J, Rink C, Cardounel AJ, et al. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. J Biol Chem. 2003;278:47129–47135. doi: 10.1074/jbc.M308703200. [DOI] [PubMed] [Google Scholar]
- 10.Sen CK, Khanna S, Roy S. Perceived hyperoxia: oxygen-induced remodeling of the reoxygenated heart. Cardiovasc Res. 2006;71:280–288. doi: 10.1016/j.cardiores.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Gnyawali SC, Roy S, McCoy M, Biswas S, Sen CK. Remodeling of the ischemia-reperfused murine heart: 11.7T cardiac magnetic resonance imaging of contrast enhanced infarct patches and transmurality. Antioxid Redox Signal. 2009;11:1829–1839. doi: 10.1089/ars.2009.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siaghy EM, Devaux Y, Sfaksi N, Carteaux JP, Ungureanu-Longrois D, Zannad F, et al. Consequences of inspired oxygen fraction manipulation on myocardial oxygen pressure, adenosine and lactate concentrations: a combined myocardial microdialysis and sensitive oxygen electrode study in pigs. J Mol Cell Cardiol. 2000;32:493–504. doi: 10.1006/jmcc.1999.1094. [DOI] [PubMed] [Google Scholar]
- 13.Khanna S, Roy S, Maurer M, Ratan RR, Sen CK. Oxygen-sensitive reset of hypoxia-inducible factor transactivation response: prolyl hydroxylases tune the biological normoxic set point. Free Radic Biol Med. 2006;40:2147–2154. doi: 10.1016/j.freeradbiomed.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn DE, Roy S, Radtke J, Gupta S, Sen CK. Laser microdissection and pressure-catapulting technique to study gene expression in the reoxygenated myocardium. Am J Physiol Heart Circ Physiol. 2006;290:H2625–H2632. doi: 10.1152/ajpheart.01346.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn DE, Roy S, Radtke J, Khanna S, Sen CK. Laser microdissection and capture of pure cardiomyocytes and fibroblasts from infarcted heart regions: perceived hyperoxia induces p21 in peri-infarct myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H1245–H1253. doi: 10.1152/ajpheart.01069.2006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Azhar G, Nagano K, Wei JY. Differential vulnerability to oxidative stress in rat cardiac myocytes versus fibroblasts. J Am Coll Cardiol. 2001;38:2055–2062. doi: 10.1016/s0735-1097(01)01665-5. [DOI] [PubMed] [Google Scholar]
- 17.Liao XD, Wang XH, Jin HJ, Chen LY, Chen Q. Mechanical stretch induces mitochondria-dependent apoptosis in neonatal rat cardiomyocytes and G2/M accumulation in cardiac fibroblasts. Cell Res. 2004;14:16–26. doi: 10.1038/sj.cr.7290198. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Khanna S, Kuhn DE, Rink C, Williams WT, Zweier JL, et al. Transcriptome analysis of the ischemia-reperfused remodeling myocardium: temporal changes in inflammation and extracellular matrix. Physiol Genomics. 2006;25:364–374. doi: 10.1152/physiolgenomics.00013.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ojha N, Roy S, Radtke J, Simonetti O, Gnyawali S, Zweier JL, et al. Characterization of the structural and functional changes in the myocardium following focal ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;294:H2435–H2443. doi: 10.1152/ajpheart.01190.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, et al. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA. 2007;104:14472–14477. doi: 10.1073/pnas.0706793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 24.Weigert C, Sauer U, Brodbeck K, Pfeiffer A, Haring HU, Schleicher ED. AP-1 proteins mediate hyperglycemia-induced activation of the human TGF-beta1 promoter in mesangial cells. J Am Soc Nephrol. 2000;11:2007–2016. doi: 10.1681/ASN.V11112007. [DOI] [PubMed] [Google Scholar]
- 25.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 26.Jibiki I, Hashimoto S, Maruoka S, Gon Y, Matsuzawa A, Nishitoh H, et al. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates nitric oxide-induced activator protein-1 activation in human bronchial epithelial cells. Am J Respir Crit Care Med. 2003;167:856–861. doi: 10.1164/rccm.2204042. [DOI] [PubMed] [Google Scholar]
- 27.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci USA. 1981;78:5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, et al. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SJ, Jeang KT, Glick AB, Sporn MB, Roberts AB. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989;264:7041–7045. [PubMed] [Google Scholar]
- 32.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- 33.Roberts AB, Sporn MB. Differential expression of the TGF-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol Reprod Dev. 1992;32:91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 34.Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- 35.Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 36.Taylor AW. Review of the activation of TGF-{beta} in immunity. J Leukoc Biol. 2009;85:29–33. doi: 10.1189/jlb.0708415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 38.Cheng F, Li Y, Feng L, Li S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant Proc. 2008;40:2167–2170. doi: 10.1016/j.transproceed.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 39.Furuichi K, Gao JL, Murphy PM. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am J Pathol. 2006;169:372–387. doi: 10.2353/ajpath.2006.060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishina H, Sato H, Suzuki T, Sato M, Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci USA. 1990;87:3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruda MC, Kovary K, Metz R, Bravo R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene. 1994;9:2537–2547. [PubMed] [Google Scholar]
- 43.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Andersen H, Mahmood S, Tkach V, Cohn M, Kustikova O, Grigorian M, et al. The ability of Fos family members to produce phenotypic changes in epithelioid cells is not directly linked to their transactivation potentials. Oncogene. 2002;21:4843–4848. doi: 10.1038/sj.onc.1205590. [DOI] [PubMed] [Google Scholar]
- 45.Zahradka P. Novel role for osteopontin in cardiac fibrosis. Circ Res. 2008;102:270–272. doi: 10.1161/CIRCRESAHA.107.170555. [DOI] [PubMed] [Google Scholar]
- 46.Ibrahim EM, Stewart RL, Corke K, Blackett AD, Tidy JA, Wells M. Upregulation of CD44 expression by interleukins 1, 4, and 13, transforming growth factor-beta1, estrogen, and progestogen in human cervical adenocarcinoma cell lines. Int J Gynecol Cancer. 2006;16:1631–1642. doi: 10.1111/j.1525-1438.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 47.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, et al. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 48.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 49.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, et al. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouschop KM, Sewnath ME, Claessen N, Roelofs JJ, Hoedemaeker I, van der Neut R, et al. CD44 deficiency increases tubular damage but reduces renal fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2004;15:674–686. doi: 10.1097/01.asn.0000115703.30835.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.